Abstract
Cerebral radiation necrosis (CRN) is a toxicity of radiation therapy that can result in significant, potentially life-threatening neurologic deficits. Treatment for CRN has included surgical resection, corticosteroids, hyperbaric oxygen therapy (HBOT), and bevacizumab, but no consensus approach has been identified. We reviewed the available literature to evaluate efficacy of treatment approaches. Using methods specified in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines when possible, we conducted searches of Ovid MEDLINE, Embase and Pubmed to identify studies reporting on outcomes for children (≤21 years old) with CRN. Eligible studies from 1990 to 2014 describing central nervous system (CNS) radiation necrosis with details of both treatment and outcomes were included. Eleven studies meeting criteria were identified. Of the nine studies with total patient denominators, 37 of 806 patients developed CRN (incidence = 4.6 %). Patients received treatment courses of steroids alone (n = 13), steroids with bevacizumab (n = 11) or HBOT (n = 12). Patients who failed to respond to steroids were more likely to be older than steroid-responsive patients (p = 0.009). With the exception of steroid-related adverse events, there was only one report of an adverse event (brainstem stroke) potentially attributable to intervention (bevacizumab). Those who received proton beam RT were both younger (p = 0.001) and had a shorter time to development of CRN (p = 0.079). The most common treatment following steroid initiation was addition of bevacizumab or HBOT, with good success and minimal toxicity. However, randomized controlled trials are needed to establish a definitive treatment algorithm that can be applied to children affected by CRN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The advent of treatment modalities including surgery, radiation therapy, and chemotherapy has brought about dramatic improvements in the survival of children with brain tumors, with survival rates for all age groups now exceeding 70 % [1]. However, with nearly 4300 children diagnosed yearly with a primary CNS tumor [2] and an increasing population of survivors, ameliorating the long-term adverse effects, ranging from stroke to neurocognitive deficits, has become a major area of study. Cerebral radiation necrosis (CRN) is a well-characterized toxicity of radiation therapy, first described in adult patients [3]. MRI findings in CRN have been well characterized and include white matter contrast enhancement with a “spreading wave front” of peripheral enhancement and a “Swiss cheese/soap bubble” appearance of interior enhancement [4]. Incidence data is primarily derived from adult studies but is thought to be 5–10 % at doses of 120 Gy (range 100–140) and 150 Gy (range 140–170) when given in fraction sizes of <2.5 Gy [5]. Retrospective studies of children reported widely variant CRN rates of 3–26 % [6–8].
No consensus treatment for pediatric CRN has been identified. Due to the paucity of pediatric data, we sought to collect the modalities used in the treatment of CRN in pediatric patients. Given the diagnostic challenge posed by new lesions in children with brain tumors and the potentially devastating long-term effects of CRN, there is a need for a successful and low-risk treatment approach; thus, a systemic review of the collective medical experience can provide guidance to practitioners in pediatric CRN therapy.
Methods
Search strategy and study eligibility
Our methodology was guided by the PRISMA checklist [9], which provides an evidence-based process for conducting systematic reviews. Given the limited number of published studies describing outcomes in children with CRN, most with very small sample sizes, many elements of the PRISMA checklist were not applicable for this review. The procedure for identification of eligible studies is detailed in Fig. 1. The following electronic databases were searched to identify reports of either relevant pediatric randomized controlled trials or case series: the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid EMBASE; and the reference sections of included studies. Search terms included: pediatric; cerebral; radiation; necrosis; treatment. These search terms resulted in 64 publications, one of which described a duplicate sample. The remaining 63 studies were screened by two authors (ND, EH) to determine which met inclusion criteria. Six studies were excluded due to their involvement of topics outside of radiation necrosis (e.g., diabetic ketoacidosis). Inclusion criteria included: (1) publication in a peer-reviewed journal, (2) availability partly or wholly in English, (3) report on pediatric patients who developed CRN from 1981 to 2014. In addition, eligible studies included patients who were ≤21 years of age with any central nervous system (CNS) lesion that received radiation therapy who developed radiation necrosis and reported details of both treatment and outcomes. The measures of primary interest were response (either clinical or radiographic), event-free survival and overall survival.
As may be seen in Fig. 2, 52 of the identified studies (82.5 %) were omitted due to incomplete demographic, treatment, or outcome data, as follows: (1) CRN-oriented report without adequate details of CRN-directed therapy, (2) Studies reporting only diagnostic radiologic studies, (3) Reports detailing radiation effects broadly without sufficient CRN-specific data (4) General studies on pediatric brain tumors (5) Publications listing CRN as an adverse effect of treatment without other information. Three included studies prospectively evaluated a treatment modality amongst patients who had already developed CRN. Although these studies did not include sufficient information needed to describe incidence of CRN, all authors were contacted and one of the three was able to provide this information after review of their records.
Reasons for study exclusion. Excluded studies fell into five broad categories: (1) those reporting CRN without reporting CRN treatment data, (2) studies which focused on the use of radiology in diagnosing CRNs without other pertinent data, (3) those which primarily discussed effects of RT outside of CRN, (4) reports which involved other topics within the subject of pediatric BTs, and (5) studies that involved topics other than treatment within the field of CRN
Data extraction and synthesis
Two authors (ND, EH) independently reviewed each study and extracted data from the reports. Patient age and tumor type, type of radiation therapy, symptoms/symptom evolution, and dose received were tabulated with those patients who developed CRN further analyzed as to the location of lesion(s), treatment(s) used, time to treatment, primary outcome of treatment, and length of follow-up. Discrepancies between authors were resolved by consensus after discussion. All treatment modalities were identified across reported studies. Eleven non-duplicative papers were included.
Statistical approach
Because the number of participants across identified studies was small and none of the eligible reports were randomized clinical trials, traditional meta-analytic strategies were not possible. Rather, we provide descriptive statistics of patients’ demographic (i.e., age, gender) and medical (i.e., tumor, treatment, timing of onset of CRN) characteristics, as well as clinical features of patients’ CRN treatment outcomes. When complete patient-level data were available, we used t tests and Pearson correlations to determine associations between demographic and medical characteristics and CRN-related outcomes. All analyses were conducted using SPSS, version 23 (SPSS Inc., Chicago, IL).
Results
CRN incidence and associated features
Eleven studies met all inclusion/exclusion criteria. Table 1 lists characteristics of the samples. Across all identified studies, forty-eight pediatric patients were reported to have developed CRN. Of these, 45 were diagnosed with primary CNS tumors, one with a metastatic brain lesion, and two with arteriovenous malformations. Thirty-nine patients were diagnosed by radiographic findings only (with confirmatory histology obtained at autopsy for one) and nine patients underwent tissue biopsy. As noted above, two of the 11 studies did not provide sufficient information to determine the incidence of CRN within a larger sample; however, using the nine studies which included information about the percentage of patients who developed CRN, 37 of 806 patients developed CRN for an incidence of 4.6 %. The ages of the children at diagnosis of CRN ranged from 2 to 17 years (median eight) with 35.4 % female. The most frequent tumor subtypes represented were low-grade gliomas outside of the brainstem (8), followed by patients with brainstem gliomas including diffuse intrinsic pontine gliomas and pilocytic astrocytomas (5). Radiation therapy utilized various doses and modalities, including external beam (EBRT) or proton beam radiation (PBT) [10] and stereotactic radiosurgery (SRT). EBRT doses ranged from 2400 to 7020 centigrays (cGy), SRT doses ranged from 800 to 2400 cGy, and PBT doses ranged from 4700 to 5240 cGy. Children who received PBT were younger than those who received EBRT (p = 0.001). CRN developed while on therapy in one patient (1 week prior to completion of RT); the remaining patients developed CRN up to 131 months after completion of radiation therapy (median 5 months); those who received PBT had a shorter timeline to development of CRN compared with those received photon beam radiation approaching statistical significance (p = 0.079). Follow up ranged from 3 months to over 4.6 years, with disparate spans in large part due to differing underlying diagnoses. Patients with poor prognoses tended to have shorter follow up times by virtue of disease progression. Screening protocols for radiation necrosis were not clearly stated across all studies, though all patients had imaging with neurologic changes as well as periodic imaging as per standard of care or clinical trial mandates.
In addition to radiation therapy, some patients also received high-dose chemotherapy in conjunction with radiation therapy [15, 20, 21] or also received therapy on Phase I protocols [14]. At least 20 patients underwent chemotherapy for diagnoses including medulloblastoma, ependymoma, and low grade glioma, either before or after radiation therapy, while at least 13 patients did not receive chemotherapy, including both patients with AVM, five with low grade gliomas, and five with high grade glioma/DIPG. Given the small number of patients comprising each of these groups (age, gender, diagnosis, type of radiation therapy, radiation dose/field, and use of additional treatment agents), no statistical analyses were performed.
Forty-five of 48 patients (94 %) presented with new neurologic symptoms prompting imaging and/or biopsy and were subsequently diagnosed with CRN. The most frequent documented symptoms included ataxia (43.8 %), cranial nerve palsies (39.6 %), weakness (37.5 %), headache (18.8 %), and vision changes (6.3 %).
CRN treatment and outcomes
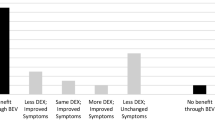
A summary of treatment modalities and outcome measures can be found in Fig. 3. All but four patients (92 %) received at least one method of pharmacotherapy. Corticosteroids were the most commonly used treatment modality with 89.6 % (n = 43) of patients receiving them as all or part of their therapy, while 35.4 % of patients received HBOT and 33.3 % received bevacizumab as part of their treatment regimens.
Long-term response to treatment. Summary of treatment modalities and outcome measures amongst patients receiving no treatment, resection alone, corticosteroids, corticosteroids + HBOT, corticosteroids + bevacizumab, and other combinations. *Other combinations included: Bevacizumab alone; corticosteroids + bevacizumab and HBOT; corticosteroids + HBOT and LMWH; corticosteroids + IVIG; corticosteroids + bevacizumab and irinotecan; corticosteroids + HBOT and bevacizumab and pentoxifylline
Table 2 shows the outcomes for different CRN treatment modalities. Twelve patients (25 %) were treated with hyperbaric oxygen therapy (HBOT) alone after an initial course of steroids. Of these, eleven patients (92 %) had initial stabilization and/or improvement in symptoms and nine (75 %) had resolution or improvement on imaging with data being unavailable for two patients. Three (25 %) of the patients who had initial improvement or stabilization of imaging and symptoms ultimately died of disease progression.
Eleven patients (23 %) received bevacizumab alone in conjunction with steroid treatment; ten of these (91 %) had symptom and imaging improvement; one patient died of disease progression. Eight patients (17 %) received other combinations of pharmacotherapy including one patient who received bevacizumab alone, and one patient each received corticosteroids in concert with IVIG; irinotecan and bevacizumab; HBOT and LMWH; and bevacizumab, HBOT and pentoxifylline. Two patients received corticosteroids, bevacizumab and HBOT.
Response to therapy was generally favorable with 52.1 % (n = 25) of patients demonstrating symptomatic and/or imaging improvement and 27.1 % (n = 13) with stabilization. One patient underwent resection due to the unclear nature of the radiographic findings with lesions that were suspicious for tumor recurrence. In this case, histological examination after resection confirmed RN rather than tumor and no further treatment was needed [22]. Fourteen patients (29.2 %) received steroids alone, and of these, six patients demonstrated improvement in symptoms. One patient with a low-grade pontine glioma had worsening neurologic symptoms later attributed to disease progression and ultimately died of disease [7]. The thirty (62.5 %) remaining patients underwent initial treatment with steroids although four had recurrence of neurologic symptoms on tapering the steroid dose. Of those patients for whom descriptive data is available [23], five had severe side effects related to long-term steroid use, including Cushingoid features, arterial hypertension, striae development, and an increase in body mass index [18, 19]. At least sixteen of the forty-three patients who received steroid treatment (37 %) failed initial treatment with steroids, typically described as neurologic symptom reemergence [12, 14, 16, 18].
Toxicities from CRN-directed treatment were relatively manageable, although steroid treatment often resulted in an unacceptable side effect profile [19]. Two children developed avascular necrosis after steroids and bevacizumab [18]. Older children were more likely to fail steroid monotherapy (p = 0.009). One patient developed a brainstem stroke while receiving bevacizumab after having received corticosteroids and HBOT for CRN; however, it was unclear whether bevacizumab played a role in this event [10]. Two patients (4 %) died of CRN, one of whom received corticosteroid treatment alone and one who received corticosteroids, bevacizumab and HBOT.
We examined the relation between symptom presentation, patient demographics, and treatment response for the 48 patients for whom these data were available. Time to development of CRN was not significantly related to age, (t = −0.06, p = 0.95), gender (t = −0.06, p = 0.96), or radiation modality (t = 1.12, p = 0.27). Weakness as a presenting symptom did not differ by age, gender, radiation modality, or time to development. There was a trend for patients presenting with headache to be older (t = 1.93, p = 0.060), and for patients presenting with ataxia to be younger (t = −1.98, p = 0.055). Failure to respond to bevacizumab was not related to age or time to development of CRN (t = −0.53, p = 0.60 and t = −0.35, p = 0.74, respectively). Patients who failed to respond to steroids, however, were significantly more likely to be older (with a mean of 9.8 years versus 6.5 years) than patients who responded to steroid treatment (t = 2.76, p = 0.009).
Discussion
CRN is a well-known complication of RT for brain tumors, primarily described in adult patients [3, 24, 25]. However, with increasing numbers of surviving pediatric brain tumor patients, there is a need for pediatric-based approaches for the treatment of late effects, including CRN; unfortunately, the outcomes of most treatment approaches are limited to small reports and case studies without any unification of findings. In the adult population, treatment of CRN has included such varied approaches as corticosteroids [10, 24, 26–30], bevacizumab [4, 20, 24], hyperbaric oxygen therapy [26, 31], NSAIDs, anticoagulation [28, 29], and oral vitamin E [30].
Through our systematic review, we were able to examine the largest aggregated sample of pediatric patients undergoing treatment for CRN in the literature to date. The incidence of CRN has been previously reported as falling between 3 and 26 % [6–8]. In our analysis, comprised from an aggregate of studies of one to 236 patients, a total of 48 children developed CRN, diagnosed by development of neurologic symptoms, imaging, or both. Of the aggregate of 806 patients described in those studies with patient denominators, 37 patients developed CRN, for an overall incidence of 4.6 %.
CRN occurs within three time periods: acute (during or immediately following therapy), early-delayed (between 3 and 6 weeks and 3–6 months after therapy is complete), and late-delayed (between 6 and 12 months to years after therapy is complete) [32]. Based on our data, the median time to development of CRN was 5 months; interestingly, those who received proton beam RT were both significantly younger and may have had a shorter time to development of CRN. Though these patients likely received proton beam RT because of their young ages in an effort to avoid the increased toxicity of external beam RT, it is possible that they are also prone to earlier development of CRN, or that proton beam radiation may hasten the development of CRN. Younger children tended to present with ataxia, and older children with headache when diagnosed with CRN, although this may be reflective of typical general tumor location (infratentorial vs. supratentorial, respectively) in the two age groups.
Thought to occur as a primary effect of cerebral vascular injury, radiation necrosis begins as acute cellular injury with endothelial cell death resulting in platelet aggregation and thrombus formation leading to occlusion of microvessels [33]. Endothelial cell dysfunction may also cause the release of vascular endothelial growth factor (VEGF) with resultant capillary leakage and brain edema [24]. Histopathologically, vascular derangement is present along with demyelination and white matter necrosis [3].It is thought that cellular injury and vascular damage from radiation leads to tissue hypoxia and initiates a cascade of events resulting in degradation of collagen, disruption of the blood–brain barrier (BBB) [3], vascular endothelial growth factor (VEGF) release and capillary leakage [24], perivascular inflammation and the production of thrombi [29]. These changes can occur diffusely throughout the white matter, leading to cerebral atrophy, or focally, causing mass lesions and resulting in confusion, seizures, and other significant neurologic effects [12].
Three different modalities of treatment were primarily used in the series reviewed; corticosteroids, bevacizumab and HBOT, either singly or in combination. Corticosteroids were used for their potent anti-inflammatory effects, but are associated with a significant adverse effect profile that often makes long-term use untenable. Bevacizumab is a humanized monoclonal antibody that blocks VEGF. HBOT and vitamin E are thought to improve tissue oxygenation leading to neovascularization and reduction of inflammation.
Corticosteroids were the initial mainstay of treatment in nearly 90 % of patients who received pharmacotherapy for their lesions. Many patients were either unable to tolerate steroid therapy on a long-term basis, or developed a recurrence of neurologic symptoms on tapering of their steroid dose. Older patients (mean 9.8 years) failed steroid monotherapy more frequently than younger patients (mean 6.5 years). Among those treated with corticosteroids in addition to either HBOT (12 patients) or bevacizumab (ten patients), 92 and 90 %, respectively, had improvement in symptoms, neuroimaging, or both. Further, each approach was generally safe, with no positively attributed severe adverse events with either methodology. These data suggest that earlier use of adjuvant agents in addition to steroids is safe, more tolerable and may be particularly important in older patients.
As with all systematic reviews, there were several limitations to this study. First, these data are likely affected by selection bias, as characterization of more severely affected patients, particularly those who require treatment, is more likely to have been prioritized both by researchers. Because of the limited number of studies and their tendency to be descriptive in nature, however, it is difficult to estimate the level of selection bias in the studies that were published. Further, given the different types of reports ranging from small sample case reports to larger scale retrospective analyses, the overall dataset is variable. Treatment bias may also have been present, as centers with ready availability of HBOT may have led to a tendency to report HBOT-related positive findings. Due to the small numbers of patients receiving a particular treatment for their tumor, it is not possible to draw conclusions regarding the effect of a particular regimen (ranging from high dose chemotherapy with autologous stem cell transplant to phase I therapies) on the development of CRN. As described, six patients died of their disease, and at least twenty-six patients had remaining neurologic deficits; whether these were attributable to CRN or their original lesion is unknown. Likewise, the duration of follow up was potentially inadequate for many patients, in part due to the poor prognosis associated with some of the tumors. As these reports span many years, there may have been changing practices amongst clinicians with a lack of rigorous and systematic documentation of adverse effects in previous years. Furthermore, there is no consensus on standard definitions for the grading of CRN itself or on the response to therapy (either radiographically and symptomatically), although neurologic exam, Lansky–Karnofsky performance status, and the NCI Common Terminology Criteria for Adverse Events, were all used to describe patients’ clinical status. Finally, it is possible that our search missed cases within larger reports of radiation therapy for CNS lesions, and the small number of cases that were described limited our ability to conduct a meta-analysis of the efficacy of treatment approaches.
CRN is a recognized complication of the different modalities of radiation therapy. Based on the results presented, there is no clear evidence that HBOT, bevacizumab or a combination of the two is more effective than corticosteroids alone, though it is worth noting that older patients were more likely to fail steroid therapy which could help to guide treatment approaches. Thus, more prospective evaluation is needed, ideally based upon a standardized definition of radiographic signs and response to treatment to delineate the most appropriate outcome measure for these patients, including overall survival, neurologic status, quality of life, and duration of steroid use. The use of bevacizumab and/or HBOT appears to be safe and effective in this population of patients. Ultimately, a better understanding of which patients are at risk for CRN and what therapies put them at highest risk is needed to effect a change in its therapeutic management and to bring about a rapid and lasting improvement to the sometimes devastating neurologic effects of CRN.
References
Smith MA, Seibel NL, Reaman GH et al (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28(15):2625–2634
Ostrom QT, Gittelman H, Farah P et al (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neurooncol 15:ii1–ii56
Greene-Schloesser D, Robbins ME, Peiffer AM et al (2012) Radiation-induced brain injury: a review. Front Oncol 2(73):1–18
Rogers LR, Gutierrez J, Scarpace L et al (2011) Morphologic magnetic resonance imaging features of therapy-induced cerebral necrosis. J Neurooncol 101:25–32
Lawrence YR, Li XA, el Naqa I et al (2010) Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 76(3 suppl):20–27
Hodgson DC, Goumnerova LC, Loeffler JS et al (2001) Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys 50(4):929–935
Kalapurakal JA, Kepka A, Bista T et al (2000) Fractionated stereotactic radiotherapy for pediatric brain tumors: the Chicago children’s experience. Child’s Nerv Syst 16:296–303
Plimpton SR, Stence N, Hemenway M, Hankinson TC, Foreman N, Liu AK (2015) Cerebral radiation necrosis in pediatric patients. Pediatri Hematol Oncol 32(1):78–83
Mohler D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 Statement. Syst Rev 4:1
Indelicato DJ, Flampouri S, Rotondo RL et al (2014) Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 53:1298–1304
Marks JE, Baglan RJ, Prassad SC, Blank WF (1981) Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys 7(2):243–252
Chuba PJ, Aronin P, Bhambhani K et al (1997) Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer 80(10):2005–2012
Beuthien-Baumann B, Hahn G, Winkler C, Heubner G (2003) Differentiation between recurrent tumor and radiation necrosis in a child with anaplastic ependymoma after chemotherapy and radiation therapy. Strahlenther Oncol 12:819–822
Liu AK, Macy ME, Foreman NK (2009) Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75(4):1148–1154
Murphy ES, Merchant TE, Wu S et al (2012) Necrosis after craniospinal irradiation: results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys 83(5):e655–e660
Wang Y, Pan L, Sheng X et al (2012) Reversal of cerebral radiation necrosis with bevacizumab in 17 Chinese patients. Eur J Med Res 17:25
Strenger V, Lackner H, Mayer R (2013) Incidence and clinical course of radionecrosis in children with brain tumors. Strahlenther Oncol 189:759–764
Foster KA, Ares WJ, Pollack IF, Jakacki RI (2014) Bevacizumab for symptomatic radiation-induced tumor enlargement in pediatric low grade gliomas. Pediatr Blood Cancer. doi:10.1002/pbc.25277
Preuss M, Hirsch W, Hoffmann KT, Bernhard MK, Siekmeyer M, Kiess W, Meixensberger J, Wurm RE, Merkenschlager A (2013) Effectiveness of bevacizumab for radiation-induced cerebral necrosis in children. Pediatr Neurosurg 49(2):81–85
Furuse M, Kawabata S, Kuroiwa T, Miyatake SI (2011) Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neuro-Oncol 102(3):471–475
Byrd SE, Tomita T, Palka PS, Darling CF, Norfray JP, Fan J (1996) Magnetic resonance spectroscopy (MRS) in the evaluation of pediatric brain tumors part II: clinical analysis. J Natl Med Assoc 88(11):717–723
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Stockham AL, Ahluwalia M, Reddy CA et al (2013) Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neurooncol 115:469–475
Sadraei NH, Dahiya S, Chao ST et al (2013) Treatment of cerebral radiation necrosis with bevacizumab: the cleveland clinic experience. Am J Clin Oncol 00:1–7
Ruben JD, Dally M, Bailey M et al (2006) Cerebral Radiation Necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65(2):499–508
Kuffler DP (2012) Hyperbaric oxygen therapy: Can it prevent irradiation-induced necrosis? Exp Neurol 235:517–527
Alessandretti M, Buzaid AC, Brandao R, Brandao E (2013) Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep Oncol 6:598–601
Happold C, Ernemann U, Roth P et al (2008) Anticoagulation for radiation-induced neurotoxicity revisited. J Neurooncol 90:357–361
Glantz MJ, Burger PC, Friedman AH et al (1994) Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology 44(11):2020–2027
Williamson R, Kondziolka D, Kanaan H et al (2008) Adverse radiation effects after radiosurgery may benefit from oral vitamin e and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg 86:359–366
Wanebo JE, Kidd GA, King MC, Chung TS (2009) Hyperbaric oxygen therapy for treatment of adverse radiation effects after stereotactic radiosurgery of arteriovenous malformations: case report and review of literature. Surg Neurol 72:162–168
Helton KJ, Edwards M, Steen G et al (2005) Neuroimaging-detected late transient treatment-induced lesions in pediatric patients with brain tumors. J Neurosurg 102:179–186
Sundgren PC, Cao Y (2009) Brain irradiation: effects on normal brain parenchyma and radiation injury. Neuroimag Clin N Am 19:657–668
Acknowledgments
This project wishes to acknowledge the support of the Tucker Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose for this project.
Rights and permissions
About this article
Cite this article
Drezner, N., Hardy, K.K., Wells, E. et al. Treatment of pediatric cerebral radiation necrosis: a systematic review. J Neurooncol 130, 141–148 (2016). https://doi.org/10.1007/s11060-016-2219-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2219-5