Abstract
A protocol was developed for shoot proliferation and plantlet formation of Khaya senegalensis, an important medicinal and timber plantation species introduced to Australia and southern Asia from western and central Africa. We assessed effects of the plant growth regulators, benzyladenine, kinetin, naphthalene acetic acid and gibberellic acid, on shoot proliferation and subsequent plantlet conversion. Shoot proliferation over four passages was higher in media containing benzyladenine than in media containing other growth regulators, and optimal proliferation from seed of three different sources was consistently obtained in medium containing 4.4 μM benzyladenine. Shoots from this medium were converted to plantlets at high frequencies (76–90%) after treatment with 19.6 μM indole-3-butyric acid, and almost all plantlets were successfully acclimatized to nursery conditions. These methods provide the means for establishing in vitro and ex vitro clone banks of juvenile K. senegalensis trees for field selection of desired genotypes and tropical plantation establishment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Khaya comprises seven species, native to tropical Africa and Madagascar, within subfamily Swietenioideae of the mahogany family Meliaceae (Pennington and Styles 1975; Styles 1981). Meliaceae contains many commercially important tree species including Azadirachta indica (neem tree), Cedrela odorata (Spanish cedar), Melia azedarach (paradise tree), Swietenia macrophylla (big-leaf mahogany) and Toona ciliata (Australian red cedar) (Vila et al. 2002; Mroginski et al. 2003; Quraishi et al. 2004; Schottz et al. 2007; Cameron 2010). The four Khaya species that occur in western Africa, K. anthotheca, K. grandifoliola, K. ivorensis and K. senegalensis, are commonly known as the African mahoganies. These species are valued for timber, medicinal products and amenity plantings throughout their natural distributions in western, central and eastern Africa (Pennington and Styles 1975; Styles 1981; Nikles et al. 2008). Khaya provides a major source of revenue to many countries because of its hard, durable timber, which is widely sought for construction, furniture and carpentry (Ofori et al. 2007; Nikles et al. 2008; Opuni-Frimpong et al. 2008a). The highly desired bark, seeds, leaves and other parts of Khaya trees are also used traditionally for treatment of diseases including cancer, diarrhoea, dysentery and malaria (Zhang et al. 2007; Makut et al. 2008; Kubmarawa et al. 2009). The African mahoganies are classified as ‘vulnerable’ because of habitat loss and over-harvesting for timber and medicinal products (Ofori et al. 2007; Nikles et al. 2008).
One of the African mahoganies, K. senegalensis, has been introduced widely across tropical Australia, China, Vietnam, Malaysia, Indonesia and Sri Lanka for forestry plantations and amenity plantings (Arnold et al. 2004). The species has proven suitable for high-value timber plantations in northern Australia, where the trees are not exposed to the mahogany shoot borer, Hypsipyla robusta (Arnold et al. 2004; Nikles et al. 2008). A genetic improvement program, aimed at selecting high-quality timber-yielding clones and families, commenced in Australia in 2001. However, limited seed production in seed orchards and plantations has limited the availability of desired genotypes of K. senegalensis, and development of micropropagation methods for mass production and germplasm storage is a main priority of the program (Nikles et al. 2008).
There are very few reports on micropropagation of Khaya species. Initial shoot induction has been described from the single-node shoots of freshly-germinated K. senegalensis seeds (Danthu et al. 2003). These authors induced axillary shoots in full-strength Murashige and Skoog (MS) medium containing 2.2 or 8.9 μM benzyladenine (BA), although the maximum number of shoots obtained after the single passage of their study was only 2.5 per seedling. Axillary shoot proliferation has been described from nodal explants of K. ivorensis stock plants, with final shoot numbers being highest in full-strength MS medium containing 22.2 μM BA, 0.5 μM α-naphthalene acetic acid (NAA) and 2.9 μM gibberellic acid (GA3) (Mathias 1988). Reports on micropropagation of other Meliaceae trees have also used MS media containing BA for initiating or proliferating shoots, with the BA frequently, though not always, supplemented with another growth regulator such as NAA, indole-3-butyric acid (IBA), GA3 or kinetin (Daniel et al. 1999; Vila et al. 2002; Mroginski et al. 2003; Tacoronte et al. 2004; Schottz et al. 2007; Shahin-uz-zaman et al. 2008; Hajari et al. 2009; Husain and Anis 2009).
The aim of our research was to develop methods for micropropagation of K. senegalensis, including (1) shoot induction and shoot proliferation to establish a laboratory archive of juvenile clones, and (2) root formation to generate plantlets of the same clones. Very high proliferation rates are not necessary for establishment of the ex vitro clone banks, because Khaya species can be propagated from cuttings of juvenile stock plants (Nikles et al. 2008; Opuni-Frimpong et al. 2008b) and so sufficient rooted cuttings for initial field tests can be obtained from just a few stock plants of each clone. Therefore, we aimed to develop methods for axillary shoot proliferation (Schottz et al. 2007; Trueman and Richardson 2007; Hajari et al. 2009; Husain and Anis 2009) rather than adventitious shoot production, which might pose a greater risk of somaclonal variation (George 1993; Pijut et al. 2007). Specifically, we present the results of large factorial experiments assessing the effects of BA, kinetin, NAA or GA3, singly or in combination, on shoot induction, shoot proliferation and subsequent IBA-induced plantlet conversion.
Materials and methods
Experiment 1: shoot induction, proliferation, and plantlet conversion
Khaya senegalensis seeds from a natural provenance in Burkina Faso were obtained from the Hardwood Tree Improvement Group, Agri-Science Queensland. Seeds were refrigerated at 4°C until use. Seven hundred and eighty seeds were used for this experiment. Batches of five seeds were washed in 70% (v/v) ethanol for 1 min in 70-ml vials containing one drop of Tween 20, rinsed in sterile distilled water for 1 min, and then transferred to new vials containing sodium hypochlorite (1, 5 or 10%; w/v) and one drop of Tween 20. The vials were swirled for 5, 10, 20 or 40 min on an orbital shaker at 110 rpm. After three subsequent rinses in sterile distilled water, seeds were plated (five seeds per 90-mm Petri dish) onto germination medium consisting of half-strength MS basal salts and 20 g l−1 sucrose, solidified with 8 g l−1 agar, with pH adjusted to 5.8 prior to autoclaving at 121°C for 20 min. Seeds were germinated at 25°C under a 16-h photoperiod (~50 μmol m−2 s−1 by cool white fluorescent tubes). Sodium hypochlorite concentration and duration had no significant effect on seed contamination or the percentage of seeds producing suitable shoots for transfer to shoot induction medium (2-way ANOVA; P > 0.05). Therefore, all upright, uncontaminated shoots were transferred randomly after 2 weeks to shoot induction media (Fig. 1).
Shoots were dissected at the point of emergence from the seed coat, and transferred to 375-ml glass jars containing 50 ml of one of the ten shoot induction treatments: 0, 1.1, 2.2, 4.4 or 8.9 μM BA combined with 0 or 0.3 μM NAA (Sigma, St. Louis, MO). All shoot induction and shoot proliferation media (see section below) consisted of full-strength MS medium containing 30 g l−1 sucrose, solidified with 8 g l−1 agar, with pH adjusted to 5.8 prior to autoclaving at 121°C for 20 min, and with jars maintained at 25°C under a 16-h photoperiod (~100 μmol m−2 s−1). The number of replicate shoots per induction treatment ranged from 65 to 75. Length of the main shoot, and numbers of nodes and macroscopic lateral shoots, were recorded after 4 weeks. Each node, and any lateral shoot >10-mm in length, was dissected for transfer, and the number of excised shoots transferred to proliferation medium was recorded.
All excised shoots from each seedling (i.e. one clone) were then transferred to a 375-ml jar containing 50 ml of one of the eight shoot proliferation treatments: (1) control (no hormones), (2) 0.3 μM NAA, (3) 1.5 μM GA3, (4) 4.7 μM kinetin, (5) 4.4 μM BA, (6) 4.4 μM BA + 0.3 μM NAA, (7) 4.4 μM BA + 1.5 μM GA3, or (8) 4.7 μM kinetin + 1.5 μM GA3. The 65–75 clones from within each of the ten shoot induction treatments were randomly transferred across the eight proliferation treatments, and so the number of replicate clones for each of the 80 induction × proliferation treatment combinations was 8–10. Each node, and any lateral shoot >10-mm in length, was dissected for transfer to fresh medium after 5 weeks. The same process was repeated after 10 weeks. After 15 weeks, shoots were dissected for root formation by only removing basal callus or existing roots. The number of shoots transferred was recorded after each of the three passages in proliferation medium.
After 15 weeks in proliferation medium, shoots were transferred to 375-ml glass jars containing half-strength MS medium supplemented with 19.6 μM IBA, and the jars were placed in darkness at 22°C for 1 week. Shoots were then transferred to hormone-free half-strength MS medium and maintained at 25°C under a 16-h photoperiod (~100 μmol m−2 s−1) for a further 3 weeks. All shoots were then gently extracted to determine the proportion of shoots from each clone that had formed roots and the number of adventitious roots per rooted shoot. Plantlets were rinsed of agar and transplanted into a potting mix containing composted pine bark, perlite, lime and slow-release Osmocote™ fertiliser (Scotts International, Heerlen, The Netherlands) in 90-cm3 propagation tubes (Trueman and Richardson 2008). Plantlets were grown in a polyethylene propagation chamber for 4 weeks, with 1-min misting provided at 10-min intervals for the first 2 weeks to maintain high relative humidity.
Experiment 2: optimised shoot induction and proliferation
This experiment assessed the optimal BA concentration for shoot induction and proliferation, using K. senegalensis seeds from three different sources: (1) seeds from a natural provenance in Burkina Faso, obtained from the Hardwood Tree Improvement Group, Agri-Science Queensland; (2) open-pollinated seeds of a selected Uganda-provenance clone (number 104) in a plantation trial in the Northern Territory, Australia, obtained from the Northern Territory Department of Resources; and (3) a mixed seedlot (number 20851) from a plantation in Weipa, Queensland, Australia, obtained from the CSIRO Australian Tree Seed Centre. The Weipa plantation had been established from open-pollinated seeds of street trees in Darwin, Australia, which had mostly been planted using seeds from Senegal provenances. The three seed sources are referred to as ‘Burkina Faso’, ‘Northern Territory’ and ‘Queensland’, respectively, because the African provenances that contributed to the pedigrees of the open-pollinated Northern Territory and Queensland seeds are not completely known. All seeds were refrigerated at 4°C until use.
The seeds were surface-sterilised with 70% ethanol for 1 min and 5% NaOCl for 10 min, and germinated using the methods described for experiment 1 (above). After 2 weeks, 100 upright shoots per seed source were dissected at the point of emergence from the seed coat and transferred randomly to one of five media for shoot induction and shoot proliferation: full-strength MS medium with 2.2, 4.4, 8.9, 17.8 or 35.5 μM BA (Fig. 1). Shoots were induced on this medium for 4 weeks, and then dissected and transferred to fresh medium of the same composition for three 5-week passages of shoot proliferation. The vessels, media volumes, culture conditions, dissection methods, and number and duration of passages were as described for experiment 1. The number of shoots transferred after each proliferation passage was recorded for each clone.
After 15 weeks of shoot proliferation, a subsample of five shoots per clone from the treatments with 2.2, 4.4, 8.9 or 17.8 μM BA was transferred to root induction medium, using the methods described for experiment 1. Insufficient shoots were available from the other treatment (35.5 μM BA) to attempt plantlet conversion. Shoots were gently extracted after 4 weeks to determine the proportion of shoots from each clone that had formed roots and the number of adventitious roots per rooted shoot. Plantlets were transplanted to potting mix and grown in a polyethylene propagation chamber, as described for experiment 1.
Statistical analyses
Data for shoot length, and numbers of nodes, lateral buds and transferred shoots after 4 weeks in shoot induction medium, were analysed by 1-way ANOVA (10 induction media). The number of shoots transferred after each passage in proliferation medium, the proportion of shoots forming roots, and root number per rooted shoot were analysed by 2-way ANOVA (10 induction media × 8 proliferation media) in experiment 1 because there were no significant interactions between these two factors, and by 1-way ANOVA (5 media) in experiment 2. Proportions were arcsine square root transformed, and lengths or numbers were square root or log transformed, when variance was heterogeneous. Duncan’s multiple range tests were performed when significant differences among treatments were detected by ANOVA. Means are reported with standard errors, and treatment differences or interactions were regarded as significant at P < 0.05.
Results and discussion
Experiment 1
Seedlings of K. senegalensis grew vigorously and formed a well-developed root system and a shoot with length > 20 mm within 2 weeks of plating (Fig. 2a). After germination, a medium containing cytokinin was the most effective for shoot induction. Addition of BA to induction medium did not affect the length of shoots or, in most cases, the number of nodes per shoot (Table 1). However, all concentrations of BA promoted development of macroscopic lateral buds (Table 1; Fig. 2b). The highest numbers of shoots transferred to the second passage (2.3 ± 0.1–2.6 ± 0.2 per explant) were obtained from media supplemented with 8.9 μM BA alone, or 4.4 or 8.9 μM BA in combination with 0.3 μM NAA, whereas the lowest shoot number (1.2 ± 0.1 per explant) was obtained in the medium containing 1.1 μM BA without NAA (Table 1). These results are similar to those of Danthu et al. (2003), who obtained highest induction (2.4 ± 0.5 shoots) on full-strength MS medium containing 8.9 μM BA and 0.3 μM IBA but also on medium containing 2.2 μM BA and 0.3 μM IBA (2.3 ± 0.4 shoots). The number of shoots induced using K. senegalensis seedling shoots is low compared with the numbers obtained from shoot tips and nodal segments of Cedrela odorata (Maruyama et al. 1989), Melia azedarach (Ahmad et al. 1990; Thakur et al. 1998; Husain and Anis 2009) and Azadirachta indica (Arora et al. 2010). Therefore, the induced shoots required subsequent proliferation before each clone could yield sufficient material for a combined clone bank of in vitro shoots and ex vitro plantlets.
Micropropagation of Khaya senegalensis from seeds: a germination on ½MS medium; b shoots induced on MS medium supplemented with 8.9 μM BA and 0.3 μM NAA; c shoots proliferating on MS medium with 4.4 μM BA; d BA-derived shoot forming roots on ½MS medium after treatment with 19.6 μM IBA; e 1-month acclimatisation of a plantlet; and f 3-month acclimatisation of plantlets. Bar 1 cm
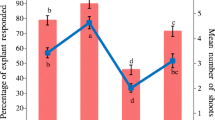
Carry-over effects of shoot induction media on shoot numbers were observed after the second and third passages but not after the fourth passage, i.e. after 5 and 10, but not after 15, weeks in proliferation medium (data not presented), and there were no significant interactions between induction and proliferation media. Consequently, mean shoot numbers for each of the eight proliferation media were pooled across ten induction media (Fig. 3). Shoot proliferation was consistently highest in media containing BA but lacking GA3, and final shoot numbers were lowest in media containing GA3. This gibberellin, in combination with BA, had previously been shown to improve shoot proliferation in cultures of Khaya ivorensis (Mathias 1988), Azadirachta indica (Chaturvedi et al. 2004), Naregamia alata (Daniel et al. 1999), Melia azedarach (Vila et al. 2002) and Cedrela odorata (Peña-Ramírez et al. 2010). The use of 4.4 μM BA in proliferation medium promoted K. senegalensis shoot production by 1.3-, 2.8- and 11.0-fold over nil-hormone medium across the three successive proliferation passages, with a cumulative increase in shoot number from 4.6 ± 0.3 to 12.4 ± 1.0 to 52.9 ± 4.9 per clone (Fig. 3a–c).
Effect of growth regulators on number of shoots transferred per Khaya senegalensis clone after a 5 weeks, b 10 weeks, and c 15 weeks in shoot proliferation medium. The growth regulators were 0.3 μM NAA (NAA), 1.5 μM GA3 (GA), 4.7 μM kinetin (Kin) or 4.4 μM BA (BA) singly or in combination. Means (+s.e.) with different letters at each time point are significantly different (2-way ANOVA and Duncan’s multiple range test; P < 0.05; n = 81–90 clones)
NAA or kinetin failed to increase shoot proliferation over any of their respective controls (Fig. 3). Indeed, addition of 0.3 μM NAA to proliferation medium containing 4.4 μM BA decreased final shoot production by 22% (Fig. 3c). These results contrast with the positive effects of combined BA and NAA treatments in Azadirachta excelsa (Liew and Teo 1998) and Azadirachta indica (Khatun et al. 1998), but they accord with the greater effectiveness of BA compared with kinetin or zeatin for shoot proliferation of Melia azedarach (Ahmad et al. 1990; Husain and Anis 2009) and Cedrela odorata (Peña-Ramírez et al. 2010). Almost all K. senegalensis shoots from all treatments were suitable for transfer to fresh medium after each passage. Callus was frequently formed at the base of shoots in the presence of BA (Fig. 2c) or kinetin, but basal callus was removed after each passage to promote nutrient and growth regulator uptake for axillary shoot proliferation or, after the fourth passage, root formation.
Conversion to plantlets was highest for shoots obtained from the optimal proliferation medium; i.e. containing 4.4 μM BA alone (Figs. 3c, 4a). This medium provided significantly higher rooting (75.7 ± 2.1%) than all of the other media containing growth regulators. Rooting was lowest (24.3 ± 2.6–44.7 ± 3.2%) for shoots obtained from the three proliferation media containing GA3, and these media also provided the lowest numbers of adventitious roots per plantlet (Fig. 4b). Adventitious root numbers among the other media (3.6 ± 0.2–4.0 ± 0.2) did not differ significantly. There was a single carry-over effect of the shoot induction treatment on the percentage of shoots forming roots: 66.5 ± 3.7% of shoots derived from the induction treatment containing 2.2 μM BA and 0.3 μM NAA produced roots, which was significantly higher than rooting of shoots from the six induction treatments containing 0, 1.1 or 8.9 μM BA with or without 0.3 μM NAA (51.0 ± 4.2–55.5 ± 4.3%; data not presented). Roots were formed at the base of shoots, with little or no callus production (Fig. 2d). Almost 100% of plantlets survived and were acclimatised successfully upon transfer to the propagation chamber (Fig. 2e, f).
Effect of growth regulators in shoot proliferation medium on a percentage of shoots with roots and b number of adventitious roots per rooted shoot for Khaya senegalensis clones after a 1-week treatment of shoots with 19.6 μM IBA followed by 3-week culture on ½MS medium. The growth regulators were 0.3 μM NAA (NAA), 1.5 μM GA3 (GA), 4.7 μM kinetin (Kin) or 4.4 μM BA (BA) singly or in combination. Means (+s.e.) with different letters are significantly different (2-way ANOVA and Duncan’s multiple range test; P < 0.05; n = 80–88 clones)
Experiment 2
The first experiment demonstrated the effectiveness of medium containing a single hormone (BA, tested only at 4.4 μM) for proliferation of K. senegalensis shoots obtained from Burkina Faso seeds. The second experiment determined the optimal BA concentration for K. senegalensis micropropagation by assessing a wider range of concentrations (2.2–35.5 μM BA) for shoots from three different seed sources (Burkina Faso, Northern Territory, and Queensland). Shoot induction media containing 4.4 μM BA consistently provided longer shoots, with more nodes, than media containing 17.8 or 35.5 μM BA (Table 2). This medium promoted lateral shoot formation on Burkina Faso and Northern Territory seedling shoots, and it consistently provided one of the highest mean shoot numbers for transfer to the second passage. This mean was significantly higher than those of all other media for Burkina Faso seedlings, and significantly higher than those of the media containing 2.2, 17.8 or 35.5 μM BA for Northern Territory seedlings (Table 2). The only previous report of K. senegalensis shoot induction tested BA at 0, 2.2, 8.9 and 22.2 μM in combination with 0.3 μM IBA, and found highest induction in the media containing 2.2 or 8.9 μM BA (Danthu et al. 2003).
Subsequent shoot proliferation was also highest in the medium containing 4.4 μM BA (Fig. 5), although the final number of Northern Territory shoots produced was not significantly higher than from the media containing 2.2 or 8.9 μM BA (Fig. 5h). The final numbers of shoots produced in this medium were 106.7 ± 21.6, 75.0 ± 15.1 and 73.8 ± 15.9 from the Burkina Faso, Northern Territory and Queensland seed sources, respectively. Proliferation from all seed sources across all three passages was lowest in medium containing 35.5 μM BA, and final shoot numbers in the media containing 17.8 or 35.5 μM BA were consistently lower than in the media containing 2.2, 4.4 or 8.9 μM BA. Similar results were found in Melia azedarach, in which nodal segments produced fewer axillary buds with greater basal callusing at BA concentrations above 8.9 μM (Ahmad et al. 1990; Shahzad and Siddiqui 2001). In fact, for K. senegalensis, the highest BA concentration, 35.5 μM, completely inhibited shoot proliferation, with the final numbers of shoots produced in this medium (1.8 ± 0.2, 2.6 ± 0.4 and 2.0 ± 0.4; Fig. 5g–i, respectively) having not increased significantly (t-tests, P > 0.05, n = 19–20 clones) from the numbers available after shoot induction (Table 2). Survival of clones in medium containing 4.4 μM BA was 100%, and coefficients of multiplication (Sánchez and Vieitez 1991) for each of the 4- or 5-week passages ranged from 2.0 ± 0.1 to 4.3 ± 0.4 (Table 3). The final coefficients of multiplication (i.e. SP3 in Table 3) would have been higher if all shoots had been dissected for further proliferation rather than a subsample being kept intact for subsequent plantlet conversion.
Effect of BA concentration on number of shoots transferred per Khaya senegalensis clone after a, b, c 5 weeks, d, e, f 10 weeks, and g, h, i 15 weeks in shoot proliferation medium. Clones were derived from seeds obtained from a, d, g Burkina Faso (BF), b, e, h Northern Territory (NT) or c, f, i Queensland (Qld). Means (+s.e.) with different letters within each time point and seed source are significantly different (ANOVA and Duncan’s multiple range test; P < 0.05; n = 19–20 clones)
Most shoots from the medium containing 4.4 μM BA formed roots, with plantlet conversion frequencies of 90.0 ± 4.9% (Fig. 6a), 82.0 ± 4.8% (Fig. 6b) and 85.0 ± 5.2% (Fig. 6c). These means did not differ significantly from those of the media containing 2.2 μM BA for Burkina Faso shoots (Fig. 6a), any other BA concentration for Northern Territory shoots (Fig. 6b), and 2.2 or 8.9 μM BA for Queensland shoots (Fig. 6c). Plantlets from the medium containing 4.4 μM BA had more adventitious roots (3.5 ± 0.2, 2.6 ± 0.2 and 2.2 ± 0.1; Fig. 6d–f, respectively) than plantlets from one or both of the media containing 8.9 or 17.8 μM BA, depending on the seed source. Plantlets from all seed sources exhibited almost 100% survival after acclimatisation in the nursery, and their morphology was very similar to those produced in experiment 1 (Fig. 2e, f). The high rooting percentages achieved in this study, following application of 19.6 μM IBA for 7 d, are comparable with those obtained for the same species (87% and 96%) by applying 260 μM IBA for 1 or 7 d, respectively (Danthu et al. 2003). The current IBA concentration and exposure period are used for successful rooting (57–100%) of eucalypt hybrids (Hung and Trueman 2010, 2011), and rooting in the current study was higher than that obtained previously for other Meliaceae species including Khaya ivorensis (Mathias 1988), Swietenia macrophylla (Maruyama 2006) and Toona ciliata (Mroginski et al. 2003).
Effect of BA concentration in the shoot proliferation medium on a, b, c percentage of shoots with roots and d, e, f number of adventitious roots per rooted shoot for Khaya senegalensis clones after a 1-week treatment of shoots with 19.6 μM IBA followed by 3-week culture on ½MS medium. Clones were derived from seeds obtained from a, d Burkina Faso, b, e Northern Territory or c, f Queensland. Means (+s.e.) with different letters within a seed source are significantly different (ANOVA and Duncan’s multiple range test; P < 0.05; n = 14–20 clones)
Conclusion
This study has developed highly effective methods for (1) shoot induction and shoot proliferation to establish a laboratory archive of juvenile clones, and (2) root formation to generate plantlets of the same clones. These methods provide a means for simultaneous in vitro and ex vitro clone banking of K. senegalensis, thus allowing simultaneous laboratory preservation, plantlet conversion, cuttings production and field testing of clones for high-value tropical timber plantations.
References
Ahmad Z, Zaidi N, Shah FH (1990) Micropropagation of Melia azedarach from mature tissue. Pak J Bot 22:172–178
Arnold R, Reilly D, Dickinson G, Jovanovic T (2004) Determining the climatic suitability of Khaya senegalensis for plantations in Australia. In: Bevege DI, Bristow M, Nikles DG, Skelton D (eds) Prospects for high-value hardwood timber plantations in the ‘dry’ tropics of northern Australia. Private Forestry North Queensland Association Inc., Kairi, Australia, 10 pp
Arora K, Sharma M, Srivastava J, Ranade SA, Sharma AK (2010) Rapid in vitro cloning of a 40-year-old tree of Azadirachta indica A. Juss. (Neem) employing nodal stem segments. Agrofor Syst 78:53–63
Cameron SI (2010) Plant regeneration in Spanish cedar, Cedrela odorata L., using zygotic embryo explants from mature seed and improvement of embryogenic nodule initiation by heat shock. In Vitro Cell Dev Biol Plant 46:126–133
Chaturvedi R, Razdan MK, Bhojwani SS (2004) In vitro clonal propagation of an adult tree of neem (Azadirachta indica A. Juss.) by forced axillary branching. Plant Sci 166:501–506
Daniel B, John S, Sonlya EV, Nair GM (1999) Micropropagation of Naregamia alata W & A—an important medicinal plant. J Plant Biochem Biotechnol 8:105–107
Danthu D, Diaité-Sanogo D, Sagna M, Sagna P, Dia-Gassama YK (2003) Micropropagation of Khaya senegalensis, an African mahogany from dry tropical zones. J Trop For Sci 15:164–175
George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Ltd, Edington, UK
Hajari E, Berjak P, Pammenter NW, Watt MP (2009) Micropropagation of recalcitrant-seeded Ekebergia capensis Sparrm. using in vitro nodal segments and roots. J Hortic Sci Biotechnol 84:87–91
Hung CD, Trueman SJ (2010) Nutrient responses differ between node and organogenic cultures of Corymbia torelliana × C. citriodora (Myrtaceae). Aust J Bot 58:410–419
Hung CD, Trueman SJ (2011) Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora. Plant Cell Tissue Organ Cult 104:69–77
Husain MK, Anis M (2009) Rapid in vitro multiplication of Melia azedarach L. (a multipurpose woody tree). Acta Physiol Plant 31:765–772
Khatun R, Islam MS, Jahan MAA, Hossain MT (1998) Mass propagation of Azadirachta indica A. Juss. (neem) through in vitro-grown seedling culture. Bangladesh J Sci Ind Res 33:46–50
Kubmarawa D, Khan ME, Punah AM, Hassan M (2009) Phytochemical screening and antimicrobial efficacy of extracts from Khaya senegalensis against human pathogenic bacteria. Afr J Biotechnol 7:4563–4566
Liew TK, Teo CKH (1998) Multiple shoot production in vitro of the tropical timber tree, sentang (Azadirachta excelsa Linn.). HortSci 33:1073–1075
Makut MD, Gyar SD, Pennap GRI, Anthony P (2008) Phytochemical screening and antimicrobial activity of the ethanolic and methanolic extracts of the leaf and bark of Khaya senegalensis. Afr J Biotechnol 7:1216–1219
Maruyama E (2006) Tissue culture of Swietenia macrophylla King (big-leaf mahogany). In: Suzuki K, Ishii K, Sakurai S, Sasaki S (eds) Plantation technology in tropical forest science. Springer-Verlag, Tokyo, pp 131–136
Maruyama E, Ishii K, Saito A, Migita K (1989) Micropropagation of Cedro (Cedrela odorata L.) by shoot tip culture. J Jpn For Soc 71:329–331
Mathias PJ (1988) Micropropagation of the tropical hardwoods, Khaya ivorensis A. Chev. and Nauclea diderrichii (De Wild and Th. Dur.) Merrill. Ph.D. dissertation, University of Nottingham
Mroginski E, Rey HY, Mroginski LA (2003) In vitro plantlet regeneration from Australian Red Cedar (Toona ciliata, Meliaceae). New For 25:177–184
Nikles DG, Bevege DI, Dickinson GR, Griffiths MW, Reilly DF, Lee DJ (2008) Developing African mahogany (Khaya senegalensis) germplasm and its management for a sustainable forest plantation industry in northern Australia: progress and needs. Aust For 71:33–47
Ofori DA, Opuni-Frimpong E, Cobbinah JR (2007) Provenance variation in Khaya species for growth and resistance to shoot borer Hypsipyla robusta. For Ecol Manag 242:438–443
Opuni-Frimpong E, Karnosky DF, Storer AJ, Cobbinah JR (2008a) Silvicultural systems for plantation mahogany in Africa: influences of canopy shade on tree growth and pest damage. For Ecol Manag 255:328–333
Opuni-Frimpong E, Karnosky DF, Storer AJ, Cobbinah JR (2008b) Key roles of leaves, stockplant age, and auxin concentration in vegetative propagation of two African mahoganies: Khaya anthotheca Welw. and Khaya ivorensis A. Chev. New For 36:115–123
Peña-Ramírez YJ, Juárez-Gómez J, Gómez-López L, Jerónimo-Pérez JL, García-Sheseña I, González-Rodríguez JA, Robert ML (2010) Multiple adventitious shoot formation in Spanish Red Cedar (Cedrela odorata L.) cultured in vitro using juvenile and mature tissues: an improved micropropagation protocol for a highly valuable tropical tree species. In Vitro Cell Dev Biol Plant 46:149–160
Pennington TD, Styles BT (1975) A generic monograph of the Meliaceae. Blumea 22:419–540
Pijut PM, Woeste KE, Vengadesan G, Michler CH (2007) Technological advances in temperate hardwood tree improvement including breeding and molecular marker applications. In Vitro Cell Dev Biol Plant 43:283–303
Quraishi A, Koche V, Sharma P, Mishra SK (2004) In vitro clonal propagation of neem (Azadirachta indica). Plant Cell Tissue Organ Cult 78:281–284
Sánchez MC, Vieitez AM (1991) In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol 8:59–70
Schottz ES, Filho ANK, Tracz AL, Koehler H, Ribas LLF, Quoirin M (2007) In vitro multiplication of Swietenia macrophylla King (Meliaceae) from juvenile shoots. Cienc Florest 17:109–117
Shahin-uz-zaman M, Ashrafuzzaman M, Haque MS, Luna LN (2008) In vitro clonal propagation of the neem tree (Azadirachta indica A. Juss.). Afr J Biotechnol 7:386–391
Shahzad A, Siddiqui SA (2001) Micropropagation of Melia azedarach L. Phytomorphol 51:151–154
Styles BT (1981) Swietenioideae. In: Pennington TD, Styles BT, Taylor DAH (eds) Meliaceae (Flora neotropica monograph, no 28). New York Botanical Garden, New York, pp 359–418
Tacoronte M, Vielma M, Mora A, Valecillos C (2004) Propagación in vitro de caoba (Swietenia macrophylla King) a partir de yemas axilares. Acta Cient Venez 55:7–12
Thakur R, Rao PS, Bapat VA (1998) In vitro plant regeneration in Melia azedarach L. Plant Cell Rep 18:127–131
Trueman SJ, Richardson DM (2007) In vitro propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via cytokinin-free node culture. Aust J Bot 55:471–481
Trueman SJ, Richardson DM (2008) Relationships between indole-3-butyric acid, photoinhibition and adventitious rooting of Corymbia torelliana, C. citriodora and F1 hybrid cuttings. Tree For Sci Biotechnol 2:26–33
Vila S, Scocchi A, Mroginski L (2002) Plant regeneration from shoot apical meristems of Melia azedarach L. (Meliaceae). Acta Physiol Plant 24:195–199
Zhang HP, Wang X, Chen F, Androulakis XM, Wargovich MJ (2007) Anticancer activity of limonoid from Khaya senegalensis. Phytother Res 21:731–734
Acknowledgments
We thank Geoff Dickinson (Agri-Science Queensland) and Don Reilly (Department of Resources, Northern Territory) for providing Khaya seeds, and Bruce Randall for critical reading of the manuscript. The project was funded by the Queensland National and International Research Alliances Program and supported by a postgraduate scholarship to CDH from the Vietnam Ministry of Education and Training.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, C.D., Trueman, S.J. In vitro propagation of the African mahogany Khaya senegalensis . New Forests 42, 117–130 (2011). https://doi.org/10.1007/s11056-010-9241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-010-9241-9