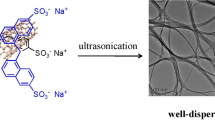
Abstract
In this article, an easy, effective, π-π conjugation non-covalent surface functionalization of multi-walled carbon nanotubes (MWCNTs) was achieved by using ball grinding technology between pristine MWCNTs and esterified styrene-maleic anhydride ester (ESMA) copolymer, resulting in the formation of modified E-MWCNTs dispersion with excellent dispersibility and long storage stability in N-methylpyrrolidone (NMP). The analysis of transmittance spectroscopy, absorption spectroscopy, sedimentation photo, and transmittance electron microscopy (TEM) confirms that the modified E-MWCNT dispersion has a homogeneous dispersion in NMP. Results obtained from thermogravimetry analysis (TGA) and Raman spectroscopy reveal that ESMA copolymer is successfully coated on pristine MWCNTs surface. Compared with the bad dispersibility of MWCNTs in NMP, the physically modified E-MWCNTs-3 dispersion mixed with 0.12wt% ESMA copolymer under the condition of total 6 h ball grinding time using paint shaker can be easily well-dispersed and has good storage stability, and no sedimentation is observed more than 3 months. Furthermore, the method is simple and economical to operate in industry.
Graphical Abstract
An easy, effective, π-π conjugation non-covalent surface functionalization of multi-walled carbon nanotubes (MWCNTs) was achieved by using ball grinding technology between pristine MWCNTs and esterified styrene-maleic anhydride ester (ESMA) copolymer, resulting in the formation of modified E-MWCNTs dispersion with excellent dispersibility and long storage stability reported firstly in N-methylpyrrolidone.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-walled carbon nanotubes (MWCNTs) have attracted great interest among researchers owing to their excellent electronic [1, 2], mechanical [3], energy conversion [4], biomedical applications [5], and more. Compared with conductive spherical particles, the high aspect ratio and high conductivity of MWCNTs show a wonderful advantage in constructing a conductive network in the lithium-ion battery positive electrode at a much lower concentration [6]. To obtain these extraordinary properties, commercial MWCNTs often need to be dispersed into a medium and should be dispersed individually. However, homogeneous dispersion with good dispersibility and long storage stability still presents a challenge, as the tube–tube contact makes them susceptible to aggregation, driven by strong van der Waals forces interaction energy of ca.500 eV/μm2.
In recent years, chemical and physical modification strategies have been commonly used to obtain abundant functionalized CNTs [7,8,9,10,11,12,13,14,15,16,17,18,19]. The chemical modification strategy is to introduce functional groups on the surface of CNTs, and then to graft soluble hydrophilic or hydrophobic polymer chains [7, 8]. On the other hand, researchers have vigorously adopted steric hindrance or π–π stacking interaction strategies to disperse carbon nanotubes (CNTs) into the aqueous or solvent system, generally with the assistance of surfactants, biomolecules, aromatic compounds, and polymers [9,10,11,12,13,14,15,16,17,18,19]. Since this modification strategy avoids hazardous chemical reactions under strong acidic conditions while preserving the conjugated structure and intrinsic properties of CNTs, it is considered to be one of the more promising methods for the dispersion of CNTs in various applications. The physical dispersion strategy except two-step method [20], however, presents a challenge in terms of the homogeneous and long-term stability of CNT dispersion which is generally inferior to that achieved by the chemical modification method [21, 22].
In this paper, we report an easy, effective one-step modification of MWCNTs by using π-π conjugation non-covalent strategy between pristine MWCNTs and the benzene type backbone of esterified SMA copolymer, as shown in Fig. 1, to prepare modified E-MWCNTs dispersion with good dispersibility and long storage stability in NMP. Thus, the ESMA copolymer was easily grafted onto the surface of MWCNTs with a single step.
This effective surface modification strategy of pristine MWCNTs can be used to prepare well-dispersed conductive dispersion for a similar application in the positive electrode of lithium-ion batteries [6, 23,24,25,26,27,28,29]. More importantly, this physical modification above is easy and economical due to easy operation and cheap raw materials in industry. We also reckon that this experimental work will promote the application of MWCNTs in high-performance lithium-ion batteries.
Experimental
Materials
Pristine MWCNTs product (black powder: MWCNTs, length: 20–50um, BET: 230–300 m2/g, MWCNTs outer mean diameter: 5–10 nm) was obtained from Hefei Aigo Additives Technology Co. Ltd. Esterified styrene-maleic anhydride ester (ESMA) copolymer bought from POLYSCOPE company was used without purification. N-methylpyrrolidone (NMP) bought from the market was used without purification.
Modification of MWCNTs with ESMA copolymer
First of all, NMP ((98-n) g) and ESMA copolymer (n = 0.03, 0.06, and 0.12 g) were added to the glass bottle and stirred with a stirrer at 300 rpm for 15 min. After that, pristine MWCNTs (2.0 g) and zirconium bead (sizer: 1 mm) were added to the pre-prepared mixture and dispersed for 6 h in a paint shaker to afford uniform E-MWCNT dispersion. The corresponding recipe of modified E-MWCNTs is shown in Table 1. Then, the above products were dried overnight at 80 °C in a vacuum. The procedure of the preparation of modified E-MWCNT dispersion is schematically displayed in Fig. 1.
Characterization
The transmittance and absorption of the MWCNTs dispersion were systematically determined with a quartz cell using a UV2600 ultraviolet spectrophotometer (Shimadzu Instruments) scanned in the range of 200–800 nm. The particle size distribution of the MWCNTs dispersion was characterized by a 90Plus PALS zeta potential (Brookhaven Instruments). Thermogravimetry analysis (TGA) of pristine MWCNTs and modified E-MWCNTs was performed under nitrogen on a 209F1 thermogravimetric analyzer (Netzsch Instruments) with a temperature range of 25–600℃ at a heating rate of 10℃/min. The nanostructures of the dried E-MWCNTs were studied using the Raman spectroscopic (Raman, Kaiser Holo-Lab) technique. The morphology of the dilute MWCNTs dispersion was investigated by transmittance electron microscopy (TEM, FEI F20). For TEM, the dilute dispersion (in NMP) was placed onto a carbon-coated copper grid.
Results and discussion
Transmittance spectroscopy
For accurately studying the dispersion effect of carbon materials, it is necessary to compare their optical transmittance [30, 31]. Figure 2a shows the transmittance spectroscopy of ESMA copolymer and diluted pristine MWCNTs and modified E-MWCNT dispersion scanned in the range of 200–800 nm. We extracted the optical transmittance data at 550 nm from ESMA and pristine MWCNTs and modified E-MWCNTs dispersion in Fig. 2a. For ESMA copolymer, its optical transmittance is 99.8% at 550 nm, showing that it has little effect on the transmittance of carbon nanotubes. Compared to 90.2% at 550 nm of pristine MWCNTs dispersion, the optical transmittance of modified E-MWCNTs dispersion is lower, indicating that the pristine MWCNTs were dispersed poorly in NMP and the serious agglomeration was formed due to its high surface area, strong van der Waals forces, and inherent hydrophobicity. The lower optical transmittance confirms that the pristine MWCNTs were dispersed significantly by ESMA copolymer.
Transmittance spectra of ESMA copolymer and dilute dispersion of pristine MWCNTs and modified E-MWCNTs with incident light wavelength ranging from 200 to 780 nm. The data in the figure indicates the optical transmittance with incident 550 nm visible light (a); effect of ESMA content on the transmittance (wavelength, 550 nm) of modified E-MWCNTs dispersion (b); pristine MWCNTs dispersion prepared using similar technology in “Modification of MWCNTs with ESMA copolymer” has the same solid content with modified E-MWCNTs dispersion
Figure 2b shows the effect of ESMA copolymer content on the transmittance of modified E-MWCNTs dispersion at 550 nm. Compared with the pristine MWCNTs, the modified E-MWCNTs showed good dispersibility in NMP, especially the E-MWCNTs-3 (32.1%). It was indicated that the dispersing effect of MWCNTs can be improved after being modified with different ESMA copolymer contents in NMP. Because of the new π-π conjugation non-covalent modification between pristine MWCNTs and ESMA copolymer, the tight agglomeration was controlled effectively. With the increasing ESMA copolymer content, the optical transmittance of E-MWCNTs dispersion decreased gradually. This may be because when the ESMA copolymer content is insufficient, the uncoated MWCNTs will be likely to agglomerate together.
Absorption spectroscopy
To take advantage of the physical properties of MWCNTs, the entangled bundles of the commercialized MWCNTs need to be modified before being used as functional additives in the various materials [2, 17, 19]. It is an accurate test to prove the modification effect of CNTs by comparing the absorbance intensity of carbon nanotubes remaining after a centrifugation [32]. Therefore, the dispersing abilities at varied ESMA copolymer concentrations were characterized using UV–vis spectroscopy by recording the absorbance values at 200–800 nm. As reported in previous studies, the greater absorbance proved to the better dispersibility because the intensity of absorbance is related to the number of particles contained in the unit volume of the dispersion [33].
Figure 3 shows the absorbance spectra of diluted MWCNTs and modified E-MWCNTs dispersion taken after centrifugation for 15 min at 900 rpm. In Fig. 3, it is clear that the modified E-MWCNTs dispersion with different contents of ESMA copolymer shows an upward shift of the baseline. The shorter the wavelength is, the larger the baseline shift is. In addition, the MWCNTs dispersion appears to peak at around 200–300 nm in the UV region, and its tail extends to the visible region. The phenomenon is owing to the wavelength dependence of light scattering by MWCNTs in NMP. As can be seen in Fig. 3, the absorbance of pristine MWCNTs dispersion taken after centrifugation is the lowest due to heavy sedimentation. Compared to that of pristine MWCNTs dispersion, the absorbance intensity of E-MWCNTs-1 dispersion is increased owing to a little sedimentation and that of E-MWCNTs-3 dispersion having redshift without sedimentation is the highest, indicating that π–π stacking interaction has formed between MWCNTs and benzene backbone of ESMA copolymer.
Absorption spectra of dispersion of pristine MWCNTs and modified E-MWCNTs with different contents of ESMA copolymer (a); optical images of the modified E-MWCNTs-3 dispersion (b), and E-MWCNTs-3 dispersion after centrifugation (c). All samples were diluted 2000 times in NMP and then centrifuged under the condition of 900 rpm for 15 min
Dispersibility and stability
On the basis of the dynamic light scattering (DLS) principle, the number average diameter and size distribution of the diluted MWCNTs dispersion were analyzed using a zeta potential analyzer as shown in Fig. 4. According to the result, the pristine MWCNTs are dispersed poorly in NMP, and serious agglomeration is formed owing to the existence of the high surface area and strong van der Waals forces [34, 35]. Its average diameter (D50) is about 4589 nm as shown in Fig. 4b. Because of the new non-covalent modification between ESMA and pristine MWCNTs, the agglomeration is controlled effectively. With the increasing content of the ESMA copolymer, the average diameter of carbon nanotubes decreased gradually.
Compared with the pristine MWCNTs, the E-MWCNTs modified with ESMA copolymer showed good dispersibility in NMP, especially the E-MWCNTs-3 (1763 nm). It was suggested that the dispersing effect of carbon nanotubes using a paint shaker can be improved after being modified with ESMA copolymer.
The simplest method evaluating the dispersibility and storage stability of MWCNTs in the solvent is the sedimentation test [31, 34]. Comparison photographs of pristine MWCNTs and modified E-MWCNTs dispersions stored for 3 months are shown in Fig. 5. The dispersibility and storage stability of the ESMA copolymer–modified MWCNTs are much better than that of pristine MWCNTs dispersion without dispersant in NMP. In contrast to pristine MWCNTs and E-MWCNTs modified with different ESMA copolymer contents, the pristine MWCNTs are insoluble and form an obvious black layer in the bottom of the glass, while three kinds of E-MWCNTs dispersion in Fig. 5A can be easily well-dispersed in NMP, and no sedimentation is found more than 3 months.
Figure 5B shows the diluted dispersibility comparison photos of pristine MWCNTs and modified E-MWCNTs dispersions for 3 months. The dispersibility of E-MWCNTs is also much better than that of their totally physical mixed dispersion. However, the diluted E-MWCNTs-1/2 dispersions have a little sediment in Fig. 5B, indicating that ESMA copolymer is not enough to bring mutual exclusion and steric hindrance effect. By comparison, E-MWCNTs-3 diluted 100 times can be easily well-dispersed in NMP, and no sedimentation is observed for more than 3 months in Fig. 5B, indicating that they are a physically stable system.
TGA analysis
Actual MWCNT compositions in the dispersions were measured by TGA analysis of pristine MWCNTs and modified E-MWCNTs after evaporation of the solvent in Fig. 6. The TGA plot of MWCNTs indicates a slight mass loss of around 1.2% as the temperature reaches 600 ℃. There is a distinct mass loss region between 250 and 600 ℃ for modified E-MWCNTs with different contents of ESMA copolymer, which was attributed to the pyrolysis of ESMA copolymer. Furthermore, we note that the more ESMA is used, the more it decomposes. The results indicate that ESMA copolymer can be adsorbed on the surface of carbon nanotubes.
Raman spectroscopy
Raman spectroscopy is usually used to distinguish the ordered and disordered carbon structures of CNTs [2]. Figure 7 displays the Raman spectra of pristine MWCNTs and modified E-MWCNTs. As shown in Fig. 7, the prominent D peak at 1337 cm−1 represented the A1g mode corresponding to the disordered graphite structure of pristine MWCNTs. The G band at 1572 cm−1 was associated with the E2g mode representing the C = C stretching in the graphitic carbon. Generally, the relative peak intensity ratio of the D band (ID) to G band (IG) represented graphitic degrees and graphitic crystallite sizes of carbon [19]. The ID/IG ratio of modified E-MWCNTs presents a slight increase from 1.14 for pristine MWCNTs to 1.20 for E-MWCNT-3, indicating that no more defects are introduced after the physical modification of pristine MWCNTs with ESMA copolymer, and the carbon nanotubes preserves its basic structural properties.
Dispersion morphology
The dispersion morphologies of pristine MWCNTs and E-MWCNTs-1–3 modified by ESMA copolymer in NMP are checked by TEM, as shown in Fig. 8. To represent detailed morphological information of the specimens, different magnifications were utilized for the diluted dispersions. The pristine MWCNTs dispersed in NMP at the carbon-coated copper grid show tight agglomeration and exist large stacked carbon nanotubes [35, 36], which is attributed to the poor dispersion of MWCNT nanotubes in Fig. 8A. In comparison, we find that the dispersibility of E-MWCNTs improves with increasing ESMA copolymer content in Fig. 8C to D. Furthermore, the E-MWCNT-3 is well-dispersed in NMP, and the aggregates of MWCNTs are successfully debundled into individually dispersed nanotubes in Fig. 8D. This observation is consistent with MWCNTs functionalization with non-covalent π-π interactions, where the molecular chains of ESMA copolymer brought mutual exclusion and steric hindrance effect. Thus, the surface free energy reduced correspondingly, and the agglomeration was controlled effectively. Thus, it can be inferred from the dispersion morphologies that E-MWCNTs-3 with good dispersibility and long storage stability is easy to disperse in lithium-ion batteries’ positive slurry and build good electrochemical properties.
TEM images of pristine MWCNTs (A) and modified E-MWCNTs with different contents of ESMA copolymer (B to D in sequence) drop-casted from the NMP, showing different dispersion morphologies. TEM images (A′-D′) on the right are magnified dispersion morphologies. All samples were diluted 2000 times in NMP
Conclusion
In summary, ESMA copolymer–modified E-MWCNTs dispersion was successfully prepared in NMP by using a one-step π-π conjugation non-covalent surface functionalization technique between pristine MWCNTs and benzene backbone. The results show that the best modified E-MWCNTs-3 with the lowest optical transmittance (32.1%) at 550 nm can be easily well-dispersed in NMP, and the aggregates of MWCNTs are successfully debundled into individually dispersed nanotubes by using paint shaker. The prepared E-MWCNTs-3 dispersion with no sedimentation more than 3 months has the potential application as a functional conductive or performance additive for lithium-ion batteries, which provided a new strategy for the high value-added use of MWCNTs.
References
Guille JF, Valdez Z, Golzio M, Flahaut E (2019) Electrical properties of double-wall carbon nanotubes nanocomposite hydrogels. Carbon 146:542–548
Liu L, Yu PX, Wu MY, Wu QY, Liu JY, Yang JJ, Zhang JA (2021) Poly(tannin urethane)-stabilized multiwalled carbon nanotube aqueous dispersion for antistatic coating. Ind Eng Chem Res 60:12353–12361
Cao M, Luo Z, Yang Y, Wang Y, Wang B (2017) In situ polymerized poly(amide imide)/multiwalled carbon nanotube composite: structural, mechanical, and electrical studies. Polym-Plast Technol Eng 56:1391–1400
Yan J, Orecchioni MF, Vitale J, Pasquali M (2021) Biocompatibility studies of macroscopic fibers made from carbon nanotubes: implications for carbon nanotube macrostructures in biomedical applications. Carbon 173:462–476
Wang Q, Cheng L, Wang J, Qian Z, Wei T, Guo W (2019) High performance antistatic HDPE composites with bridging effect of hybrid carbon black and multi-walled carbon nanotubes fillers. Adv Eng Mater 21:1800609
Landi BJ, Ganter MJ, Cress CD, DiLeo RA, Raffaelle RP (2009) Carbon nanotubes for lithium ion batteries. Energy Environ Sci 2:638–654
Li MH, Xu ZY, Chen JY, Zhu SE (2018) Covalent functionalization of multiwalled carbon nanotubes with super hydrophobic property. J Polym Eng 38(6):537–543
Fernando S, Lin Y, Sun YP (2004) High aqueous solubility of functionalized single-walled carbon nanotubes. Langmuir 20:4777–4778
Zou J, Khondaker S, Huo Q, Zhai L (2009) A general strategy to disperse and functionalize carbon nanotubes using conjugated block copolymers. Adv Funct Mater 19:479–483
Hamedi MM, Hajian A, Fall AB, Hakansson K, Salajkova M, Lundell F, Wagberg L, Berglund LA (2014) Highly conducting, strong nanocomposites based on nanocellulose-assisted aqueous dispersions of single-wall carbon nanotubes. ACS Nano 8:2467–2476
Liebscher M, Lange A, Schrofl C, Fuge R, Mechtcherine V, Plank J, Leonhardt A (2017) Impact of the molecular architecture of polycarboxylate superplasticizers on the dispersion of multi-walled carbon nanotubes in aqueous phase. J Mater Sci 52:2296–2307
Bai L, Bai Y, Zheng J (2017) Improving the filler dispersion and performance of silicone rubber/multi-walled carbon nanotube composites by noncovalent functionalization of polymethylphenylsiloxane. J Mater Sci 52:7516–7529
Peter WM, Pawel W, David LO, Dirk MG (2018) Use of alkylated, amphiphilic zinc porphyrins to disperse individualized SWCNTs. J Porphyrins Phthalocyanines 22:1–8
Han Y, Cai C, Lin J, Gong S, Xu W, Hu R (2018) Self-assembly of rod-coil block copolymers on carbon nanotubes: a route toward diverse surface nanostructures. Macromol Rapid Commun 39:1880–1889
Liu G, Liu N, Lόpez-Moreno A, Zhao P, Dai WB (2018) Efficient production of single-walled carbon nanotube aqueous dispersion using hexahydroxytriphenylene as a dispersant and stabilizer. Chem Select 3:6081–6086
Kim Y, Hong JS, Moon SY, Hong JY, Lee JU (2021) Evaluation of carbon nanotubes dispersion in aqueous solution with various dispersing agents. Carbon Lett 31:1327–1337
Wang T, Jing L, Zhu Q, Ethiraj A, Fan X (2020) Tannic acid modified graphene/CNT three-dimensional conductive network for preparing high-performance transparent flexible heaters. J Colloid Interface Sci 577:300–310
Li X, Chen W, Zou C (2020) The stability, viscosity and thermal conductivity of carbon nanotubes nanofluids with high particle concentration: a surface modification approach. Powder Technol 361:957–967
Hussain T, Bashir F, Mujahid A (2022) Highly stable APTES incorporated CNTs based ternary polymer composites with improved dielectric and thermal properties. SILICON 14:10807–10816
Sharma B, Sharma SK, Gupta SM, Kumar A (2018) Modified two-step method to prepare long-term stable CNT nanofluids for heat transfer applications. Arab J Sci Eng 43:6155–6163
Czech B, Oleszczuk P, Wiącek A (2015) Advanced oxidation(H2O2 and/or UV) of functionalized carbon nanotubes (CNT-OH and CNT-COOH) and its influence on the stabilization of CNTs in water and tannic acid solution. Environ Pollut 200:161–167
Qiao L, Liang C, Du K (2021) Homogeneous and stable carbon nanotube dispersion assisted by cellulose in NaOH/thiourea aqueous solution. Cellulose 28:5421–5431
Li L, Yang H, Zhou D, Zhou Y (2014) Progress in application of CNTs in lithium-ion batteries. J Nanomater 2014:1–8
Mordkovich VZ, Sergeyev VG, Antipov EV (2023) Single-, double-, and multi-walled carbon nanotubes as electrically conductive additives to lithium-ion battery cathodes. Dokl Chem 508:1–9
Kim JH, Kim S, Han JH, Seo SB, Choi YR, Lim J, Kim YA (2023) Perspective on carbon nanotubes as conducting agent in lithium-ion batteries: the status and future challenges. Carbon Lett 33:325–333
Hakimian A, Kamarthi S, Erbis S, Abraham KM, Cullinane TP, Isaacs JA (2015) Economic analysis of CNT lithium-ion battery manufacturing. Environ Sci: Nano 2:463–476
Chen T, Liu BC, Zheng ML, Luo YS (2023) Suspensions based on LiFePO4/carbon nanotubes composites with three-dimensional conductive network for lithium-ion semi-solid flow batteries. J Energy Storage 57:106300
Kim JH, Ahn J, Kim HM, Cho JY, Lee DG, Oh Y, Park JH, Kim JS, Yoo JK, Han JT (2023) Highly efficient oxidation of single-walled carbon nanotubes in liquid crystalline phase and dispersion for applications in Li-ion batteries. Chem Eng J 458:141350
Choi JH, Lee C, Park S, Hwang M, Embleton TJ, Ko K, Jo M, Saqib KS, Yun J, Jo M, Son Y, Oh P (2023) Improved electrochemical performance using well-dispersed carbon nanotubes as conductive additive in the Ni-rich positive electrode of lithium-ion batteries. Electrochem Commun 146:107419
Li MH, Lu XY, Jiang JJ, Gao L, Gao J, Jiang DM (2022) Green modification of graphene dispersion with high nanosheets content and good dispersibility and long storage stability. RSC Adv 12:6037
Li MH, Jiang DM, Du ZQ, Yu SJ, Ge XJ, He Y (2023) Green modification of single-walled carbon nanotubes dispersion with good dispersibility and long storage stability. J Nanopart Res 25:85
Li MH, Xu P, Yang JG, Ying H, Haubner K, Dunsch L, Yang SF (2011) Synthesis of pyrene-substituted poly(3-hexylthiophene) via postpolymerization and its noncovalent interactions with single-walled carbon nanotubes. J Phys Chem C 115:4584–4593
Si SX, Gao TT, Wang JH, Liu QZ, Zhou GW (2018) Mussel inspired polymerized P(TA-TETA) for facile functionalization of carbon nanotube. Appl Surf Sci 433:94–100
Babita, Sharma SK, Gupta SM (2018) Synergic effect of SDBS and GA to prepare stable dispersion of CNT in water for industrial heat transfer application. Mater Res Express 5:055511
Rehman AU, Abbas SM, Ammad HM, Badshah A, Ali Z, Anjum DH (2013) A facile and novel approach towards carboxylic acid functionalization of multiwalled carbon nanotubes and efficient water dispersion. Mater Lett 108(1):253–256
Gupta R, Singh B (2020) Chemical modification of carboxylated MWCNTs for enhanced electrical conducting and magnetic properties. Mater Sci Eng B 262:114730
Funding
This work was mainly supported by the Hefei Municipal Natural Science Foundation (No. 202337) and the Anhui Provincial Key Research and Development Program (No. 2023z04020004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Du, Z., Fang, X. et al. Esterified styrene-maleic anhydride ester copolymer–modified MWCNTs dispersion with excellent dispersibility and long storage stability. J Nanopart Res 25, 216 (2023). https://doi.org/10.1007/s11051-023-05866-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05866-4