Abstract
The Fibroblast Growth Factor (FGF) signaling pathway is reported to stimulate glioblastoma (GBM) growth. In this work we evaluated the effect of FGF2, FGF receptor (FGFR), and small molecule inhibition on GBM cells grown in traditional media, or cultured directly in stem-cell media. These lines each expressed the FGFR1, FGFR3 and FGFR4 receptors. Addition of FGF2 ligand showed significant growth stimulation in 8 of 10 cell lines. Disruption of FGF signaling by a neutralizing FGF2 monoclonal antibody and FGFR1 suppression by RNA interference both partially inhibited cell proliferation. Growth inhibition was temporally correlated with a reduction in MAPK signaling. A receptor tyrosine kinase inhibitor with known FGFR/VEGFR activity, PD173074, showed reproducible growth inhibition. Possible mechanisms of growth suppression by PD173074 were implicated by reduced phosphorylation of AKT and MAPK, known oncogenic signal transducers. Subsequent reduction in the cyclin D1, cyclin D2 and CDK4 cell cycle regulators was also observed. Our results indicate that FGF signaling pathway inhibition as a monotherapy will slow, but not arrest growth of glioblastoma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite modest increases in survival for glioblastoma (GBM) patients, unfortunately the majority die within 2 years of diagnosis. Receptor tyrosine kinase (RTK) genomic alterations are implicated in GBM growth and have been investigated as therapeutic targets. The most common altered RTK is the Epidermal Growth Factor Receptor (EGFR). The vast majority of EGFR alterations in GBM are by genomic amplification, which is observed on average in about 32% of GBMs [1–3]. GBMs with EGFR amplification commonly undergo gene rearrangement that activates the gene further, with the most common rearrangement known as deltaEGFR or EGFRvIII [4, 5]. There are other RTKs, such as platelet derived growth factor α (PDGFRA) and MET that undergo gene amplification in GBM, but at a low frequency [1–3]. Overall certain RTKs are implicated by genomic alteration in GBM.
Altered expression of other RTKs, including the fibroblast growth factor receptors (FGFRs), have also been implicated in GBMs. Increased expression of basic FGF (bFGF) and acidic FGF (aFGF) have been detected in malignant astrocytomas relative to normal brain [6–8], parallel to the expression of the FGFR1 receptor and particularly in an alternative splice form FGFR1-β that is higher in GBM compared with normal white matter [9, 10]. Recently, we have identified a very low incidence of FGFR1 point mutations in the tyrosine kinase domain in GBMs [11]. Although individually it is not clear the biological significance of this low frequency of mutations in the growth of GBMs, others have shown that suppression of bFGF [12] or FGFR1 [13] expression by antisense oligodeoxynucleotides reduces GBM cell line growth. Taken together the evidence suggests that inhibition of the FGF pathway may reduce glioblastoma growth, and there is reason to further investigate FGFR1 as a possible therapeutic target.
The purpose of the present study was to determine if GBM growth, modeled by GBM stem-like cells and traditional cultures, is altered by disruption of the fibroblast growth factor signaling pathway. We also sought to determine if a small molecule inhibitor of FGFR can inhibit GBM cell growth and predict if this mode of inhibition has a direct effect on the tumor cell. For this study we employed traditionally cultured GBM cell lines and GBM stem-like cell lines, also known as oncospheres or cancer neurospheres. These stem-like cells are grown as suspension clusters in stem cell media, and evidence indicates this type of culture better maintains the phenotype and genotype of primary tumors, compared to traditional cell lines [14].
We investigated the expression of FGFR proteins and found that FGFR1, FGFR3 and FGFR4 but not FGFR2 were expressed in GBM and GBM stem-like cells. Recombinant human FGF2 showed growth stimulatory effect on GBM cells whereas a neutralizing antibody against FGF2 and siRNA against FGFR1 resulted in down-regulation of FGFR1 expression and subsequent decreased proliferation. Moreover, the small molecule inhibitor of FGFR/VEGFR, PD173074, inhibited glioblastoma cell proliferation. A possible mechanism of this inhibition is by suppressing the PI3 Kinase/AKT and MAPK signaling pathways, which leads to down regulation of cyclin D1, cyclin D2, CDK4 and c-MYC.
Materials and methods
Cell lines and cell culture
The following glioblastoma and glioblastoma stem-like cell lines used in this study included U87, D54, 898 (which expresses EGFRvIII in a U87 background), 897 (the wild-type EGFR expressing control for 898), D54E (which expresses EGFRvIII in a D54 background), H392, 020913, 040622, 040922 and 060919 cell lines. 020913, 040622, 040922 stem-like cell lines [15] were kindly provided by Angelo Vescovi whereas 060919 cell line was established from surgical specimen of GBM patient in our laboratory by GLG. These lines all form invasive intracranial tumors with the same histopathological features of glioblastoma. Traditional glioblastoma cell lines were maintained as adherent cultures in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 100 units/ml penicillin, 100 μg/ml streptomycin, in humidified 5% CO2 at 37°C whereas glioblastoma stem-like cell lines were cultured as suspension spheroids in stem/progenitor cell media containing 20 ng/ml EGF (PeproTech Inc.) and 10 ng/ml FGF (PeproTech Inc.) and 0.0004% heparin (Stem Cell Technologies Inc.).
Growth factor, inhibitors, antibodies
Recombinant hFGF2 was purchased from PeproTech Inc. Neutralizing antibody against FGF2, 3H3 mouse monoclonal antibody (3H3 MoAb mouse IgG class I) was purchased from Takeda Chemical Inc. The FGFR inhibitor SU5402 and the FGFR/VEGR inhibitor PD173074 were purchased from Calbiochem. Inhibitors were dissolved in dimethylsulfoxide (DMSO) at stock concentration of 10 mM and stored at −20°C until used. Antibodies used for western blotting were as follows: FGFR1 (ab10646, Abcam), FGFR2/Bek (sc-6930, Santa Cruz Biotechnology), FGFR3 (sc-123, Santa Cruz Biotechnology), FGFR4 (sc-124, Santa Cruz Biotechnology), p44/42 MAP Kinase (#4695, Cell Signaling Technology), phospho-p44/42 MAP Kinase (#4377, Cell Signaling Technology), AKT (#9272, Cell Signaling Technology), phospho-AKT (#9271, Cell Signaling Technology), cyclin D1 (#2926, Cell Signaling Technology), cyclin D2 (sc-181, Santa Cruz Biotechnology), CDK4 (#2906, Cell Signaling Technology), c-MYC (sc-764, Santa Cruz Biotechnology) and GAPDH (sc-25778, Santa Cruz Biotechnology).
Cell proliferation assay
Cell proliferation was determined by using alamarBlue® assays (Invitrogen). To determine the effect of FGF2 on cell proliferation, cells (5 × 102/well) were seeded in black clear bottom 96 well plates (Becton Dickinson) and incubated overnight with serum-free media or growth factor-free stem cell media. Recombinant hFGF2 was added at the designated concentration followed by 20 μl of 1× alamar blue. The volume in each well was raised to 200 μl with serum-free media or growth factor-free stem cell media. After 72 h incubation, fluorescence was measured on a Victor3 multiwell plate reader (Perkin Elmer) with a 540 nm excitation filter and a 590 nm emission filter. Experiments were performed as three independent experiments, with six replicates per experiment. To measure the effect of the neutralizing monoclonal antibody against FGF2, and FGFR inhibitors on cell proliferation, experiments were carried out as described above, except in medium containing 10% FBS or in growth factor containing stem cell media.
siRNA transfection
Cells (1.5 × 105) were plated 24 h before transfection. Cells were transfected with the pre-designed FGFR1 siRNA or non-targeting control siRNA (Dharmacon) at a final concentration of 100 nM using Lipofectamine 2000 (Invitrogen) per the manufacturer’s instruction. After 24 h of transfection, cells were plated and subjected to cell proliferation assay as described above. The efficiency of siRNA transfection was assessed using western blot after 72 h of transfection as described below.
Stable FGFR1 knockdown GBM cell line
A Mission™ TRC-Hs (Human) clone set of sequence-verified shRNA lentiviral plasmid vectors against FGFR1, TRCN00001212307-11, were obtained from the JHU High Throughput Biology Center. VSV-G pseudotyped virus was produced by co-transfecting 293T cells with a shRNA transducing vector and two packaging vectors: psPAX2 and PMD2.G using Lipofectamine 2000 (Invitrogen). Infectious virus was harvested at 36 and 48 h after transfection and filtered through 0.22 micron pore size cellulose acetate filter. The virus was concentrated by centrifuging through a Millipore Centricon Plus-70.
U87 GBM cells (1 × 105) were plated in six well plates (Falcon). After 24 h, 100 μl of concentrated virus was added in DMEM containing 8 μg/ml polybrene. Cells were incubated at 37°C and 5% CO2. Growth media was replaced 24 h post-transduction. FGFR1 knockdown cells were selected with 50 μg/ml puromycin. The efficiency of shRNA transfection was assessed by western blot.
Apoptosis assays
Apoptosis assays were performed using the caspase-Glo® 3/7 assay (Promega) according to manufacturer’s instructions. Briefly, cells (2 × 103) were plated in 100 μl media and incubated overnight. Then, drug was added at the designed final concentration. After the cells were incubated for 24 h the plates were removed from the incubator and equilibrated to room temperature. Fifty microliter of caspase-Glo® 3/7 reagent was added and mixed to each well, followed by a 1 h incubation at room temperature. Luminescence was measured on a Victor3 multiwell plate reader (Perkin Elmer) per the manufacturer instructions. Experiments were performed twice with three replicates per experiment.
Western blotting
Cells (2 × 105) were seeded in six well plates (Becton Dickinson) overnight. The following day, cells were treated with 2 μM of PD173074. Cells were harvested at the indicated times and protein lysates were prepared using radioimmuno-precipitation assay (RIPA) buffer containing 1× Complete Mini Protease Inhibitor (Roche), 50 mM NaF and 1 mM heat-activated Na3VO4. Protein extracts were quantified using the Pierce BCA™ Protein Assay Kit (Pierce Biotechnology). Sixty micrograms of protein were loaded onto NuPage® Novex 4–12% Bis-Tris gels (Invitrogen) using MES running buffer (Invitrogen) and blotted onto PVDF membranes (Bio-Rad). Blots were stained with the primary antibodies and GAPDH antibody was used as an internal loading control. Appropriate horseradish peroxidase-conjugated secondary antibodies and SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) were used for chemiluminescent detection.
Statistic analysis
An independent samples t-test was used to detect significant differences in proliferation between treated and untreated control cells. All statistical tests were two-tailed. A P-value of less than 0.05 was considered statistically significant.
Results
Fibroblast growth factor receptor expression
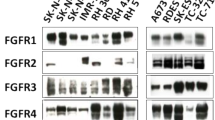
The protein expression of four isoforms of FGFR genes was analyzed by western blotting in six GBM traditional adherent lines and four GBM stem-like cell lines. The cell lines used for this study all clearly expressed FGFR1, whereas FGFR2 protein was undetectable in any of the examined cell lines (see Fig. 1a). The glioblastoma cell lines also exhibited varying levels of FGFR3 and FGFR4 expression.
FGF2 stimulates GBM cell growth. a Expression of different FGFR family members in GBM and GBM stem-like cell lines determined by western blotting. b Growth stimulatory effect of FGF2 in GBM and GBM stem-like cell lines. GBM and GBM stem-like cell lines were treated with FGF2 at the indicated concentration, and cell proliferation was determined by using an alamarBlue assay. Fold change in cell proliferation was normalized to the untreated control. Significant growth stimulation (P < 0.05) compared with each control sample is denoted by an asterisk (*)
Growth stimulatory effect of FGF2
In order to determine the role of fibroblast growth factor signaling pathway in GBM cell proliferation, the growth stimulatory effect of FGF2 protein was analyzed under serum or growth factor-starved condition. Recombinant FGF2 showed significant growth-promoting effect in 8 out of 10 GBM and GBM stem-like cell lines in a dose-dependent manner except for 897 and 060919 cell lines (Fig. 1b). However, at 100 ng/ml concentration of FGF2, the result showed a growth inhibitory effect in some GBM cell lines.
Disruption of fibroblast growth factor signaling pathway suppresses GBM cell growth
To confirm whether fibroblast signaling pathway contributes to the growth of GBM and GBM stem-like cells, we examined the effect of a neutralizing antibody against FGF2 on GBM cell proliferation. Anti-FGF2 IgG showed an inhibitory effect on the proliferation of adherent (U87 and D54) and stem-like (020913) GBM cells (P < 0.05) compared to those with normal mouse IgG1 (Fig. 2a). Furthermore, transient transfection of GBM cell lines (D54 and H392) with FGFR1 siRNA reduced cell proliferation in comparison to a scramble control (P < 0.05, Fig. 2b, c). Additionally, the effect of suppressing FGFR1 prevented GBM (D54) cell proliferation in response to FGF2 stimulation (P < 0.05, Fig. 2d). Stable FGFR1 knockdown also significantly reduced U87 cell growth (Fig. 2e) which seems to be correlated with the inhibition of MAPK but not the PI3 Kinase/AKT signaling pathway (Fig. 2f).
Inhibition of FGFR1 decreases GBM cell growth. a The inhibitory effect of anti-FGF2 on the growth of U87, D54 and 020913 cell lines. *, P < 0.05 compared to cells treated with normal mouse IgG. b Western blot analysis of D54 and H392 cell lines after transfection with siRNA directed against FGFR1 or scramble control siRNA. c Transfection of D54 and H392 cells with FGFR1 siRNA reduced cell growth and d suppression of FGFR1 prevented GBM (D54) cell proliferation even in the presence of FGF2 stimulation (100 ng/ml). e Decreased cell growth is observed in U87 with a stable FGFR1 knockdown using lentiviral infection, which is consistent with transient FGFR1 knockdown. f FGFR1 stable knockdown inhibits P44/42 MAPK, but not AKT phosphorylation. *, P < 0.05 compared to scramble control siRNA transfection
Small molecules inhibitors of FGFR inhibited growth
We next sought to determine if known FGFR inhibitors SU5402 and PD173074 [16, 17] would inhibit GBM and/or GBM stem-like cell proliferation. Proliferation of both GBM and GBM stem-like cells was slightly inhibited when treated with 50 μM of SU5402 (data not shown). A significant dose-dependent inhibition of growth was observed in all the cells studied in the presence of PD173074, a FGFR/VEGR inhibitor (Fig. 3a).
The FGFR inhibitor PD173074 reduces cell proliferation. a PD173074 shows growth reduction of GBM cells with micromolar concentrations. b Caspase 3/7 expression was induced in GBM and GBM stem like cell lines, but only at higher concentrations. Relative cell proliferation and caspase levels were assayed in comparison to DMSO-treated cells. Results shown are the mean of six replicates in two independent experiments and error bars show 95% confidence interval of mean. *, P < 0.05 compared with DMSO-treated cells
To test if PD173074 inhibited cell growth at least in part by inducing apoptosis, we tested if mediators of apoptosis, caspase 3/7, were induced. As shown in Fig. 3b apoptosis was only slightly increased at the higher drug concentration of 10 μM in GBM (U87) and GBM stem-like cells (020913).
The PI3 Kinase/AKT and MAPK signaling pathway are suppressed by PD173074
To explore the molecular mechanism by which PD173074 inhibited GBM cell growth, we used western blotting with phosphorylation specific antibodies to determine if the PI3 Kinase/AKT and MAPK pathways were altered. These pathways are likely downstream signal transducers of the FGFRs [18]. The level of phospho-AKT and phospho-p44/42 MAPK was significantly reduced in both GBM (U87) and the GBM stem-like cell line, 020913, following PD173074 treatment. The down regulation of AKT and p44/42 MAPK-phosphorylation appears to have resulted in decreased expression of cell cycle regulation proteins including cyclin D1, cyclin D2, CDK4 and the transcription factor, c-MYC, respectively (Fig. 4).
AKT and MAPK phosphorylation is reduced with an FGFR inhibitor. 020913 and U87 cells were treated with PD173074 (2 μM). Cells were harvested at indicated times and protein lysates were analyzed by western blotting with phospho-specific antibodies against AKT and P44/42 MAPK. The membranes were re-probed to test for total AKT and P44/42 MAPK protein levels. The protein levels of cyclin D1, cyclin D2, CDK4 and c-MYC were evaluated by immunoblotting with protein specific antibodies. Equivalent loading was confirmed by re-probing with a GAPDH antibody
Discussion
In this study we investigated how the growth of GBM and GBM stem-like cells is altered by changes in fibroblast growth factor signaling. We initially examined FGFR expression in six traditional GBM and four GBM stem-like cell lines and found that all cell lines studied express FGFR1, FGFR3 and FGFR4, whereas FGFR2 protein was undetectable in these cell lines. Previous studies [9, 19] have shown that FGFR1 expression was absent or barely detectable in normal white matter but was significantly elevated in malignant tumor. Conversely, FGFR2 expression was abundant in normal white matter and in all low-grade astrocytomas but was not seen in malignant astrocytomas [9, 19]. The glioblastoma cell lines exhibited varying levels of FGFR3 and FGFR4 expression which is also consistent with a previous report [13].
We have confirmed the contribution of fibroblast growth factor signaling pathway in GBM cell proliferation and found that FGF2, the ligand which can activate any of the FGF receptors, FGFR1-4 [20], showed growth-promoting effect in all GBM and GBM stem-like cell lines in a dose-dependent manner except 897 and 060919 cell lines. Presumably, FGF2 is acting on FGFR1, as FGFR2 is not expressed, and inhibition of FGFR1 slows growth. At 100 ng/ml concentration of FGF2 stimulation, however, there were some growth inhibitory effects in U87, 898 and D54E cell lines, likely due to toxic effects of the higher concentration. Although we found that FGF2 induces growth in various GBM cell lines, FGF2 acted as a growth inhibitor of Ewing’s sarcoma cells [21, 22], so it may play a different role in different cancers. It is also important to note that the oncosphere lines used in this study are normally grown already in the presence of FGF2.
Our further investigation showed that a neutralizing antibody against FGF2 showed partial inhibition of GBM (U87 and D54) and GBM stem-like (020913) cell proliferation (P < 0.05) compared to those treated with normal mouse IgG1. Furthermore, transient transfection of GBM cell lines (D54 and H392) with FGFR1 siRNA significantly reduced cell proliferation (P < 0.05) in comparison to the scramble control siRNA. The stable FGFR1 knockdown with a lentiviral construct in the U87 cell line also showed cell growth inhibition consistent with the transient knock down. These results support the previous studies that inhibiting of the FGF pathway reduces GBM cell growth [13, 23].
The fact that FGF2 and the FGF pathway can stimulate glioblastoma cell growth is not too surprising for those investigators culturing stem-like cancer cells, because FGF2, along with EGF, are the main recombinant growth factors used in most formulations of neural ‘stem-cell’ media. It appears from our experiments that FGFR1 probably signals through the MAPK pathway, rather than PI3 kinase/AKT pathway. However, activation and interference of FGF signaling pathway showed effects on GBM cell proliferation that are small in magnitude suggesting that this pathway is not alone in driving GBM cell growth.
Proliferation of the GBM cell lines was significantly suppressed in the presence of the small molecule inhibitor of FGFR/VEGFR, PD173074, whereas only a slight growth inhibitory effect was observed in the presence of the FGFR inhibitor SU5402 even at a 50 μM concentration. Higher doses of PD173074 were required to inhibit the growth of all of the cancer cell lines and it is possible that these higher doses of PD173074 are acting on targets other than FGFR. Although, PD173074 was found to be highly selective for the FGF receptor with a nanomolar inhibitor of FGFR1 and submicromolar inhibitor of VEGFR2, with little effect against EGFR, InsR, MEK and PKC at concentration as high as 50 μM [17]. The other FGFR inhibitor, SU5402 showed a very small effect on GBM cell growth inhibition, which is consistent with its with higher IC50 for FGFR1 tyrosine phosphorylation inhibition [16].
Inhibition of PD173074 through a FGFR1 target is partially consistent with the findings that more specific inhibition, such as with FGF2 antibody and FGFR1 siRNA, similarly inhibits growth. Although PD173074 biochemically appears to be most specific for inhibiting FGFR, off-target effects of the drug can not be excluded. The superior inhibition of the small molecule compared to the antibody or siRNA may be due to other important targets of inhibition, a general toxicity of the drug, and/or a better ability of the drug to reach the receptor and sustain inhibition.
The downstream molecular mechanism by which PD173074 suppressed GBM cells appeared to be due to the inhibition of the PI3 Kinase/AKT and MAPK signaling pathways. This appears to have led also to decrease expression of G1-S transition regulatory proteins that included cyclin D1, cyclin D2, and CDK4. Although, PD173074 initially induce cyclin D1 and cyclin D2 expression in the U87 line, after 8 h there was an overall decrease in expression. The 020913 stem-like line showed decreased cyclin expression initially and throughout treatment. We also found that PD173074 caused a decrease of c-MYC levels in the cell lines we examined, suggesting that c-MYC might be involved in proliferation of these cells, although this decrease came late in treatment after 48 h. The use of c-MYC to induce gliomas in transgenic mice [24, 25], and its amplification in some GBMs suggest c-MYC has an oncogenic role in GBMs.
Our results showed that cell growth inhibition, rather than apoptosis, was the main mechanism by which PD173074 inhibited cell proliferation in GBM and GBM stem-like cells. Other investigators have recently found that PD173074 inhibited breast cancer cell proliferation by suppressing the PI3 Kinase/AKT and MAPK signaling pathways as well as the down-regulation of D-type cyclins [26]. This is similar to our results.
We conclude that the fibroblast signaling pathway can be used by GBM cells to aid in increased growth. Inhibition of this particular signaling pathway by anti-FGF2 antibody, siRNA against FGFR1 and the PD173074 small molecule inhibitor of FGFR/VEGFR produced a small but significant growth inhibition of GBM cells in vitro. Our results suggest that targeting the FGF signaling pathway may be a useful approach for GBM therapy. However, the survival and complete growth suppression of GBM cells did not depend on the FGF signaling pathway alone; hence suppression of FGF signaling by itself will likely not be sufficient to stop tumor cell proliferation in patients. Therefore, other growth stimulatory pathways that are essential for tumor cell viability must be included for drug targeting in order to obtain the best chances for a therapeutic response. Combined targeting of GBMs might include the FGF pathway.
References
Korshunov A, Sycheva R, Golanov A (2006) Genetically distinct and clinically relevant subtypes of glioblastoma defined by array-based comparative genomic hybridization (array-CGH). Acta Neuropathol 111:465–474. doi:10.1007/s00401-006-0057-9
Misra A, Pellarin M, Nigro J, Smirnov I, Moore D, Lamborn KR, Pinkel D, Albertson DG, Feuerstein BG (2005) Array comparative genomic hybridization identifies genetic subgroups in grade 4 human astrocytoma. Clin Cancer Res 11:2907–2918. doi:10.1158/1078-0432.CCR-04-0708
Ruano Y, Mollejo M, Ribalta T, Fiano C, Camacho FI, Gomez E, de Lope AR, Hernandez-Moneo JL, Martinez P, Melendez B (2006) Identification of novel candidate target genes in amplicons of Glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer 5:39. doi:10.1186/1476-4598-5-39
Schwechheimer K, Huang S, Cavenee WK (1995) EGFR gene amplification—rearrangement in human glioblastomas. Int J Cancer 62:145–148. doi:10.1002/ijc.2910620206
Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP (1991) Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 51:2164–2172
Maxwell M, Naber SP, Wolfe HJ, Hedley-Whyte ET, Galanopoulos T, Neville-Golden J, Antoniades HN (1991) Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res 51:1345–1351
Morrison RS, Gross JL, Herblin WF, Reilly TM, LaSala PA, Alterman RL, Moskal JR, Kornblith PL, Dexter DL (1990) Basic fibroblast growth factor-like activity and receptors are expressed in a human glioma cell line. Cancer Res 50:2524–2529
Zagzag D, Miller DC, Sato Y, Rifkin DB, Burstein DE (1990) Immunohistochemical localization of basic fibroblast growth factor in astrocytomas. Cancer Res 50:7393–7398
Morrison RS, Yamaguchi F, Bruner JM, Tang M, McKeehan W, Berger MS (1994) Fibroblast growth factor receptor gene expression and immunoreactivity are elevated in human glioblastoma multiforme. Cancer Res 54:2794–2799
Morrison RS, Yamaguchi F, Saya H, Bruner JM, Yahanda AM, Donehower LA, Berger M (1994) Basic fibroblast growth factor and fibroblast growth factor receptor I are implicated in the growth of human astrocytomas. J Neurooncol 18:207–216. doi:10.1007/BF01328955
Rand V, Huang J, Stockwell T, Ferriera S, Buzko O, Levy S, Busam D, Li K, Edwards JB, Eberhart C, Murphy KM, Tsiamouri A, Beeson K, Simpson AJ, Venter JC, Riggins GJ, Strausberg RL (2005) Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci USA 102:14344–14349. doi:10.1073/pnas.0507200102
Murphy PR, Sato Y, Knee RS (1992) Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage-independent growth of a malignant glioblastoma cell line. Mol Endocrinol 6:877–884. doi:10.1210/me.6.6.877
Yamada SM, Yamaguchi F, Brown R, Berger MS, Morrison RS (1999) Suppression of glioblastoma cell growth following antisense oligonucleotide-mediated inhibition of fibroblast growth factor receptor expression. Glia 28:66–76. doi:10.1002/(SICI)1098-1136(199910)28:1<66::AID-GLIA8>3.0.CO;2-M
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403. doi:10.1016/j.ccr.2006.03.030
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64:7011–7021. doi:10.1158/0008-5472.CAN-04-1364
Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276:955–960. doi:10.1126/science.276.5314.955
Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR (1998) Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J 17:5896–5904. doi:10.1093/emboj/17.20.5896
Klint P, Claesson-Welsh L (1999) Signal transduction by fibroblast growth factor receptors. Front Biosci 4:D165–D177. doi:10.2741/Klint
Yamaguchi F, Saya H, Bruner JM, Morrison RS (1994) Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci USA 91:484–488. doi:10.1073/pnas.91.2.484
Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16:139–149. doi:10.1016/j.cytogfr.2005.01.001
Kim MS, Kim CJ, Jung HS, Seo MR, Juhnn YS, Shin HY, Ahn HS, Thiele CJ, Chi JG (2004) Fibroblast growth factor 2 induces differentiation and apoptosis of Askin tumour cells. J Pathol 202:103–112. doi:10.1002/path.1497
Sturla LM, Westwood G, Selby PJ, Lewis IJ, Burchill SA (2000) Induction of cell death by basic fibroblast growth factor in Ewing’s sarcoma. Cancer Res 60:6160–6170
Takahashi JA, Fukumoto M, Kozai Y, Ito N, Oda Y, Kikuchi H, Hatanaka M (1991) Inhibition of cell growth and tumorigenesis of human glioblastoma cells by a neutralizing antibody against human basic fibroblast growth factor. FEBS Lett 288:65–71. doi:10.1016/0014-5793(91)81004-R
Jensen NA, Pedersen KM, Lihme F, Rask L, Nielsen JV, Rasmussen TE, Mitchelmore C (2003) Astroglial c-Myc overexpression predisposes mice to primary malignant gliomas. J Biol Chem 278:8300–8308. doi:10.1074/jbc.M211195200
Lassman AB, Dai C, Fuller GN, Vickers AJ, Holland EC (2004) Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Akt signaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol 1:157–163. doi:10.1017/S1740925X04000249
Koziczak M, Holbro T, Hynes NE (2004) Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D-type cyclins. Oncogene 23:3501–3508. doi:10.1038/sj.onc.1207331
Acknowledgements
We thank Brenda Raymond for administrative support. This project was supported by NIH Grant R01 NS052507, the Johns Hopkins Department of Neurosurgery and the Virginia and the D. K. Ludwig Fund for Cancer Research. W. L. is supported by the Faculty of Medicine, Khon Kaen University, Thailand. A. J. is supported by an American Brain Tumor Association (ABTA) postdoctoral fellowship and G. J. R. is the Irving J. Sherman M.D. Neurosurgery Research Professor.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11060-010-0155-3
Rights and permissions
About this article
Cite this article
Loilome, W., Joshi, A.D., ap Rhys, C.M.J. et al. Glioblastoma cell growth is suppressed by disruption of fibroblast growth factor pathway signaling. J Neurooncol 94, 359–366 (2009). https://doi.org/10.1007/s11060-009-9885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9885-5