Abstract
Ba0.5-xSr0.5PbxFe12O19 hexaferrites (x = 0, 0.1, 0.2, 0.3, and 0.5) prepared by the co-precipitation method, revealed the formation of M-type hexaferrites with crystallite sizes varying from 42.81 to 60.96 nm. Fourier transform infrared spectra (FTIR) indicated the formation of hexaferrites. Scanning electron microscope (SEM) analysis confirmed hexagonal morphology. Transmission electron microscopy (TEM) micrographs, high-resolution transmission electron microscopy (HRTEM) pictures, and selected area electron diffraction (SAED) patterns further supported the nanoparticle characteristics. SAED analysis showed clear and well-defined circular rings, corresponding to the reflection planes observed in X-ray powder diffraction (XRD) analyses. X-ray photoelectron spectroscopy (XPS) was performed to examine the electronic structure, while energy-dispersive X-ray spectroscopy (EDX) investigation proved the elements’ existence. The direct optical energy band gaps (\({\mathrm{E}}_{\mathrm{g}}\)), as determined through Tauc plots, decreased from 3.11 to 2.95 eV in an inversely proportional manner to D, indicating the quantum confinement effect. Photoluminescence (PL) spectra showed emissions at 335 nm for all synthesized compounds. The vibrational sample magnetometer (VSM) measurements showed strong ferromagnetic behavior, with a decrease in saturation magnetization (\({\mathrm{M}}_{\mathrm{s}}\)) from 56.33 to 38.83 emu/g, coercive fields (\({\mathrm{H}}_{\mathrm{c}}\)) from 4388.3 to 3289.3 G, and squareness ratios from 0.49 to 0.43. The decrease in coercivity (\({\mathrm{H}}_{\mathrm{c}}\)) with Pb incorporation is attributed to significant demagnetizing-like interactions caused by a rise in particle size and a reduction in anisotropy energy rising from Pb doping. Effective crystalline anisotropy constants (\({\mathrm{K}}_{\mathrm{eff}}\)) decreased from 2.37 × 105 to 1.03 × 105 Erg/g, categorizing the materials as hard magnets ideal for high-density magnetic recording plus permanent magnet production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, nanosized spinel-type ferrites have garnered significant scientific interest across various fields. These nanomaterials exhibit exceptional properties, including superparamagnetic, quantum size effects, and magnetic tunneling [1, 2]. Additionally, they possess a larger surface-to-volume ratio, chemical stability, and ease of synthesis. Ferrites fall into four crystal structure categories: Spinel, Garnet, Ortho, and Magneto plumbite. Based on their magnetic behavior, they are classified as either hard or soft [3, 4]. Specifically, magnetic nanoparticles with a spinel structure are referred to as spinel ferrites, while those with a hexagonal structure are known as hexaferrites or magneto plumbite ferrites [5, 6].
M-type hexagonal ferrites, known as hexaferrite, with a chemical expression of \({\mathrm{MFe}}_{12}{\mathrm{O}}_{19}\), where M represents divalent cations like \({\mathrm{Ba}}^{2+}\), \({\mathrm{Sr}}^{2+}\), or \({\mathrm{Pb}}^{2+}\) [7, 8]. These ferrimagnetic oxides possess unique magnetic properties, including high saturation magnetization, high uniaxial magnetocrystalline anisotropy, also high Curie temperature, which have piqued the interest of researchers since their discovery in 1951 [9, 10]. Hexaferrites offer magnetic, chemical stability, corrosion resistance, high coercivity, and cost-effectiveness, suitable for diverse industry applications like Plasto-ferrites, permanent magnets, DC motors, switch mode materials, transistors, and recording devices [11,12,13,14,15,16,17].
The addition of different cations through doping can alter the magneto-dielectric besides microwave characteristics of M-hexaferrite, just like Sr, Ba, Pb, and others [18]. These changes in properties can affect the suitability of hexaferrites for numerous tools and devices. Numerous researches have been conducted on cationic exchanges in M-type hexaferrite, including the Pb-doped Sr-ferrites at room temperature by Hussain et al. [19], the exchange of Ni \(-\) Ce ions in Sr-Ba hexaferrite by Iqbal et al. [20], the substitution of La and Ni ions in \({\mathrm{Sr}}_{0.5}{\mathrm{Ba}}_{0.5}{\mathrm{Fe}}_{12}{\mathrm{O}}_{19}\) to improve electrical resistivity by Iqbal et al. [21], the influence of Pb exchange on the physical, magnetic, and microwave characteristics of \({\mathrm{Sr}}_{0.5}{\mathrm{Ba}}_{0.5}{\mathrm{Fe}}_{12}{\mathrm{O}}_{19}\) by Baykal et al. [22], the impact of Pb on the magnetic interactions of the M-type hexaferrite by Guerrero et al. [23], and a study by Haq et al. [24] on the magnetic characteristics and frequency-based uses of Pb-substituted \({\mathrm{BaFe}}_{12}{\mathrm{O}}_{19}\) hexaferrite. It was found that Lead doping resulted in a coercivity decrease in the material due to an increase in particle size and decreased anisotropy energy [24]. However, there is limited literature available on lead-doped hexaferrites. Despite the scarcity of studies, Pb-doped nanoferrites have shown intriguing properties. The substitution of lead in the crystal structure can also influence the S block, occasionally leading to the formation of impurity phases [24]. Nonetheless, Pb-doped materials have gained attention and acceptance for their lower anisotropic field and faster crystallization process [24].
Various approaches have been employed for the production of M-type hexaferrite specimens, for example, co-precipitation, microemulsion, solid-state route, hydrothermal, metal–organic decomposition, and sol–gel auto-combustion methods [25]. Among these methods, the self-propagating co-precipitation method stands out as an advanced route due to its simplicity and ease of use. The self-propagating co-precipitation method allows for easy molecular-level mixing of precursors without the need for intense mechanical mixing. Another advantage is that this method does not demand prolonged high-temperature calcination, unlike the solid-state route. Throughout the co-precipitation process, the local temperature generated aids in the creation of the requested product. A significant benefit of this technique is the ability to minimize the evaporation of lead. The powders obtained from the co-precipitation technique frequently exhibit nanoscopic dimensions with a narrow size distribution. This characteristic leads to a high surface area, which can be valuable in catalytic products.
This research aims to optimize the synthesis of phase-pure barium strontium hexaferrite and investigate the impact of lead doping on its physical, optical, morphological, and magnetic properties. By focusing on lead-doped strontium-barium hexaferrite in the hexagonal phase, this study aims to fill a gap in existing knowledge. The research emphasizes the synthesis of magnetic materials suitable for applications such as high-density magnetic recording and permanent magnet production.
Experimental
Substances
The elements utilized in this examination were \({\mathrm{BaCl}}_{2}.2{\mathrm{H}}_{2}\mathrm{O}\), and \({\mathrm{Cl}}_{3}\mathrm{Fe}.6{\mathrm{H}}_{2}\mathrm{O}\) from Sigma-Aldrich, \({\mathrm{Cl}}_{2}\mathrm{Sr}.6{\mathrm{H}}_{2}\mathrm{O}\) from FisherScientific, and \({\mathrm{PbCl}}_{2}\) from ThermoScientific (Table 1).
Preparation of \({{\varvec{B}}{\varvec{a}}}_{0.5-{\varvec{x}}}{{\varvec{S}}{\varvec{r}}}_{0.5}{{\varvec{P}}{\varvec{b}}}_{{\varvec{x}}}{{\varvec{F}}{\varvec{e}}}_{12}{{\varvec{O}}}_{19}\) hexaferrite
\({\mathrm{Ba}}_{0.5-\mathrm{x}}{\mathrm{Sr}}_{0.5}{\mathrm{Pb}}_{\mathrm{x}}{\mathrm{Fe}}_{12}{\mathrm{O}}_{19}\) (\(\mathrm{x }= 0, 0.1, 0.2, 0.3,\mathrm{ and }0.5\)) samples were produced via a coprecipitation approach. The synthesis process involved dissolving the right amounts of elements (Table 1) in deionized water while vigorously stirring. The pH of the solution was adapted to 13 by a 4M sodium hydroxide solution. The resulting precipitate was then washed with a mixture of 50% deionized water and 50% ethanol until the pH reached 7. Afterward, the precipitant was dried out at 80 ℃ for 2 h, followed by a 2-h calcination for the n0-n5 samples and a 6-h calcination for the B0-B5 samples, both conducted at 950 ℃ (Table 2).
Characteristics techniques
The crystal structures and phases were analyzed using a Bruker D8 Advance Powder XRD instrument equipped with a Cu-Kα radiation source. The instrument employed a step size of \(0.02^\circ\) and a time interval of \(0.4\) ss. The acquired patterns underwent refinement and analysis using the MAUD software for phase identification. XPS spectra were recorded via a ThermoScientific K-Alpha X-ray photoelectron spectrometer system, with different pass energies for regular and high-resolution spectra. FTIR spectra were done using a Thermo Scientific Nicolet iS5 FTIR spectrometer with KBr pellets as a medium, covering a range from \(4000\) to \(350\) \({\mathrm{cm}}^{-1}\). SEM–EDX analysis was performed using a Seron AIS \(2100\) C instrument to characterize the elemental composition and morphology. TEM, HRTEM, and SAED images were acquired on a JEM \(100\) CX instrument operating at 80 kV with high resolution. Particle size and distribution analysis were conducted using ImageJ and Origin Lab software. PL and UV–vis spectroscopy were conducted at room temperature via a Fluorescence Spectrometer and a Jasco V-\(670\) spectrophotometer, respectively. The magnetic properties were examined using a VSM model Lake Shore \(7410\).
Results and discussion
XRD
The XRD powder patterns of the samples calcined for 2 and 6 h were refined using the Rietveld method with MAUD software. Figure 1 illustrates these patterns, displaying reflection planes such as (107), (110), (112), (008), (114), (203), (205), (217), (206), (1011), (219), (2011), (220), and (2014) present across all samples. This confirms the absence of any impurity phases. The identified (hkl) planes align with ICDD Card no 84–0757 for BaFe12O19. The X-ray diffraction (XRD) patterns obtained from the synthesized samples closely match the reference diffraction data found in the JCPDS database (JCPDS: 27–1029). Crystal structure constraints were determined from the Rietveld refinements and are detailed in Table 3. It is evident from the data obtained during refinement and experimentation that there is a strong correlation. The difference between the two datasets exhibits a random distribution around zero. This observation implies that there are no unused peaks, and the most recently refined shape closely resembles the actual basic cell structure of the sample [26, 27]. The percentage of hematite was noticeably higher in samples subjected to 6-h calcination compared to those calcined for 2 h, as indicated in Table 3. Therefore, the samples calcined for 2 h were selected for further analysis using subsequent techniques outlined in the paper. Table 3 demonstrates a positive correlation between an increase in the Pb ratio and a higher hematite percentage in samples calcined for 2 h. Particularly, in the n5 sample with the highest Pb concentration, intense XRD peaks revealing the presence of the hematite phase were observed. This suggests that the Lead hexaferrite phase transformed into hematite due to the evaporation of PbO at the elevated calcination temperature of 950 ℃ [25]. In Lead-rich samples such as n1, n2, and n3, there was a significant increase in the presence of the hematite impurity phase. These observations indicate that at higher calcination temperatures, the PbO phase vanishes from the Lead hexaferrite, resulting in the creation of α-Fe2o3residues. This alteration could potentially affect the magnetic properties of the specimen [25].
The mean crystallite size of the specimens was estimated via Scherrer’s expression [28]:
where k=0.9, λ =1.5456 Å, β=FWHM and θ= Bragg’s angle. The average full width at half maximum (FWHM) was determined from the major peaks in the XRD pattern and utilized in the formula to derive the average crystallite size (D), which showed a rise with higher Pb concentration (refer to Table 3). This finding was corroborated by SEM and TEM analyses. The lattice parameters ‘a’ and ‘c’ for each sample were calculated using the following relation [28]:
The Miller indices (hkl) are indicative of the plane’s interplanar spacing, represented by \({d}_{hkl}\). The lattice parameter values exhibited a decreasing trend as the Pb ratio in the samples increased (refer to Table 3). This decrease could be attributed to the larger radius of \({\mathrm{Ba}}^{2+}\) ions (1.49 Å) compared to \({\mathrm{Pb}}^{2+}\) ions (1.19 Å) [25, 29]. The variation in lattice parameters observed in our synthesized hexaferrite samples could be attributed to the presence of additional phases, such as α- Fe2O3, within the X-ray diffraction (XRD) patterns [30]. Additionally, the (c/a) ratio values are presented in Table 3. It is suggested in the literature that a hexagonal M-type hexaferrite structure typically forms when the c/a value is below 3.98, in accordance with Vegard’s law for unit cell volume [31]. Furthermore, the dislocation density \(\left(\delta =1/{D}^{2}\right)\) value decreased from 4.28 × 10−4 to 2.66 × 10−4 as the Pb concentration increased (refer to Table 3). This indicates that a higher Pb concentration leads to a more compact lattice. Additionally, the lattice strain, calculated using the Stokes-Wilson method, is detailed in Table 3. It is evident that the lattice strain diminishes as the Pb content rises. This reduction in lattice strain can be attributed to the weaker bond strength of the lead-oxygen (Pb–O) bonds compared to the barium-oxygen (Ba–O) bonds [32]. Following the Hall–Petch relation [32], the increase in crystallite size corresponds to a decrease in yield strength and lattice strain with higher Pb doping content. Moreover, \(({\uprho }_{\mathrm{x}})\) represents the X-ray density which can be calculated as [33]:
where Z is the number of formula units in unit cell (Z = 2), M stands for the molecular mass of the sample, \({\mathrm{N}}_{\mathrm{A}}\) is the Avogadro’s number, and \({V}_{cell}\) is the volume of the cell calculated as \({V}_{cell}=0.8666{a}^{2}c\) [33, 34]. The increase in Pb doping corresponds to a rise in X-ray density, from 5.15 to 5.34 g/cm3 (refer to Table 3). This change can be linked to lattice contraction [34].
XPS
XPS was operated on the prepared hexaferrite specimens to analyze the elemental composition and oxidation states. The resulting spectra confirmed the presence of all constituent elements within the hexaferrite. Figures 2, 3, 4 depict the different binding energy (B.E.) ranges corresponding to the Ba 3d, Sr 3d, Pb 4f, O in 1s, and Fe in 2p states, as illustrated in the figures.
Figures 2b and 3b display the deconvoluted spectrum standing for the Ba \(3{\mathrm{d}}_{5/2}\) and Ba \(3{\mathrm{d}}_{3/2}\) states at binding energies around \(779\) and \(794\) \(\mathrm{eV}\), respectively. The Sr spectrum shows peaks near \(133\) and \(134\) \(\mathrm{eV}\), corresponding to the \(3{\mathrm{d}}_{5/2}\) and \(3{\mathrm{d}}_{3/2}\) states of \({\mathrm{Sr}}^{2+}\) cations (Figs. 2c, 3c, and 4b). For Fe, the spectrum shows peaks at \(712\) and \(727\) \(\mathrm{eV}\), along with satellite peaks at \(719.5\) and \(733.5\) \(\mathrm{eV}\). The peak at \(710.4\) eV can be further settled into \(710.5\) \(\mathrm{eV}\) (\({\mathrm{Fe}}^{2+}\)) and \(712.6\) eV (\({\mathrm{Fe}}^{3+}\)), while the peak at \(724.1\) \(\mathrm{eV}\) can be settled into \(723.9\) \(\mathrm{eV}\) (\({\mathrm{Fe}}^{2+}\)) and \(726\) \(\mathrm{eV}\) (\({\mathrm{Fe}}^{3+}\)) (Figs. 2d, 3e, and 4d). The deconvoluted spectrum of O 1s can be analyzed to reveal three distinct peaks. These peaks appear at binding energies of \(529.8\) and \(531.8\) \(\mathrm{eV}\), ascribed to \({\mathrm{O}}^{2-}\) (Ob) and oxygen vacancy (Oc) (Figs. 2e, 3f, and 4e) [35]. Additionally, the Pb 4f doublet forms of two peaks, Pb \({4\mathrm{f}}_{7/2}\) (\(138\mathrm{ eV}\)) and Pb \({4\mathrm{f}}_{5/2}\) (\(142.9\mathrm{ eV}\)), which show the existence of Pb in a \(+2\) state. Similar Pb peaks with comparable binding energies have been previously detected in other studies [36].
EDX
EDX analysis was employed for the elemental analysis of the \({\mathrm{Ba}}_{0.5-\mathrm{x}}{\mathrm{Sr}}_{0.5}{\mathrm{Pb}}_{\mathrm{x}}{\mathrm{Fe}}_{12}{\mathrm{O}}_{19}\) hexaferrites, as shown in Fig. 5. The spectrum displays distinct energy lines. The weight (%) of Sr, Ba, Pb, Fe, and O detailed in Table 4 corresponds well with the overall ratios of these elements used in the sample preparation, affirming the successful production of the hexaferrite samples. Figure 5 further illustrates this pattern, with the size of the Pb-related peaks increasing while the Ba-related peaks gradually decreasing across the series of specimens. The atomic percentages provided in Table 4 support this observation.
TEM, HRTEM, and SAED
In Fig. 6a, TEM images of the Pb-substituted Ba/Sr-hexaferrite samples exhibited agglomerated particles with a hexagonal shape. The TEM analysis indicated that the average grain size surpassed the average crystallite size determined by XRD, suggesting the presence of multiple crystallites within each grain. The introduction of Pb followed a consistent trend in Scherrer’s model, with values ranging from 89.99 to 101.41 nm (Fig. 6b). Additionally, HRTEM images supported the crystal structure by displaying lattice fringes that corresponded to the earlier XRD results (Fig. 6c). These lattice fringes represented various d-spacing values corresponding to (hkl) planes such as (101), (102), (107), (008), and (006). SAED patterns for \({\mathrm{Ba}}_{0.5-\mathrm{x}}{\mathrm{Sr}}_{0.5}{\mathrm{Pb}}_{\mathrm{x}}{\mathrm{Fe}}_{12}{\mathrm{O}}_{19}\) (x = 0.0, 0.1, 0.2, 0.3, and 0.5) provided further insight into the crystal structure, exhibiting distinct circular rings aligned with XRD reflection planes (Fig. 6d). These outcomes confirmed the polycrystalline nature of the nanostructures and validated the presence of the hexagonal phase.
SEM
The study employed scanning electron microscopy to examine the morphology of Pb-substituted Ba/Sr hexaferrite nanoparticles. The images in Fig. 7 portrayed small-sized particles with some aggregation, highlighting the uniform distribution and hexagonal platelet shape of the grains. Variations in grain boundary density were noted, influenced by accelerated crystallization facilitated by Pb2+ ions. Higher Pb concentrations were associated with the presence of small, rounded hematite particles. Changes in particle size, distribution, grain quantity, boundaries, and morphology were observable with increasing Pb concentration, resulting in sharper hexagonal structures and varying grain boundary densities linked to composition and the rapid crystallization induced by Pb2+ ions.
FTIR
The composition analysis and confirmation of the M-type hexaferrite phase in Pb-substituted Ba, Sr-hexaferrite were conducted using FTIR spectra, in addition to the XRD characterization (Fig. 8a). The obtained FTIR spectra between 400 and 800 \({\mathrm{cm}}^{-1}\) are presented in Fig. 8b. The presence of hexagonal ferrite was verified through absorption bands centered at 593 cm, arising from the stretching vibrations of metal–oxygen (M–O) ions [1, 37]. The obtained FTIR spectra between 400 and 800 \({\mathrm{cm}}^{-1}\) are presented in Fig. 8b. The spectra clearly reveal two vibration modes known as \({\upnu }_{1}\) and \({\upnu }_{2}\), which correspond to oxygen-metal bond vibrations at the tetrahedral (\({\mathrm{M}}^{2+}-{\mathrm{O}}^{2-}\)) and octahedral (\({\mathrm{M}}^{3+}-{\mathrm{O}}^{2-}\)) sub-lattice sites, correspondingly. These two vibrational frequencies in the interval of 430–600 \({\mathrm{cm}}^{-1}\) are characteristic of M-type hexaferrite. The tetrahedral vibrational frequency band ranges from 586 to 592 \({\mathrm{cm}}^{-1}\), while the octahedral vibrational frequency band is noted between 438 and 443 \({\mathrm{cm}}^{-1}\) (Table 5). In the current samples, replacing the \({\mathrm{Ba}}^{2+}\) ion with the \({\mathrm{Pb}}^{2+}\) ion, which has a slighter ionic radius and atomic mass, causes variations in the vibrational frequency (\({\upnu }_{1}\)). This discrepancy is assigned to the bigger ionic radius and atomic mass of \({\mathrm{Pb}}^{2+}\) compared to Ba2+.
The bands detected around \(900\) \({\mathrm{cm}}^{-1}\) verify the occurrence of \(\mathrm{M}-\mathrm{O}\) stretching band in hexaferrites [38]. The bands observed in the sort of 1620-1680 cm-1ascribe to the stretching vibration of \(\mathrm{C}=\mathrm{C}\) and \(\mathrm{C}=\mathrm{O}\), respectively [39]. The peak observed near 2354 \({\mathrm{cm}}^{-1}\) is attributed to the presence of \({\mathrm{CO}}_{2}\) gas in the atmosphere during the FT-IR analysis [8]. The very weak absorption bands observed in the region of \(2975\) \({\mathrm{cm}}^{-1}\) are assigned to the \(\mathrm{O}-\mathrm{H}\) stretching band of \({\mathrm{H}}_{2}\mathrm{O}\), indicating the presence of water molecules [40]. The peaks seen at \(3438\) \({\mathrm{cm}}^{-1}\) show the presence of surface hydrogen groups (\(\mathrm{O}-\mathrm{H}\)) and ascribe to the stretching and bending vibrations of these groups. These hydrogen groups are believed to be acquired from the surrounding environment [38, 41].
PL
The photoluminescence (PL) emission spectra of the samples, as shown in Figure 9, were analyzed at room temperature (27 ℃) with an excitation wavelength of 300 nm. These spectra offer valuable insights into the absorption and emission mechanisms of the materials, facilitating the determination of bandgaps as detailed in Table 6 using the formula, E= hc/λ, where E represents the bandgap energy, c is the speed of light, h denotes Planck’s constant, and \(\uplambda\) represents the wavelength [42]. Pb doping introduces additional energy levels into the band structure. When these levels closely align with existing energy states, they may not significantly alter the photoluminescence spectra. The electronic transitions responsible for photoluminescence remain largely unaffected. Furthermore, Pb ions can create defect states or act as recombination centers. If these states exhibit similar energies across all samples, the photoluminescence behavior remains consistent. Consequently, the defect energy levels may not exhibit significant variation with increasing Pb concentration [43]. The 335-nm peak corresponds to long-wave ultraviolet (UV) light, often referred to as blacklight or near ultraviolet (N-UV) because of its resistance to ozone layer absorption. The absence of additional peaks suggests the lack of defect level emission (DLE), commonly associated with oxygen vacancies or surface defects within the crystal structure [44]. Defects and impurities within the oxygen lattices of M-type ferrites contribute to the photoluminescence properties of these hexagonal ferrites. Lower photoluminescence levels may indicate a lesser presence of oxygen defect structures in M-type ferrites [45]. Additionally, the Pb-doped samples exhibited higher intensity compared to the pure Ba-Sr sample (n0), potentially indicating an enhancement in electron–hole recombination [46]. This could further imply the absence of additional energy levels between the valence and conduction bands, which might be influenced by intrinsic defects [47].
UV–Vis
In this study, the UV–visible absorption spectrum of the materials was analyzed, as shown in Fig. 10a. The spectra were recorded within the wavelength range of \(300\mathrm{ to }600\mathrm{ nm}\). The determination of the optical energy band gaps (Eg) was conducted using Tauc plots. These plots involve plotting the square of the product of the absorption coefficient (α) and photon energy(Hu) against the photon energy graph. It was assumed that the direct allowed transition is the predominant mechanism [48]. By extending the tangent to the axis standing for (α h v)2, the band gap energy (Eg) was obtained. The Tauc plots and correspondingEg values for the Pb-substituted Ba, Sr-hexaferrite nanoparticles samples are presented in Fig. 10b. The Tauc relation between (α) and (hv) is used to calculate the energy band gap (Eg) [49]:
where (h) is Planck’s constant, \((\nu )\) is the incident photon frequency, and (A) is a characteristic parameter of the transition, which depends on the value of (n). The value of (n) can be 1/2, 2, 3/2, or 3 for allowed direct, allowed indirect, forbidden direct, and forbidden indirect transitions, respectively [50]. Another method can be used to calculate \({\mathrm{E}}_{\mathrm{g}}\) where Jani et al. [51] used the Kubelka–Munk function to determine it.
a Absorbance versus wavelength, (b) (α h u)2 versus energy (hu) plots for Pb-substituted Ba/Sr hexaferrite nanoparticles. Linear regression analysis was performed to figure out the band gap energy (Eg) by extending the regression line to the (hu) axis, and (c) alteration of crystallite size D (nm) and band gap Eg (eV) with substituent concentration
In Fig. 10b, the band gap energy decreases from 3.11 to 2.95 eV with an increase in Pb ion concentration. This decrease is due to Pb doping, which enhances the material’s conductivity by reducing the gap between the conduction and valence bands [39]. Additionally, the decrease in optical band gap (Eg) values can be attributed to the increase in crystallite size (Fig. 10c), as observed from TEM analysis. Previous studies have also shown an inverse relationship between crystallite size and band gap energy, which is attributed to quantum confinement effects [52]. The optical band gap diminishes due to heightened energy levels and orbital overlap caused by the abundant atoms in larger grains. This results in broader bandwidth and lower band gap energy [39]. Materials exhibiting these characteristics hold promise for applications in light-emitting diodes, solar cell absorbing layers, and magnetic recording media.
There is limited literature on the optical band gap study for Pb-substituted Ba/Sr hexaferrite nanoparticles. However, for the closely related hexaferrite \({\mathrm{BaFe}}_{12}{\mathrm{O}}_{19}\) and \({\mathrm{SrFe}}_{12}{\mathrm{O}}_{19}\), reported band gap values to range from \(3.18\) to \(3.92\mathrm{ eV}\) and \(1.80\) to \(2.10\mathrm{ eV}\), respectively [48]. This is satisfactory with the results obtained in this study.
VSM
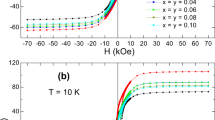
In the conducted study, the magnetization loops of the prepared hexaferrites were analyzed, unveiling strong ferromagnetic behavior at 27 ℃ (refer to Fig. 11). The remnant magnetizations \(({\mathrm{M}}_{\mathrm{r}})\) and saturation magnetizations \(({\mathrm{M}}_{\mathrm{s}})\) consistently decreased from 27.83 to 17.97 emu/g and 56.33 to 38.83 emu/g, respectively. Pb2+ ions likely occupy Fe3+ ion sites, contributing to the decrease in (Ms) attributed to the weakened superexchange interaction between Fe3+ and O2− ions [32]. With an increase in nonmagnetic Pb dopant, the superexchange interaction between Fe3+ ions in two sub-lattices of the octahedral holes results in opposite spins [24]. This can lead to the formation of vacancies, causing a spin-canting effect and contributing to a decrease in (Ms) [53]. The variation in magnetic saturation (Ms) as a function of lead (Pb) dopant content could be attributed to the presence of α- Fe2O3 in all the synthesized samples. Notably, the x = 0.00 sample exhibits the highest Ms value, likely due to the minimal α- Fe2O3 content in this composition. As the Pb content increases, the concentration of α- Fe2O3 also rises, potentially contributing to the reduction in Ms alongside changes in spin site occupancy. Also, this behavior can be explained by the magnetic nature of hematite and barium hexaferrite. The hematite and the barium hexaferrite are, respectively, antiferromagnetic and ferromagnetic. The antiferromagnetic hematite has a net magnetization nearly zero when the external magnetic field is absent. When it is combined with a ferromagnetic hexaferrite, it does not contribute to the net magnetic moment. Instead, it dilutes the magnetization, causing a reduction in the total saturation magnetization of the formed material. Additionally, it is worth noting that coercivity tends to decrease with increasing crystallite size, while the magnetocrystalline anisotropy constant shows a positive correlation with Pb dopant concentration [30]. The change in \(({\mathrm{M}}_{\mathrm{s}})\) also affects the remnant magnetization \(({\mathrm{M}}_{\mathrm{r}})\) of all samples. The squareness ratio (SQR) is an important parameter in various applications, representing the \({\mathrm{M}}_{\mathrm{r}}/{\mathrm{M}}_{\mathrm{s}}\) ratio, and indicating the squareness of the magnetic hysteresis loop. The decreasing SQR values from 0.49 to 0.46, all below 0.5, suggest the presence of a single magnetic domain. As per the Stoner-Wohlfarth (S-W) theory, the single-domain nature with uniaxial anisotropy is established by a theoretical limit of 0.50 [54]. Furthermore, the magneton number \(({\upeta }_{\mathrm{B}})\), calculated as \({\upeta }_{\mathrm{B}}=(\) Molecular weight \(\times\) \({\mathrm{M}}_{\mathrm{s}})/\) 5585, was also determined [48]. The initial decrease in \(({\upeta }_{\mathrm{B}})\) values can be attributed to the weakening of superexchange interactions between distinct sites [55]. Further details of \(({\mathrm{M}}_{\mathrm{r}})\), \(({\mathrm{M}}_{\mathrm{s}})\), SQR, and \(({\upeta }_{\mathrm{B}})\) can be found in Table 7.
The coercivity (HC) of the Pb-substituted Ba/Sr hexaferrite nanoparticles, as derived from the (M-H) curves, decreased from 4388.3 to 3289.3 G. This reduction can be attributed to factors such as particle size and the decrease in anisotropic energy, leading to a decline in magnetocrystalline anisotropy [32]. The addition of Pb, as shown in Table 3, resulted in an increase in the ‘c-axis’ length and a decrease in the ‘a-axis’ length, leading to the formation of hexagonal plates. Changes in grain morphology and boundary quantity due to varying Pb2+ ion concentrations also influence magnetic coercivity through grain size alterations. Lead substitution shifted the anisotropy from the ‘c-axis’ to the ‘c-plane’, decreasing coercivity by impeding domain wall motion and increasing crystallite size [24]. SEM micrographs demonstrated that particle size increases with higher Pb concentration, contributing to reduced coercivity. Moreover, the relationship between coercivity and crystallite size is inversely proportional (Fig. 12) [24]. The decrease in \(({\mathrm{H}}_{\mathrm{c}})\) may also be related to the diamagnetic properties of Pb in the hexaferrite material, influencing both \(({\mathrm{M}}_{\mathrm{s}})\) and coercivity through magnetizing and demagnetizing interactions. Changes in magnetization could be attributed to demagnetizing interactions from dipolar interactions resulting from Pb exchange in the hexaferrite materials [23]. Additionally, the reduction in coercivity might be associated with a decrease in the strength of intergranular pores due to grain growth [56]. Since barium hexaferrite is ferromagnetic, then it has a high coercivity because of its strong anisotropy. The presence of the antiferromagnetic hematite can disrupt the uniform magnetic interactions between the barium hexaferrite grains. Therefore, the coercivity will be reduced due to this disruption. The average magnetic domain size \(({\mathrm{d}}_{\mathrm{s}})\) can be calculated using the following equation [57]:
where (\(\frac{\Delta \mathrm{M}}{\Delta \mathrm{H}}\)) is the slope of the M-H line at \(\mathrm{H}=0\mathrm{ G}\), \(\mathrm{T}=300\mathrm{ K}\), (\({\mathrm{k}}_{\mathrm{B}}\)) is Boltzmann constant, \({\uprho }_{\mathrm{x}}\) represents the density, and \({\mathrm{M}}_{\mathrm{s}}\) is the saturation magnetization (Table 7) [57]. The determined magnetic domain sizes of 37.69, 41.95, 42.09, 42.75, and 43.32 nm, corresponding to the respective crystallite sizes of 42.81, 45.41, 53.00, 57.07, and 60.96 nm (Fig. 13), suggesting the presence of a single magnetic domain in the samples, as the crystallite sizes closely align with the magnetic domain sizes. This observation is further supported by the SQR values, all of which are near 0.5 [54].
During the formation of Ba-hexaferrite, the Fe3+ ions occupying the ‘4f2’ octahedral site exhibit a spin opposite to that of the Fe3+ ions in the ‘2a’ and ‘12k’ octahedral sites. The Fe3+ ions in the ‘4f2’ sites significantly contribute to the anisotropy constant ‘K1’ [24]. The introduction of dopant ions has caused modifications in the lattice parameters, elongating the regular structure along the ‘c-axis’ and compressing it along the ‘a-axis’, aligning with conclusions drawn by Teh and Jefferson [58]. The increase in ‘c’ with ‘x’ influences the spacing between magnet ions, affecting the exchange interaction and subsequently the magnetic properties. The ‘c-axis’ plays a pivotal role in the magnetic properties and anisotropy of Ba-hexaferrite, crucial for coercivity. The substitution of Ba2+ ions has a noticeable impact on the lattice parameters, specifically on the ‘2b’, ‘12k’, and ‘4f2’ sites, followed by ‘2a’. When Ba2+ ions are replaced by Pb2+ ions, energy perturbations, and lattice distortions can occur due to the differences in their ionic radii [24]. This can lead to variations in anisotropy energy and magnetic properties, particularly in coercivity, which decreases with Pb doping. Materials with high coercivity fields typically exhibit improved thermal stability compared to those with lower coercivity levels. The high coercivity of such materials makes them suitable for applications in recording media and permanent magnets requiring a highly coercive medium [24, 47].
Thongmee et al. [59] suggest that large-sized dopant ions tend to occupy octahedral sites, while small-sized ions prefer tetrahedral sites. When the saturation magnetization '\({\mathrm{M}}_{\mathrm{s}}\)' increases, the dopants are likely to be in spin-down sites. Conversely, when the saturation magnetization decreases, the dopants are situated in spin-up sites. However, in cases where '\({\mathrm{M}}_{\mathrm{s}}\)' remains constant, indicating no change, it is inferred that the dopants are evenly distributed among possible sites [59]. Ongoing research is being conducted to gain a deeper understanding of these phenomena, with findings to be shared at the right time. Moreover, in Ba0.5Sr0.5Fe12 O19, the cation distribution across octahedral and tetrahedral sites affects the magnetic properties. The overall saturation magnetization may remain unchanged in the case where the substituted Pb2+ ions prefer sites that do not significantly change the net magnetic moment of the unit cell. This can occur if Pb2+ substitutes Ba2+ in the crystal lattice without significantly affecting the Fe sublattice responsible for the material’s magnetization [24].
The saturation magnetization (\({\mathrm{M}}_{\mathrm{s}}\)), and effective crystalline anisotropy parameter (\({\mathrm{K}}_{\mathrm{eff}}\)) for each specimen were figured out using the law of approach to saturation (LAS) [48]:
where A stands for the inhomogeneity parameter, B is a factor associated with magnetocrystalline anisotropy, H denotes the applied magnetic field, and \({\upchi }_{\mathrm{p}}\) refers to the high field differential susceptibility. Corresponding to the law of approach to saturation, the M − H loops of the specimens have been fitted, and the results are compatible with the experimental data, as depicted in Fig. 14. The products consistently demonstrate SQR values within the range of 0.46–0.49, suggesting that the nano-sized hexaferrite particles possess a uniaxial anisotropy. In the case of uniaxial magnetic nanoparticles, the relationship between the effective magnetic anisotropy constant (\({\mathrm{K}}_{\mathrm{eff}}\)), saturation magnetization (\({\mathrm{M}}_{\mathrm{s}}\)), and the parameter B is expressed as follows [47, 48]:
in this study, \({\mathrm{K}}_{\mathrm{eff}}\) values were found to be in the same range of \({10}^{5}\) \(\mathrm{Ergs}/\mathrm{g}\) (Table 8). The anisotropic constant exhibits a decline, decreasing from 2.37 × 105 to 1.03 × 105 erg/g with increasing dopant composition. This reduction arises due to the direct correlation between the anisotropic constant and coercivity (Hc). As coercivity diminishes, it indicates that a smaller magnetizing field suffices to demagnetize the sample, resulting in reduced energy requirements to overcome the anisotropic barrier [26]. The noted attitude in \({\mathrm{K}}_{\mathrm{eff}}\) with x is ascribed to the migration of Fe3+ cations between octahedral and tetrahedral sites [55]. As shown in Fig. 15 [25, 32], a decrease in magnetocrystalline anisotropy can be observed in \({\mathrm{H}}_{\mathrm{c}}\) at x = 0. This is attributed to the difference in lattice size between Pb2+ and Ba2+ ions, as shown in Table 3, which directly impacts the magnetocrystalline anisotropy [32]. The primary source of uniaxial magneto-crystalline anisotropy in hexagonal ferrites is the presence of Fe3+ ions on 4f2 and 2b sites [54]. Dopant ions occupying the 4f2 sites lead to a reduction in super-exchange interactions between various sites, consequently lowering the magnetocrystalline anisotropy field (Ha) [54], which in turn leads to a decrease in coercivity [28]. \({\mathrm{H}}_{\mathrm{a}}\) can be calculated as follows:
with the rise in Pb concentration, there is a corresponding decrease in \({\mathrm{H}}_{\mathrm{a}}\) (Table 8). Additionally, the larger values of \({\mathrm{H}}_{\mathrm{a}}\) indicate that all the magnetic nanoparticles (MNPs) exhibit magnetic hardness [47]. From this, it can be inferred that the increased Pb content leads to a decrease in the hard nature of the hexaferrite. Therefore, by controlling the Barium/Lead content, we can tailor the magnetic saturation and coercivity values of the hexaferrite sample to meet the specific needs and applications.
Conclusion
The investigation involved a comprehensive analysis of the physical, optical, and magnetic properties of Pb-substituted Ba and Sr-hexaferrite nanoparticles. Through meticulous Rietveld refinement analysis, all samples exhibited a pure hexagonal structure, confirmed by distinct absorption bands in FTIR spectra, validating successful hexagonal ferrite formation. High-resolution TEM images and SAED configurations aligned with XRD results, corroborating the nanoparticle structure.
Energy band gap calculations revealed the superior direct optical properties of the synthesized compounds, indicating potential utilization in solar light-based devices. Strong emissions at 335 nm in PL spectra pointed to promising applications in optoelectronics and photonics. SEM images depicted a hexagonal basal plane, while EDX spectroscopy affirmed effective hexaferrite production. XPS results confirmed the complete formation of the hexagonal crystal structure.
Regarding magnetic properties, coercivity Hc decreased with Pb substitution due to significant demagnetizing-like interactions. This decrease can be attributed to particle size increases and anisotropy energy reductions resulting from Pb doping. The declining coercivity suggests potential uses in high-density recording media.
These findings provide valuable insights into the structural, optical, and magnetic attributes of Pb-substituted Ba and Sr-hexaferrite nanoparticles, underscoring the importance of understanding the composition-product relationship in magnetic materials. The doped samples exhibit moderate saturation magnetization values around 40 emu/g, making them promising candidates for biomedical imaging applications, such as magnetic resonance imaging (MRI).
Data availability
No datasets were generated or analysed during the current study.
References
Chandel M, Singh VP, Jasrotia R (2020) Fabrication of Ni2+ and Dy3+ substituted Y-Type nanohexaferrites: a study of structural and magnetic properties. Phys B Condens Matter 595:412378. https://doi.org/10.1016/j.physb.2020.412378
Jasrotia R, Prakash J, Himanshi, (2023) Advancements in doping strategies for enhancing applications of M-type hexaferrites: a comprehensive review. Prog Solid State Chem 72:100427. https://doi.org/10.1016/j.progsolidstchem.2023.100427
Himanshi JR, Prakash J (2023) Synthesis, characterization, and applications of doped barium hexaferrites: a review. Phys B Condens Matter 667:415202. https://doi.org/10.1016/j.physb.2023.415202
Jasrotia R, Pratap Singh V, Kumar R (2019) Analysis of Cd2+ and In3+ ions doping on microstructure, optical, magnetic and mossbauer spectral properties of sol-gel synthesized BaM hexagonal ferrit based nanomaterials. Results Phys 12:1933–1941. https://doi.org/10.1016/j.rinp.2019.01.088
Jasrotia R, Singh VP, Kumar R, Singh M (2020) Raman spectra of sol-gel auto-combustion synthesized Mg-Ag-Mn and Ba-Nd-Cd-In ferrite based nanomaterials. Ceram Int 46:618–621. https://doi.org/10.1016/j.ceramint.2019.09.012
Jasrotia R, Singh VP, Sharma B, Verma A, Puri P, Sharma R, Singh M (2020) Sol-gel synthesized Ba-Nd-Cd-In nanohexaferrites for high frequency and microwave devices applications. J Alloys Compd 830:154687. https://doi.org/10.1016/j.jallcom.2020.154687
Braun PB (1952) Crystal structure of BaFe18O27. Nature 170:708–708. https://doi.org/10.1038/170708a0
Sözeri H, Durmuş Z, Baykal A, Uysal E (2012) Preparation of high quality, single domain BaFe12O19 particles by the citrate sol–gel combustion route with an initial Fe/Ba molar ratio of 4. Mater Sci Eng B 177:949–955. https://doi.org/10.1016/j.mseb.2012.04.023
Rathenau GW, Smit J, Stuyts AL (1952) Ferromagnetic properties of hexagonal iron-oxide compounds with and without a preferred orientation. Zeitschrift für Phys A Hadron Nucl 133:250–260. https://doi.org/10.1007/BF01948700
Sharma R, Agarwala RC, Agarwala V (2008) Development of radar absorbing nano crystals by microwave irradiation. Mater Lett 62:2233–2236. https://doi.org/10.1016/j.matlet.2007.11.076
Speliotis DE (1989) High density recording on particulate and thin film rigid disks. IEEE Trans Magn 25:4048–4050. https://doi.org/10.1109/20.42518
Gerber R, Atkinson R, Šimša Z (1997) Magnetism and magneto-optics of hexaferrite layers. J Magn Magn Mater 175:79–89. https://doi.org/10.1016/S0304-8853(97)00151-0
Kang YM, Moon KS (2015) Magnetic properties of Ce–Mn substituted M-type Sr-hexaferrites. Ceram Int 41:12828–12834. https://doi.org/10.1016/j.ceramint.2015.06.119
Onreabroy W, Papato K, Rujijanagul G, Pengpat K, Tunkasiri T (2012) Study of strontium ferrites substituted by lanthanum on the structural and magnetic properties. Ceram Int 38:S415–S419. https://doi.org/10.1016/j.ceramint.2011.05.023
Iqbal MJ, Farooq S (2009) Extraordinary role of Ce–Ni elements on the electrical and magnetic properties of Sr–Ba M-type hexaferrites. Mater Res Bull 44:2050–2055. https://doi.org/10.1016/j.materresbull.2009.07.015
Ashiq MN, Iqbal MJ, Gul IH (2009) Structural, magnetic and dielectric properties of Zr–Cd substituted strontium hexaferrite (SrFe12O19) nanoparticles. J Alloys Compd 487:341–345. https://doi.org/10.1016/j.jallcom.2009.07.140
Litsardakis G, Manolakis I, Efthimiadis K (2007) Structural and magnetic properties of barium hexaferrites with Gd–Co substitution. J Alloys Compd 427:194–198. https://doi.org/10.1016/j.jallcom.2006.02.044
Bakış Y, Auwal IA, Ünal B, Baykal A (2016) Maxwell-Wagner relaxation in grain boundary of BaBixLaxYxFe12−3xO19 (0.0 ≤ x ≤ 0.33) hexaferrites. Compos Part B Eng 99:248–256. https://doi.org/10.1016/j.compositesb.2016.06.047
Hussain S, Shah NA, Maqsood A, Ali A, Naeem M, Ahmad Adil Syed W (2011) Characterization of Pb-doped Sr-Ferrites at room temperature. J Supercond Nov Magn 24:1245–1248. https://doi.org/10.1007/s10948-010-1115-z
Iqbal MJ, Farooq S (2010) Suitability of Sr0.5Ba0.5−xCexFe12−yNiyO19 co-precipitated nanomaterials for inductor applications. J Alloys Compd 493:595–600. https://doi.org/10.1016/j.jallcom.2009.12.163
Iqbal MJ, Farooq S (2009) Enhancement of electrical resistivity of Sr0.5Ba0.5Fe12O19 nanomaterials by doping with lanthanum and nickel. Mater Chem Phys 118:308–313. https://doi.org/10.1016/j.matchemphys.2009.07.056
Baykal A, Ünver İS, Topal U, Sözeri H (2017) Pb substituted Ba, Sr-hexaferrite nanoparticles as high quality microwave absorbers. Ceram Int 43:14023–14030. https://doi.org/10.1016/j.ceramint.2017.07.134
Guerrero AL, Mirabal-García M, Palomares-Sánchez SA, Martínez JR (2016) Effect of pb on the magnetic interactions of the M-type hexaferrites. J Magn Magn Mater 399:41–45. https://doi.org/10.1016/j.jmmm.2015.09.052
Haq A, Anis-ur-Rehman M (2012) Effect of Pb on structural and magnetic properties of Ba-hexaferrite. Phys B Condens Matter 407:822–826. https://doi.org/10.1016/j.physb.2011.11.038
Choudhary HK, Kumar R, Anupama AV, Sahoo B (2018) Effect of annealing temperature on the structural and magnetic properties of Ba-Pb-hexaferrite powders synthesized by sol-gel auto-combustion method. Ceram Int 44:8877–8889. https://doi.org/10.1016/j.ceramint.2018.02.074
Sharma A, Jasrotia R, Kumari N, Kumar S, Suman VA, Kumar Godara S, Ahmed J, Alshehri SM, Tamboli AM, Kalia S, Batoo KM, Kumar R (2022) Tailoring the structural and magnetic traits of copper modified BaFe12O19 nanostructured hexaferrites for recording media application. J Magn Magn Mater 564:170124. https://doi.org/10.1016/j.jmmm.2022.170124
Thakur N, Sharma I, Thakur P, Mahajan M, Bhardwaj S, Jasrotia R, Dahshan A, Elsaeedy HI, Kaur S, Anand G, Sharma P, Batoo KM, Kumar G (2024) Improvement in the structural, magnetic and electromagnetic behaviour of barium hexaferrites with yttrium doping for EMI shielding. J Alloys Compd 976:173042. https://doi.org/10.1016/j.jallcom.2023.173042
Sharma I, Sharma S, Thakur P, Bhardwaj S, Mahajan M, Kaur S, Anand G, Jasrotia R, Dahshan A, Elsaeedy HI, Sharma P, Kumar G (2024) Improved magneto-dielectric response and dielectric characteristics of rare earth doped Ba and Co based U-type hexaferrite. Mater Chem Phys 316:129016. https://doi.org/10.1016/j.matchemphys.2024.129016
Soman VV, Nanoti VM, Kulkarni DK (2013) Dielectric and magnetic properties of Mg–Ti substituted barium hexaferrite. Ceram Int 39:5713–5723. https://doi.org/10.1016/j.ceramint.2012.12.089
Ashima A, Sanghi S, Agarwal A, Reetu, Ahlawat N, Monica (2012) Structure refinement and dielectric relaxation of M-type Ba, Sr, Ba-Sr, and Ba-Pb hexaferrites. J Appl Phys 112:. https://doi.org/10.1063/1.4734002
Denton AR, Ashcroft NW (1991) Vegard’s law. Phys Rev A 43:3161–3164. https://doi.org/10.1103/PhysRevA.43.3161
Kumar P, Gaur A, Kotnala RK (2017) Magneto-electric response in Pb substituted M-type barium-hexaferrite. Ceram Int 43:1180–1185. https://doi.org/10.1016/j.ceramint.2016.10.060
Alange RC, Khirade PP, Birajdar SD, Humbe AV, Jadhav KM (2016) Structural, magnetic and dielectrical properties of Al–Cr Co-substituted M-type barium hexaferrite nanoparticles. J Mol Struct 1106:460–467. https://doi.org/10.1016/j.molstruc.2015.11.004
Kaur T, Kaur B, Bhat BH, Kumar S, Srivastava AK (2015) Effect of calcination temperature on microstructure, dielectric, magnetic and optical properties of Ba0.7La0.3Fe11.7Co0.3O19 hexaferrites. Phys B Condens Matter 456:206–212. https://doi.org/10.1016/j.physb.2014.09.003
Pawar RA, Patange SM, Dighe PM, Meena SS, Al-Sehemi AG, Pannipara M, Jadhav SS (2023) Influence of cerium ions on structure-dependent magnetic properties of Ba–Sr M-type hexaferrite nanocrystals. J Rare Earths. https://doi.org/10.1016/j.jre.2023.01.020
Rehman M, Rehman W, Waseem M, Hussain S, Haq S, Rehman MA (2019) Adsorption mechanism of Pb2+ ions by Fe3O4, SnO2, and TiO2 nanoparticles. Environ Sci Pollut Res 26:19968–19981. https://doi.org/10.1007/s11356-019-05276-x
Singha K, Jasrotia R, Singh VP, Chandel M, Kumar R, Kalia S (2021) A study of magnetic properties of Y-Ni–Mn substituted Co2Z-type nanohexaferrites via vibrating sample magnetometry. J Sol-Gel Sci Technol 97:373–381. https://doi.org/10.1007/s10971-020-05412-x
El-Sayed SM, Meaz TM, Amer MA, El Shersaby HA (2013) Magnetic behavior and dielectric properties of aluminum substituted M-type barium hexaferrite. Phys B Condens Matter 426:137–143. https://doi.org/10.1016/j.physb.2013.06.026
Anjum S, Hameed S, Awan MS, Amed E, Sattar A (2017) Effect of strontium doped M-Type bariam hexa-ferrites on structural, magnetic and optical properties. Optik (Stuttg) 131:977–985. https://doi.org/10.1016/j.ijleo.2016.11.205
Anand S, Pauline S, Maria Vinosel V, Asisi Janifer M (2019) Structural Rietveld refinement and vibrational study of M-type BaFe12O19 nanoparticles. Mater Today Proc 8:476–483. https://doi.org/10.1016/j.matpr.2019.02.141
SM Ramay S Atiq M Saleem A Mahmood SA Siddiqi S Naseem AL-Zeghayer N, Shahabuddin M, 2014 Enhanced magnetization of Sol-Gel synthesized Pb-Doped strontium hexaferrites nanocrystallites at low temperature J Nanomater 2014 1 7 https://doi.org/10.1155/2014/452468
Raza Q, Bibi I, Majid F, Kamal S, Ata S, Ghafoor A, Arshad MI, Al-Mijalli SH, Nazir A, Iqbal M (2023) Solar light-based photocatalytic removal of CV and RhB dyes using Bi and Al doped SrFe12O19 NPs and antibacterial properties. J Ind Eng Chem 118:469–482. https://doi.org/10.1016/j.jiec.2022.11.030
Wang X, Wei S, Wang B, Cheng X, Wang Y, Liang Y (2023) Doping sites, magnetic, and microwave absorption properties of barium ferrites with multiple magnetic resonances. J Mater Sci: Mater Electron 34:310. https://doi.org/10.1007/s10854-022-09738-w
Jamshaid M, Rehman A, Kumar OP, Iqbal S, Nazir MA, Anum A, Khan HM (2021) Design of dielectric and photocatalytic properties of Dy–Ni substituted Ca0.5Pb0.5−xFe12−yO19 M-type hexaferrites. J Mater Sci Mater Electron 32:16255–16268. https://doi.org/10.1007/s10854-021-06173-1
Gulbadan S, Khan MA, Ashraf GA, Mahmood K, Shahid M, Irfan M, Ahmad A (2023) Insight of structural, dielectric and spectroscopic characteristics of Ba0.6Sr0.4-xYbxFe12-yCoyO19 M-type hexaferrite. Ceram Int 49:6487–6499. https://doi.org/10.1016/j.ceramint.2022.10.162
Mahapatro J, Meena SS, Agrawal S (2024) Effect of Eu–Er substitution on structural, optical, dielectric, and electrical properties of Ba0.5Sr0.5EuxErxFe12–2xO19 hexaferrite. J Rare Earths. https://doi.org/10.1016/j.jre.2024.01.010
Almessiere MA, Slimani Y, Baykal A (2018) Structural and magnetic properties of Ce-doped strontium hexaferrite. Ceram Int 44:9000–9008. https://doi.org/10.1016/j.ceramint.2018.02.101
Baykal A, Yokuş S, Güner S, Güngünes H, Sözeri H, Amir M (2017) Magneto-optical properties and Mössbauer investigation of BaxSryPbzFe12O19 hexaferrites. Ceram Int 43:3475–3482. https://doi.org/10.1016/j.ceramint.2016.10.013
Zielińska-Jurek A, Bielan Z, Dudziak S, Wolak I, Sobczak Z, Klimczuk T, Nowaczyk G, Hupka J (2017) Design and application of magnetic photocatalysts for water treatment. The effect of particle charge on surface functionality. Catalysts 7:360. https://doi.org/10.3390/catal7120360
Anjum S, Seher A, Mustafa Z (2019) Effect of La3+ ions substituted M-type barium hexa-ferrite on magnetic, optical, and dielectric properties. Appl Phys A 125:664. https://doi.org/10.1007/s00339-019-2937-6
Jani P, Desai H, Madhukar S, Tanna A (2021) Investigations of calcium ferrite nanoparticles synthesized by sol-gel auto combustion and solution mixture methods. Mater Res Innov 26:189–195. https://doi.org/10.1080/14328917.2021.1932318
Rema Devi BS, Raveendran R, Vaidyan AV (2007) Synthesis and characterization of Mn2+-doped ZnS nanoparticles. Pramana 68:679–687. https://doi.org/10.1007/s12043-007-0068-7
Shalini MG, Sahoo SC (2016) Magnetic studies of cobalt doped barium hexaferrite nanoparticles prepared by modified sol-gel method. AIP Conf Proc 1728:020445. https://doi.org/10.1063/1.4946496
Güner S, Almessiere MA, Slimani Y, Baykal A, Ercan I (2020) Microstructure, magnetic and optical properties of Nb3+ and Y3+ ions co-substituted Sr hexaferrites. Ceram Int 46:4610–4618. https://doi.org/10.1016/j.ceramint.2019.10.191
Atif M, Hanif Alvi M, Ullah S, Ur Rehman A, Nadeem M, Khalid W, Ali Z, Guo H (2020) Impact of strontium substitution on the structural, magnetic, dielectric and ferroelectric properties of Ba1-xSrxFe11Cr1O19 (x=0.0–0.8) hexaferrites. J Magn Magn Mater 500:166414. https://doi.org/10.1016/j.jmmm.2020.166414
Mahmood SH, Ghanem AA, Bsoul I, Awadallah A, Maswadeh Y (2017) Structural and magnetic properties of BaFe12−2 xCux MnxO19 hexaferrites. Mater Res Express 4:036105. https://doi.org/10.1088/2053-1591/aa646c
Ozkaya T, Toprak MS, Baykal A, Kavas H, Köseoğlu Y, Aktaş B (2009) Synthesis of Fe3O4 nanoparticles at 100 °C and its magnetic characterization. J Alloys Compd 472:18–23. https://doi.org/10.1016/j.jallcom.2008.04.101
Teh GB, Jefferson DA (2002) High-resolution transmission electron microscopy studies of Sol–Gel-derived cobalt-substituted barium ferrite. J Solid State Chem 167:254–257. https://doi.org/10.1006/jssc.2002.9659
Thongmee S, Winotai P, Tang IM (2003) Boron site preference in B-doped barium hexaferrite. ScienceAsia 29:51–55. https://doi.org/10.2306/scienceasia1513-1874.2003.29.051
Acknowledgements
The authors express their appreciation to the nanomaterial labs at Beirut Arab University in Lebanon and Alexandria University in Egypt.
Author information
Authors and Affiliations
Contributions
M. Farhat: Writing original draft, Data Curation, Software, Investigation, Formal analysis
R. Yassine: Contributing in writing the original draft
R. Awad: Review and Supervision
Z. Bitar: Co-supervision, Review and Editing, Conceptualization, Validation
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farhat, M.A., Yassine, R., Awad, R. et al. Investigation of Pb’s impact on the physical, optical, and magnetic characteristics of \({\mathbf{B}\mathbf{a}}_{0.5-\mathbf{x}}{\mathbf{S}\mathbf{r}}_{0.5}{\mathbf{P}\mathbf{b}}_{\mathbf{x}}{\mathbf{F}\mathbf{e}}_{12}{\mathbf{O}}_{19}\) hexaferrite. J Nanopart Res 26, 219 (2024). https://doi.org/10.1007/s11051-024-06130-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-06130-z