Abstract
The present study investigates optimal conditions for the vegetative propagation of Himalayan yew Taxus wallichiana Zucc., an important medicinal tree, during spring. Effect of four treatments: (a) sex of donor plant (male and female), (b) age/type of shoot (1, 2, 3 year old, long and dwarf shoots), (c) auxin treatment (IBA and NAA at 0, 0.5, 1.25, 5.0 & 50.0 mM) and (d) rooting environment (raised beds/polythene bags) on percentage rooting in stem cuttings was studied. Randomized complete block (RBD) designs were used for experimentation. Rooting ability of cuttings was significantly influenced by all these treatments. The overall rooting response was higher in long shoot cuttings taken from female tree. Age of shoot also influenced the rooting response and was highest in 1 year old long shoot cuttings of female tree. Exogenous application of auxin, α-naphthalene acetic acid (NAA) and indole-3 butyric acid (IBA), had significant positive effect on the percentage rooting. IBA significantly enhanced the rooting percentage in 1 year old long and dwarf shoots at lower doses and 2 and 3 year old long shoots from female tree at higher doses. Maximum percent rooting (90% ± 2.8) was obtained with interactive effect of 0.5 mM, NAA (22 h) × 1 year old long shoot from female tree; followed by the interactive effect of 50 mM IBA (5 s) × 3 year old long shoot from female tree (83% ± 4.1). Cuttings planted in soil: sand medium in polythene bags showed earlier rooting response (12 weeks) than cuttings planted in raised nursery beds (24 weeks). Overall, the findings of this study suggest that 0.5 mM IBA treatment is suitable for enhancing adventitious rooting in 1 year old long and dwarf shoots of male and female trees. IBA at higher doses is suitable for enhancing the rooting percentage of 2 and 3 year old long shoots from female tree. This study provides a significant lead towards the development of a simple and inexpensive technique for large scale propagation, aforestation of elite genotypes and raising of bush type plantation under ex-situ conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxus wallichiana Zucc. (syn T. baccata ssp. wallichiana (Zucc.) Pilg.) or Himalayan yew is the only Taxus species in India (Rikhari et al. 1998).The species has gained considerable importance as a source of various taxoids with anticancer properties (Busing et al. 1995; Nemecek 1996) including paclitaxel (better known under its commercial name Taxol); a diterpene amide which was first isolated from the stem bark of the pacific yew T. brevifolia Nutt.(Wani et al. 1971).The needles, stem and bark of other Taxus species have been reported to contain taxol and 10-deacetylbaccatin III—starting material for the synthesis of taxol (Witherup et al. 1990). Use of material from these species has taken some pressure off T. brevifolia, but has led to the overexploitation of T. wallichiana along the Himalayas (Nandi et al. 1994; Shukla et al. 1994; Behera et al. 2000). Widely distributed in temperate zone of Himalayas from Afganistan to Butan at altitudes between 1,800 and 3,400 m amsl (Purohit et al. 2001), this species commonly occurs in patches under Betula utilize, Abies pindrow, Acer cesium, Pinus wallichiana, Quercus semecarpifolia and Rhododendron arboretum (Rikhari et al. 1998; Purohit et al. 2001). Taxus wallichiana is an extremely slow growing tree and its regeneration through seed is very poor (Rajewski et al. 2000; Chee 1994) and requires complex pre-treatments for germination (Gamble 1922; Nicholson and Munn 2003; Pilz 1996a, b). Therefore vegetative multiplication through cuttings remains the only practical option for augmenting its regeneration for ex-situ conservation, aforestation and large scale cultivation programmes. The effect of auxin in promoting rooting of cuttings is well known (Nanda 1970; Hartmann et al. 1997; Husen and Mishra 2001; Husen 2003; Husen and Pal 2006), however, very little information is available on the effectiveness of auxin in relation to the branch/shoot type and sex of donor plant.
Keeping in view the increasing demand of taxol, poor regeneration through seeds and slow growing nature of the species, present study was carried out during 1997 under sub temperate humid climatic conditions of Palampur, Himachal Pradesh, India (1,300 m amsl). The study was designed to evaluate some of the major factors reported to affect the rooting capacity of cuttings namely origin of cuttings, auxin treatments (IBA and NAA) and rooting environment on rooting of stem cuttings of T. wallichiana obtained from Dauladhar range forest (Barot, Himachal Pradesh, India).
Materials and methods
Experimental site
The experiments were conducted in experimental farm of Regional Research Laboratory, extension centre, Palampur (Himachal Pradesh, India) situated 32°6′ North and 76°3′ East at an elevation of 1,300 m amsl, having temperature range 30.2–4.10°C and annual rainfall 2,677 mm.
Collection and preparation of cuttings
Branches were collected (during first week of March) from T. wallichiana trees (male and female, age unknown) found in natural stands in Dauladhar range forest (Barot, Himachal Pradesh, India). Leafy cuttings (15–20 cm) were excised from long (1, 2 and 3 year old) and dwarf (1 year old) shoots and kept separately. Needles were removed from basal 2 cm portion of cuttings and the cuttings were stored in polythene bags under moist conditions.
Treatments
The main treatments were (a) sex of donor plant, (b) age/type of shoot, and (c) auxin treatments. Two types of auxins were used at different concentrations. Auxins and their concentrations used were NAA and IBA at 0.5 and 1.25 mM (22 h) and IBA at 5 (1 and 3 h) and 50 mM (5 s). An earlier trial conducted had shown that higher concentrations of NAA were not effective in rooting of T. wallichiana cuttings. The auxins were applied in 1.5% v/v aqueous ethanol solution; basal portion of cuttings was dipped in auxin solution at room temperature. Cuttings from various age groups from long and dwarf shoots of male and female trees were treated separately and for each treatment three replications were taken with 60 cuttings per replication.
Planting
Treated cuttings were divided into two lots; lot 1 was planted in soil: sand medium (1:1) in polythene bags (6.25 cm wide × 12 cm deep) and lot 2 in raised nursery beds under artificial shade. The experiments were laid in randomized complete block design with three replications per treatment and 30 shoot cuttings per replication. The cuttings were given intermittent mist spray, 4–5 times a day.
Observation on rooting
After every 30 days cuttings from each treatment were carefully removed from the rooting medium and observations were recorded on percent rooting.
Statistical analysis
Statistical analysis was carried out with Assistat—statistical assistance computer software version 7.4. For lower doses of IBA and NAA randomized complete block design with three factor factorial arrangement was used for statistical analysis. Similarly for high doses of IBA with different age of cuttings same ANOVA method was followed. Analysis was performed on untransformed data. In the analysis of variance (ANOVA) for studied parameters, the mean values of each replication were estimated. For the comparison of different means of different treatments the critical difference were calculated based on student t-test at P ≤ 0.05.
Results
Effect of sex of donor plant
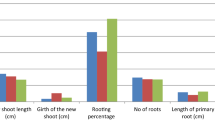
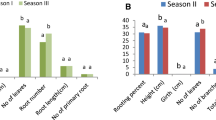
Sex of donor plant significantly (P < 0.01) influenced rooting of cuttings (Figs. 1, 2; Tables 1, 2). Rooting response was generally higher in cuttings obtained from female tree than from male tree (Figs. 1, 2).
Effect of type of shoot
Shoot type also influenced significantly the rooting percentage (Fig. 2; Table 1), which was maximum in long shoots (63.3%) followed by dwarf shoots (40%).
Effect of age of cutting
Age of the cutting also influenced the percentage rooting with maximum in 2 year old cutting (32%) followed by 1 year (28%) and 3 year old cutting (12%).
Effect of auxin treatment
In general treatment with auxin exhibited significant variation at P < 0.01 (Table 1). However, treatment with lower doses of IBA increased percentage rooting followed by NAA. Higher doses of IBA (50 mM for 5 s) resulted in maximum rooting followed by (5 mM IBA for 1 h).
Interactive effects
Interactive effect of sex and auxin treatment was significant at P < 0.01 (Tables 1, 2). The combination of female tree cuttings × 0.5 mM NAA resulted in maximum rooting response followed by female × 0.5 mM IBA and female × 50 mM IBA (Fig. 1, 2). Response of female cuttings observed was significantly superior to male cuttings at all levels of IBA and NAA treatment. Interactive effect of age and IBA was significant at P < 0.01 (Table 2). The combination of 2 years growth × 5 mM IBA (for 1 h) showed maximum rooting response followed by 3 years growth × 50 mM IBA. IBA treatment for 3 h inhibited rooting in cuttings from all the 3 years of growth. Interactive effect of shoot type and auxin (IBA and NAA) was also significant at P < 0.01 (Table 1). Long shoots showed higher response to auxin than dwarf shoots. Rooting response observed was maximum in long shoot × 0.5 mM NAA combination followed by long shoot × 0.5 mM IBA combination. Interaction between sex and shoot type was found insignificant (Table 1) however interaction between sex and age was found significant and 2 year old female were found superior followed by 1 year old female cuttings. The three factor interaction (sex, age and higher doses of IBA) was significant at P < 0.01 (Table 2) and maximum rooting response was observed with female (long shoot) × 3 years growth × 50 mM IBA (5 s) combination (83.3%) (Fig. 1). Another three factor interaction (sex × shoot type × lower auxin concentration) was also significant (Table 1) and maximum rooting response was observed with female × long shoot × 0.5 mM NAA (90%) followed by female × long shoot × 0.5 mM IBA (80%) (Fig. 2). Cuttings planted in soil: sand medium (1:1) in polythene bags (lot 1) rooted in 12 weeks, whereas the cuttings planted in raised nursery beds (lot 2) rooted in 24 weeks. However, no significant difference was observed in final rooting percentages, when comparing treatments of lot 1 and 2. Therefore, experimental data of only one lot (lot 1) is reported here.
Discussion
Sex of donor plant has an important effect on rooting of T. wallichiana cuttings. Cuttings taken from the female tree had higher rooting percentage than cuttings taken from male tree. The reason for this difference in root formation is not clear and needs further investigation. No significant difference in rooting percentages between male and female trees has been observed in pacific yew (T. brevifolia. Nutt.), (Mitchell 1997). In contrast cuttings from female Japanese yew (T. cuspidata) rooted with higher success rates than from male tree (Davidson and Olney 1964). Nandi et al. (1996) has also reported the effect of sexual differences on propagation of Taxus baccata. Shoot type (long shoot, dwarf shoot) also has an important effect on rooting of Himalayan yew. Cuttings from long shoots exhibited higher rooting response to auxins than dwarf shoots. The age of shoot growth also affects rooting of Himalayan yew. Best rooting response was exhibited by 2 year’s growth followed by 1 year’s and least in 3 year’s growth. In most tree species rooting ability of cuttings has been reported to increase from apical to basal part of the shoots which has been attributed to accumulation of carbohydrates at the base of shoot (Hartmann et al. 1997). However, there are many deviations from this general trend. For example, in Triplochiton scleroxylon, rooting percentage of cuttings from different node positions was found to decline basipetally (Leakey and Mohammed 1985). Cuttings originating from the apical position of shoots of Milicia excelsa (Ofori et al. 1997), T. scleroxylon (Leakey 1983) and Nauclea diderrichii (Matin 1989) displayed higher rooting percentages than those taken from the basal portions. Such effects on rooting may be caused by variation in the physiological status of the cutting tissues on stock plants resulting in occurrence of gradients in the cellular activity or in the level of assimilates or growth regulators or in the level of lignification (Hartmann et al. 1997). Among the auxin treatments generally, application of IBA and NAA (0.5 mM) maximally promoted rooting percentage for cuttings, which were taken from 1 year’s growth. However effect of NAA (0.5 mM) treatment was lower in 1 year old cuttings taken from dwarf shoots. Higher doses of IBA promoted rooting for cuttings which were taken from 2 and 3 year’s growth from long shoots of female tree. In other Taxus species Kim and Nam (1985) and Eccher (1988) have reported that only IBA helps in enhancing rooting process. Whereas Istas and Meneve (1977) showed that β-IBA gave better rooting results than α-NAA and tip cuttings gave better rooting results than stem cuttings in T. bacatta. In this respect it is worthwhile to mention that sensitivity to applied growth regulators does vary with status of tissue (Trevavas and Cleland 1983). Spring season cuttings root earlier than summer and autumn season cuttings (unpublished data); this may be due to the fact that cuttings were raised during spring to early summer when temperature conditions ranged from 22 to 32°C (max.) and 14 to 20°C (min.). This suggests that temperature of 21–29°C during day combined with lower night temperature (14–20°C) has worked well. A lower air temperature reduces transpiration rate and suppresses bud development. In T. cuspidata highest percentage of rooting with IBA was also obtained at 20°C rooting bed temperature (Eccher 1988).
This study provides significant information towards development of a simple and inexpensive technique for large scale propagation, aforestation of elite genotypes and raising of bush type plantation under ex-situ conditions. Thousands of plantlets raised by this technique were transferred to their natural habitat in various forest areas in Jammu & Kashmir (India). Nearly 200 cutting raised plants were planted in our experimental farm at Palampur, under shade and sun shine conditions resulting in 95–98% survival (Fig. 3). Therefore, this can be utilized for large scale propagation and raising of bush type plantation under ex-situ conditions. However, further physiological/biochemical investigations are still needed in this species to understand the detailed mechanism of this rooting response.
References
Behera MD, Srivastava S, Kushwaha SPS, Roy PS (2000) Stratification and mapping of Taxus baccata L. bearing forests in Talle Valley using remote sensing and GIS. Curr Sci 78(8):1008–1013
Busing RT, Halpernand CB, Spies TA (1995) Ecology of pacific yew (Taxus brevifolia) in western Oregoa and Washington. Conserv Biol 9:1199–1207
Chee PP (1994) In vitro culture of zygotic embryos of Taxus species. Hortic Sci 29:695–697
Davidson H, Olney A (1964) Clonal and sexual differences in the propagation of Taxus cuttings. Comb Proc Int Plant Propag Soc 14:156–160
Eccher T (1988) Response of cuttings of 16 Taxus cultivars to rooting treatments. Acta Hortic 227:251–253
Gamble JS (1922) A manual of Indian timbers. Simpson Low, Maston & Company, Limited, London, pp 868
Hartmann HT, Kester DE, Davies FT, Geneve RL (1997) Plant propagation principle and practices, 6th edn. Prentice-Hall of India Private Limited, New Delhi, pp 276–328
Husen A (2003) Effect of IBA and NAA treatments on rooting of Rauvolfia serpentina Benth. ex Kurz shoot cuttings. Ann For 11(1):88–93
Husen A, Mishra VK (2001) Effect of IBA and NAA on vegetative propagation of Vitex negundo L. through leafy stem cuttings from hedged shoots during rainy season. Ind Perf 45(20):83–87
Husen A, Pal M (2006) Variation in shoot anatomy and rooting behaviour of stem cuttings in relation to age of donor plants in teak (Tectona grandis Linn. f.). New For 31(1):57–73
Istas W, Meneve I (1977) Verbonsniews voor de Belgische Sierteelt 21:54
Kim CH, Nam JC (1985) Effect of some environmental factors on Japanese Yew (Taxus cuspidata Sieb. Et. Zucc.). J Korean For Soc 70:1–76
Leakey RRB (1983) Stock plant factors affecting root initiation in cuttings of Triplochiton Scleroxylon K. Schum an indigenous hardwood of West Africa. J Hortic Sci 58:227–290
Leakey RRB, Mohammed HRS (1985) Effects of stem length on root initiation in seqential single node cuttings of Triplochiton scteroxylon K. schum. Comm For Rev 60:117–126
Matin MA (1989) Carbon economy during rooting of cuttings of Nauclea diderrichii (De. Wild and Th. Dus.) Merill. M. Phil. thesis, University of Edinburgh, United Kingdom
Mitchell AK (1997) Propagation and growth of pacific Yew (Taxus brevifolia Nutt) cuttings. Northwest Sci 71(1):56–62
Nanda KK (1970) Investigations of on the use of auxins in vegetative reproduction of forest plants. Final Report of PL 480. Research Project A 7FS-11 (FG In 255), pp 1–215
Nandi SK, Pandey A, Palni LMS (1994) Cancer cure from Himalayan yew. Cent Himalayan Environ Assoc Bull 7:41–47
Nandi SK, Palni LMS, Rikhari HC (1996) Chemical induction of adventitious root formation In Taxus baccata cuttings. Plant Growth Regul 19:117–122
Nemecek S (1996) Rescuing an endangered tree. Sci Am 10:22
Nicholson R, Munn DX (2003) Observation on the propagation of Taxus globosa Schltdl. Bol Soc Bot Mex 72:129–130
Ofori D, Newton AC, Leakey RRB, Grace J (1997) Vegetative propagation of Milicia excelsa by leafy stem cuttings: effects of maturation, coppicing, cutting length and position on rooting ability. J Trop For Sci 10(1):115–129
Pilz D (1996a) Propagation of Pacific Yews from seed, (part I) American Conifer Society Bulletin. Winter Issue 13:13–18
Pilz D (1996b) Propagation of Pacific Yews from seed, (part II) American Conifer Society Bulletin. Spring Issue 13:80–83
Purohit A, Maikhuri RK, Rao KS, Nautiyal S (2001) Impact of bark removal on survival of Taxus bacatta L. (Himalayan yew) in Nanda Devi Biosphere Reserve, Garwal Himalaya, India. Curr Sci 81(5):586–590
Rajewski M, Lange S, Hattemer HH (2000) Problems of reproduction in the genetic conservation of rare tree species: the example of common yew (T. baccata L.). For Snow Landsc Res 75:251–266
Rikhari HC, Palni LMS, Sharma S, Nandi SK (1998) Himalayan yew: stand structure, canopy damage, regeneration and conservation strategy. Environ Conserv 25:334–341
Shukla GP, Rao K, Haridasan K (1994) Taxus baccata in Arunachal Pradesh. Arunachal For News 12:1–7
Trevavas AJ, Cleland RE (1983) Is plant development regulated by changes in the concentration of growth substances or by changes in the sensitivity to growth substances? Trends Biochem Sci 8:354–357
Wani MC, Taylor HL, Wall ME, Coggen P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM (1990) Taxus spp.: needles contain amounts of taxol comparable to the bark of Taxus brevifolia: analysis and isolation. J Nat Prod 53:1249–1255
Acknowledgement
The author is thankful to Director RRL, Jammu for providing necessary facilities. The author is also grateful to Sh. Rajmal and Sh. Suresh Sood for collection of plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaul, K. Variation in rooting behavior of stem cuttings in relation to their origin in Taxus wallichiana Zucc.. New Forests 36, 217–224 (2008). https://doi.org/10.1007/s11056-008-9094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-008-9094-7