Abstract
Background and purpose
Radiation necrosis is a possible adverse event after cranial radiation therapy and can cause severe symptoms, such as an increased intracranial pressure or neurological deterioration. The vascular endothelial growth factor (VEGF) inhibitor bevacizumab (BEV) has been shown to be a feasible therapeutic option for symptomatic radiation necrosis, either when traditional antiedematous steroid treatment fails, or as an alternative to steroid treatment. However, to the best of our knowledge, only one randomized study with a rather small cohort exists to prove a beneficial effect in this setting. Therefore, further real-life data are needed. This retrospective monocentric case study evaluates patients who received BEV due to radiation necrosis, with a specific focus on the respective clinical course.
Methods
Using the internal database for pharmaceutical products, all patients who received BEV in our department were identified. Only patients who received BEV as symptomatic treatment for radiation necrosis were included. Patient characteristics, symptoms before, during, and after treatment, and the use of dexamethasone were evaluated using medical reports and systematic internal documentation. The symptoms were graded using CTCAE version 5.0 for general neurological symptoms. Symptoms were graded directly before each cycle and after the treatment (approximately 6 weeks). Additionally, the daily steroid dose was collected at these timepoints. Patients who either improved in symptoms, received less dexamethasone after treatment, or both were considered to have a benefit from the treatment.
Results
Twenty-one patients who received BEV due to radiation necrosis were identified. For 10 patients (47.6%) symptoms improved and 11 patients (52.4%) remained clinically stable during the treatment. In 14 patients (66.7%) the dexamethasone dose could be reduced during therapy, 5 patients (23.8%) received the same dose of dexamethasone before and after the treatment, and 2 patients (9.5%) received a higher dose at the end of the treatment. According to this analysis, overall, 19 patients (90.5%) benefited from the treatment with BEV. No severe adverse effects were reported.
Conclusion
BEV might be an effective and safe therapeutic option for patients with radiation necrosis as a complication after cranial radiation therapy. Patients seem to benefit from this treatment by improving symptomatically or through reduction of dexamethasone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation necrosis (RN) is a severe adverse effect after cranial radiation therapy and is one of the main dose-limiting factors [1, 2]. The total radiation dose, the size of the target volume, and fractionation regime are the main risk factors for RN [2, 3]. Especially patients undergoing reirradiation or radiosurgery are at risk for RN [3]. Due to higher frequencies of reirradiation and radiosurgery, the incidence of RN has also risen, which makes the management of RN an important clinical issue. RN typically appears at least 3 months after radiation therapy [2, 3]. Symptoms of RN mostly consist of cephalgia, nausea, vertigo, seizures, or other area related neurological symptoms [4].
The pathogenesis of RN has been well described by Gonzalez et al. as “a continuous process in which endothelial cell dysfunction leads to tissue hypoxia and necrosis, with the concomitant liberation of a vasoactive compound, such as the vascular endothelial growth factor (VEGF)” [5]. The higher levels of VEGF in the surrounding areas of RN are mainly caused by hypoxemia [6]. VEGF leads to a dysfunction of the blood–brain barrier and therefore causes cerebral edema [4].
So far, the primary treatment for RN consists of antiedematous drugs, such as dexamethasone or mannitol [7]. Alternatively, surgical decompression can also serve as an invasive but beneficial palliative treatment option in well-selected cases when non-invasive treatments fail [7, 8]. There are other alternative treatments such as hyperbaric oxygen, antiplatelet antibodies, or high-dose tocopherols, but these showed only limited efficacy [3]. As an additional treatment option, the VEGF antibody bevacizumab (BEV) has been discussed and examined in recent years [5, 9]. So far, however, BEV only can be applied as an off-label treatment. In order for insurance companies to cover the costs of BEV as a treatment for RN, this cost coverage must be applied for first, by proving the treatment’s necessity for this individual patient. Therefore, the diagnosis of RN must be secured and traditional treatment must have been tried previously.
The indication for BEV is symptomatic RN diagnosed either by biopsy or imaging, which usually consists of contrast media-enhanced MRI or (in selected cases and according to availability) fluoroethyl-L-tyrosine positron emission tomography (FET-PET) [10, 11]. Differentiation between RN and progressive tumor is still a clinical challenge, since therapeutic approaches are dependent on the diagnostic validity [12, 13].
Clinical experience with BEV is still quite scarce. To the best of our knowledge, the study of Levin et al. is the only randomized study regarding this topic [9]. However, a few studies, both retrospective and prospective, and case reports have already demonstrated the clinical or radiological benefit [5, 14,15,16,17]. To support these data, real-life studies, which report on clinical experience with BEV, are needed. This retrospective study analyzed all patients with cerebral radiation necrosis who were treated with BEV at a single center.
Methods
Progressive tumor and RN often cannot be distinguished easily on follow-up MRI following radiotherapy. For further clarification and in equivocal cases, a FET-PET was usually added at our institution in order to differentiate a vital tumor lesion from necrotic tissue. If a final diagnosis could still not be made, a biopsy was performed. After diagnosis of RN, symptomatic patients first received traditional treatments such as dexamethasone. As far as no response was seen or a steroid-refractory state was reached, the interdisciplinary tumor board assessed whether BEV application could be an alternative treatment option.
For data acquisition, the internal database for pharmaceutical products of the University Hospital of Munich, Zenzy® (Dr. Heni SOFTWARE, Kirchzarten, Germany), was searched for all patients who received BEV. For these patients, the indication was cross-checked using the internal radiotherapy database system MOSAIQ® (Elekta, Stockholm, Sweden). Only patients who received BEV for radiation necrosis were included. Patients who received BEV therapeutically, e.g., as concurrent systemic therapy during reirradiation in recurrent malignant glioma, were excluded.
General patient characteristics, treatment parameters for BEV, and dosimetric data of the radiation therapy were derived from patient charts. For evaluation of the clinical symptoms before, during, and after BEV, treatment medical reports and documentation were searched. The symptoms were graded using the CTCAE version 5.0. All patients were graded regarding neurological symptoms (0 = no symptoms; 1 = light symptoms; 2 = moderate symptoms; 3 = severe symptoms; 4 = life-threatening symptoms; 5 = death). Additionally, the daily dose of dexamethasone before, during, and after treatment was assessed, as an additional parameter for edema reduction. These clinical data were acquired for the time period immediately before the initiation of each therapy cycle and approximately 6 weeks after the last BEV treatment. Patients who either improved symptomatically or received less steroids after treatment with BEV, or both, were considered having benefited from therapy. All patients in the cohort were retrospectively assessed for severe adverse effects associated with BEV, such as hemorrhage, proteinuria, abscesses, lung embolisms, et cetera. Follow-up MRIs, if available, were analyzed for the change of perifocal edema. The collected data were evaluated using descriptive statistics.
Results
In total, 21 patients treated with BEV due to radiation necrosis after cranial radiation therapy were identified. The cohort consisted of 9 women (42.9%) and 12 men (57.1%). The median age at the fist BEV cycle was 56 years (range 31–82 years). Overall, 11 patients (52.4%) were irradiated for cerebral metastases, 7 (33.3%) had the diagnosis of glioma, 2 (9.5%) patients presented with a meningioma, and 1 patient (4.7%) was treated for a nasopharyngeal carcinoma with brain invasion. Concerning definitive radiotherapy, 6 patients (28.6%) were treated with conventional three-dimensional conformal radiotherapy (3D-CRT) (mostly gliomas), 1 patient (4.7%) with volumetric arc therapy (VMAT), 10 patients (47.6%) with stereotactic radiosurgery (SRS), and 3 patients (14.3%) with brachytherapy using iodine-125 seeds. Five patients received an additional RT prior to the RN (1 VMAT, 2 SRT, and 2 3D-RT) and 1 patient received two additional RTs (both seeds). All patients received a BEV dosage of 7.5 mg/kg every 3 weeks. The median number of BEV therapy cycles was 2 (range 1–4). The patient characteristics are presented in Table 1.
In 10 patients (47.6%) all symptoms improved, while 11 patients (52.3%) did not improve symptomatically during treatment. The main symptoms were high intracranial pressure with cephalgia, nausea, and vertigo in 8 patients (38.1%), paresis and muscle weakness in 11 patients (52.4%), seizures in 1 patient (4.8%), and mainly neurocognitive impairment in 1 patient (4.8%). Symptomatic improvement was seen in 4 patients with symptoms of intracranial pressure (3 recovered completely), 5 patients with paresis (3 recovered completely, 2 only partially), and the patient with seizures (no further seizures). Nevertheless, in 14 patients (66.7%), the dexamethasone dose could be reduced during therapy, 5 patients (23.8%) received the same dose of dexamethasone before and after the treatment, and 2 patients (9.5%) received a higher dose after the end of BEV treatment. These last 2 patients (9.5%) improved in symptoms and were also counted to the success group. Taken together, 19 patients (90.5%) benefited from treatment with BEV. Three patients (14.3%) did not show any benefit following the treatment. These results are depicted in Fig. 1. Concerning adverse effects, 2 patients (9.5%) had an episode of epistaxis (CTCAE grade I and grade II) during BEV therapy, one of whom was treated with electrocoagulation. No other severe adverse effects were reported in the cohort.
Patient outcome depending on dexamethasone use and neurological symptoms. The leftblack column represents patients who were considered to have benefited from the treatment; the rightblack column shows the patients who did not benefit. The gray columns show the according subgroups of patients. BEV Bevacizumab, DEX Dexamethasone
19 patients (90.5%) were examined with follow-up MRIs to observe the course of edema. The 2 patients who did not receive a follow-up MRI had a reduced performance status and received best supportive care without any further MR imaging. 13 patients (68.4%; 61.9% of all patients with follow-up MRI) showed a reduction of the edema and 6 patients (31.6%; 28.6% of all patients with follow-up MRI) had stable disease. Of the 19 patients (90.5%) who had a clinical benefit from BEV, 12 patients (57.5%) showed an associated reduction of edema on MRI. 1 patient (4.7%) had a reduction of the edema on imaging but did not show any clinical improvement. 9 patients (42.9%) had more than two cycles of BEV and were examined after 2 BEV administrations. All of these patients showed a reduction of the edema on MRI and had a clinical benefit according to the criteria mentioned above. An example is given in Fig. 2. Overall, 6 patients (28.6%) received a further two cycles and 3 patients (14.3%) received a total of four BEV administrations. The administration of two further cycles (after the first two) was decided upon according to the trial of Levin et al. [9]. Patients who did not receive more than two cycles either showed sufficient improvement or showed no benefit at all. 3 of the 4 patients who received four cycles showed complete response; therefore, the interdisciplinary tumor conference decided not to administer any further cycles. After these cycles, no patient received any further cycles similar to the patients in the trial of Levin et al., in which not more than four cycles were applied [9]. Except for patients who had a recurrence of their primary decease, no related symptoms returned after BEV treatment.
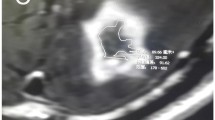
Example of a case with radiation necrosis. The patient was diagnosed with meningioma in 11/2014 and treated with stereotactic radiosurgery with 18 Gy to the 80% isodose. The MRI images (T1 and T2 sequences) show the initial images (a,e), the diagnosis of radiation necrosis in 07/2015 (b,f), the state after two applications of BEV in 10/2015 (c,g), and the state after two further applications of BEV in 12/2015 (d,h). The bottom pictures show the radiation plan. The red x in picture i indicates the isocenter, the blue dot in picture j indicates the dose reference point. The patient suffered from headache and vertigo and needed 12 mg dexamethasone/day at the time of diagnosis. After receiving four applications of BEV she recovered from the symptoms
Discussion
Necrotic areas in the brain are typically highly hypoxic. In these hypoxic areas, the hypoxia-inducible factor‑1 (HIF-1) is released and regulates the expression of pro-angiogenic factors, VEGF being the most important one. VEGF causes further growth of blood vessels with damaged blood–brain barriers, and thus causes leakage in the areas around the radiation necrosis [6]. It was hypothesized that this main effect of radiation necrosis can be blocked by inhibiting the expression of VEGF. Due to the pathophysiological association with VEGF, its antibody BEV seems to be a viable treatment method for RN [5].
Duan et al. investigated the effect of the VEGF inhibitor in mice, in which RN was artificially induced. The VEGF inhibitors could significantly revert the histological and radiological effect of RN [18]. The biological hypothesis of the exact effect is reduction of the vascular leakage by preventing VEGF from reaching its vascular target and thus reducing the cerebral edema.
Due to this promising theoretical effect, BEV has been tested in recent years in clinical practice. Clinical evidence mostly consists of retrospective studies with small cohorts or non-randomized prospective studies or case reports. These, however, showed promising clinical and radiological improvements [5, 14,15,16,17, 19]. The only randomized placebo-controlled double-blind study was done by Levin et al. in 2011 [9]. Although including only a small number of patients (n = 14), this study was able to deliver good evidence on the treatment of RN with BEV. Only patients who received BEV instead of placebo showed a clinical and radiological improvement. In contrast to our study, only patients with a Karnofsky index of 60 or higher were included into the prospective study of Levin et al., which might explain the better overall outcome concerning clinical improvement. Delishaj et al. reviewed the existing literature (in total 21 reports) and found an overall positive effect of BEV in patients with RN [3]. Of all 125 cases reviewed, 91.2% (114 cases) had an improvement in neurological symptoms. In comparison, only 47.6% of our cohort improved regarding clinical endpoints. The reason for this divergence might probably be due to the way that patients were collected for this literature review. The authors state that “only patients who responded to bevacizumab were likely to be included in the published literature” [3]. In the present study, however, all patients with RN who were treated with BEV were included, which results in a less biased cohort. Similarly, the prospective study of Furuse et al. reported a symptomatic improvement of 42.1% [17]. The low number of adverse events (2.4%) described by Delishaj et al. was, however, quite consistent with our data (9.5%) [3]. In contrast, the percentage of radiographic response was significantly smaller in the present study (68.4%; N = 19) than in comparable studies, as in Sadraei et al. or Furuse et al., with 95.8 and 78.9%, respectively.
In addition to the treatment of RN, BEV also has promising data for patients who receive reirradiation due to a recurrence of glioblastoma, as a concomitant systemic therapy during re-irradiation and following sequential administration every 2 weeks [20, 21]. In the study by Gutin et al., no cases of RN were reported and no additional dexamethasone was administered [22]. In this context, it is important to note that the incidence of RN is more frequent in patients treated with re-irradiation and that BEV could possibly reduce this risk and result in a more favorable outcome. Moreover, the inhibition of VEGF could increase the radiosensitivity of the tumor cells and therefore lower the required radiation dose [23]. The addition of BEV to first-line radiation therapy with concomitant temozolomide was also investigated by Chinot et al. in the AVAglio trial [24]. However, besides an improvement in progression-free survival and quality of life, no significant overall survival benefit was identified. On the other hand, the number of adverse effects was higher in the treatment arm with BEV. Another study by Pitter et al. was able to demonstrate that dexamethasone intake compromised overall survival in patients with glioblastoma undergoing radiochemotherapy and that BEV did not have any influence on overall survival, while having a good antiedematous effect [25]. Thus, BEV might not only be of use for prophylactic or therapeutic treatment for radiation necrosis, but also for management of cerebral edema in general.
While this study shows promising results, further data are needed to demonstrate an overall benefit of BEV in the treatment of radiation necrosis. To date, disadvantages like potential side-effects or also the high treatment costs limit the widespread usage for BEV in clinical practice. In future, it might also be possible that drugs are able to target proteins which regulate VEGF expression, like hypoxia-inducible factor 1‑alpha (HIP-1 α) or the VEGF receptors, directly [18]. But until then, BEV seems to be a reasonable treatment option for RN.
Conclusion
Of all patients who received BEV for the treatment of radiation necrosis, the majority had a clinical benefit, either in terms of improvement of symptoms or a reduction of dexamethasone or both. In the context of the present experience and current data, BEV might be offered as a treatment option to patients with symptomatic radiation necrosis.
References
Marks JF, Baglan RJ, Prassad SC, Blank WF (1981) Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 7(2):243–252. https://doi.org/10.1016/0360-3016(81)90443-0
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 65(2):499-508. https://doi.org/10.1016/j.ijrobp.2005.12.002
Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, Paiar F (2017) Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res 9(4):273–280. https://doi.org/10.14740/jocmr2936e
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9(4):180-188. https://doi.org/10.1097/01.nrl.0000080951.78533.c4
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326. https://doi.org/10.1016/j.ijrobp.2006.10.010
Kim JH, Chung YG, Kim CY, Kim HK, Lee HK (2004) Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci 19(6):879–886. https://doi.org/10.3346/jkms.2004.19.6.879
Sarin R, Murthy V (2003) Medical decompressive therapy for primary and metastatic intracranial tumours. Lancet Neurol 2(6):357–365. https://doi.org/10.1016/S1474-4422(03)00410-1
McPherson CM, Warnick RE (2004) Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 68(1):41–47. https://doi.org/10.1023/b:neon.0000024744.16031.e9
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Zhuang H, Yuan X, Yuan Z, Wang P (2017) Indication of Bevacizumab for cerebral radiation necrosis. Recent Pat Anticancer Drug Discov 12(3):272–277. https://doi.org/10.2174/1574892812666170425124430
Galldiks N, Langen KJ, Albert NL, Chamberlain M, Soffietti R, Kim MM, Law I, Le Rhun E, Chang S, Schwarting J, Combs SE, Preusser M, Forsyth P, Pope W, Weller M, Tonn JC (2019) PET imaging in patients with brain metastasis – report of the RANO/PET group. Neuro-Oncology 21(5):585–595. https://doi.org/10.1093/neuonc/noz003
de Wit MC, Eijkenboom W, Sillevis Smitt PAE, van den Bent MJ (2004) Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63(3):535–537. https://doi.org/10.1212/01.wnl.0000133398.11870.9a
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Yang TJ, Rosenblum MK, Ballangrud A, Young RJ, Zhang Z, Beal K (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156. https://doi.org/10.1007/s11060-015-1881-3
Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, Suh JH, Peereboom DM, Stevens GHJ, Ahluwalia MS (2015) Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol 38(3):304–310. https://doi.org/10.1097/COC.0b013e31829c3139
Liu AK, Macy ME, Foreman NK (2009) Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75(4):1148–1154. https://doi.org/10.1016/j.ijrobp.2008.12.032
Matuschek C, Bolke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, Gerber PA, Rusnak E, Lammering G, Budach W (2011) Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol 187(2):135–139. https://doi.org/10.1007/s00066-010-2184-4
Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, Miwa K, Iuchi T, Tsuboi K, Houkin K, Terasaka S, Tabei Y, Nakamura H, Nagane M, Sugiyama K, Terasaki M, Abe T, Narita Y, Saito N, Mukasa A, Ogasawara K, Beppu T, Kumabe T, Nariai T, Tsuyuguchi N, Nakatani E, Kurisu S, Nakagawa Y, Miyatake SI (2016) A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis. Neurooncol Pract. 3(4):272–280. https://doi.org/10.1093/nop/npv064
Duan C, Perez-Torres CJ, Yuan L, Engelbach JA, Beeman SC, Tsien CI, Rich KM, Schmidt RE, Ackerman JJH, Garbow JR (2017) Can anti-vascular endothelial growth factor antibody reverse radiation necrosis? A preclinical investigation. J Neurooncol 133(1):9–16. https://doi.org/10.1007/s11060-017-2410-3
Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, Wang X, Yuan Z, Wang P (2016) A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. https://doi.org/10.1038/srep24364
Flieger M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, la Fougere C, Ertl L, Linn J, Herrlinger U, Belka C, Niyazi M (2014) Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117(2):337–345. https://doi.org/10.1007/s11060-014-1394-5
Schnell O, Thorsteinsdottir J, Fleischmann DF, Lenski M, Abenhardt W, Giese A, Tonn JC, Belka C, Kreth FW, Niyazi M (2016) Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130(3):591–599. https://doi.org/10.1007/s11060-016-2267-x
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75(1):156–163. https://doi.org/10.1016/j.ijrobp.2008.10.043
Kozin SV, Boucher Y, Hicklin DJ, Bohlen P, Jain RK, Suit HD (2001) Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res 61(1):39–44
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. https://doi.org/10.1056/NEJMoa1308345
Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M, Chan TA, Beal K, Bishop AJ, Barker CA, Jones TS, Hentschel B, Gorlia T, Schlegel U, Stupp R, Weller M, Holland EC, Hambardzumyan D (2016) Corticosteroids compromise survival in glioblastoma. Brain 139(Pt 5):1458–1471. https://doi.org/10.1093/brain/aww046
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Bodensohn, I. Hadi, D.F. Fleischmann, S. Corradini, N. Thon, J. Rauch, C. Belka, and M. Niyazi declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bodensohn, R., Hadi, I., Fleischmann, D.F. et al. Bevacizumab as a treatment option for radiation necrosis after cranial radiation therapy: a retrospective monocentric analysis. Strahlenther Onkol 196, 70–76 (2020). https://doi.org/10.1007/s00066-019-01521-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01521-x