Abstract
In this work, an easy, green, π-π conjugation non-covalent surface modification of pristine single-walled carbon nanotubes (SWCNT) was carried out by using ultrasonic technology between pristine SWCNT and anionic surfactant (sodium dodecyl benzene cyclate (SDBS) and sodium polynaphthalene sulfonate (SPS)), resulting in the formation of modified SWCNT dispersion with good dispersibility and long storage stability in water. Results obtained from transmittance spectroscopy, absorption spectroscopy, zeta potential analyzer, sedimentation photo, and transmittance electron microscopy (TEM) reveals that the modified SWCNT dispersion has a high degree of dispersion. The analysis of thermogravimetry analysis (TGA) and scanning electron microscope (SEM) confirm that two kinds of dispersants are successfully decorated on pristine SWCNT surface. More importantly, the pristine SWCNT is insoluble, while the modified S2-SWCNT dispersion mixed with 1.25 wt% SDBS and 1.00 wt% SPS correspondingly under the condition of total 3 h ultrasonic time can be easily well-dispersed in water and has good storage stability, and no sedimentation is observed more than 3 months. More importantly, this strategy is green and economical owing to its easy operation in industry.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNT) in general, and single walled carbon nanotubes (SWCNT) in particular, have attracted a lot of research attention owing to their special structure and excellent mechanical, optical, and electrical properties [1, 2], and thus have been used in various commercial applications such as conductive inks for flexible displays [3], low-weight conductive reinforcements in polymers [4], transparent electrodes [5], and adhesive materials [6]. To obtain these excellent properties, SWCNT often need to be dispersed into a medium (either liquid or solid) and should be dispersed individually. However, dispersion with good dispersibility and long storage stability still presents a challenge, as the high surface area of SWCNT makes them susceptible to aggregation, driven by strong van der Waals forces [7].
Therefore, many efforts are invested in achieving the good dispersion of CNT on an industrial scale by using chemical and physical strategies. The chemical dispersion strategy is to introduce functional groups on the surface of CNT, and then to graft soluble functional groups or hydrophilic chains [8, 9]. Although this has been recognized as an effective strategy for stable dispersion of CNT in a medium, often results in disruption of the intrinsic sp2 hybridized network and reduce mechanical and electrical properties of pristine CNT [10, 11]. On the other hand, the physical dispersion strategy is to utilize various dispersants including surfactants, biomolecules, aromatic compounds, and polymers onto the CNT surface by using van der Waals forces or π-π stacking interactions [12,13,14,15,16]. Since this strategy avoid tedious chemical reactions under strong acidic conditions and preserves the conjugated structure and the intrinsic properties of SWCNT, it has been considered to be a more promising tool in light of the application of SWCNT dispersion. However, the physical dispersion strategy has problems that the long-term stability of CNT dispersion is generally inferior to that of the chemical method.
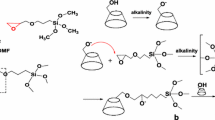
In this paper, we report an easy, green modification of SWCNT by using π-π conjugation non-covalent strategy between pristine SWCNT and the benzene type backbone of anionic surfactant, as shown in Fig. 1, to prepare modified SWCNT dispersion with good dispersibility and long storage stability in water.
This green surface modification method of pristine SWCNT can be used to prepare SWCNT/waterborne coating nanocomposites. Importantly, this strategy above is simple and economical due to easy operation in industry. We also believe that this green experimental work will promote the application of SWCNT in waterborne coatings.
Experimental
Materials
Pristine SWCNT product (black powder: SWCNT length > 5 µm, BET: 300 m2/g, SWCNT outer mean diameter: 1.6 ± 0.4 nm) was obtained from OCSiAl (Novosibirsk, Russia). Sodium dodecyl benzene cyclate (SDBS, anionic surfactant) and sodium polynaphthalene sulfonate (SPS, anionic surfactant) bought from market were used without purification. Deionized water was prepared using corresponding equipment.
Modification of SWCNT with anionic surfactant
First of all, deionized water ((98.55 − n) g) and SDBS anionic surfactant (n = 1.00, 1.25 and 1.50 g) were added to the three-necked flask and stirred with a stirrer at 300 rpm for 15 min. After that pristine SWCNT (0.2 g) was added to the preprepared mixture and sonicated for different time controlling temperature around 30 °C in a ultrasonic cleaner (400 W) to afford uniform water-based S1-SWCNT dispersion.
In the process of dispersing SWCNT, we find that some particles are existed in the dispersion using 1.00 wt% SDBS and good appearance is shown in the dispersion using 1.25 wt% SDBS and a large amount of bubbles occur in the dispersion using 1.50 wt% SDBS, respectively. To simplify the comparison, S1-SWCNT dispersion modified with 1.25 wt% SDBS was chosen to analyze the influence of three different ultrasonic time (x = 0.5, 1, and 2 h) on dispersion.
Then, SPS anionic surfactant (1.0 g) was added into above water-based S1-SWCNT-x dispersion with the lowest transmittance via 1-h sonication to obtain stable water-based S2-SWCNT dispersion. The above modified S2-SWCNT dispersion is mixed with 1.25 wt% SDBS and 1.00 wt% SPS by calculation. Then, the above products were purified after each modification by PVDF micro-filtration membrane. The resulting S1-SWCNT and S2-SWCNT solid powders were dried overnight at 80 °C in vacuum. The procedure of the preparation of green modified water-based SWCNT dispersion was schematically displayed in Fig. 1.
Characterization
The transmittance and absorption of the SWCNT dispersion were investigated by an ultraviolet spectrophotometer (UV2600). Thermogravimetry analysis (TGA) of pristine SWCNT and modified SWCNT was performed under nitrogen on a STA409PC thermogravimetric analyzer (Netzsch Instruments) with a temperature range of 25–600 °C at a heating rate of 10 °C/min. The zeta potential of the SWCNT dispersion was characterized by a 90Plus PALS Zeta Potential (Brookhaven Instruments). Morphology of the SWCNT dispersion was investigated by field emission scanning electron microscopy (FE-SEM, SU8010) and transmittance electron microscopy (TEM, FEI F20).
Results and discussion
Transmittance spectroscopy
For quickly evaluating the dispersion effect of SWCNT, it is necessary to compare their optical transmittance [17]. Figure 2a shows a transmittance spectroscopy of dispersion of pristine SWCNT and modified water-based S1-SWCNT dispersion with incident light wavelength ranging from 400 to 800 nm. We extracted the experimental data at 550 nm from pristine SWCNT and modified S1-SWCNT-2 h in Fig. 2a. We can conclude that the modified S1-SWCNT dispersions have the different optical transmittance. Compared to 97.1% at 550 nm of pristine SWCNT dispersion, the optical transmittance of modified water-based S1-SWCNT dispersion is lower, indicating that the pristine SWCNT was dispersed poorly in water and the serious agglomeration was formed because of the existence of the high surface area and strong van der Waals forces and intrinsic hydrophobicity. The lower optical transmittance also indicates that the bundle of SWCNT was dispersed significantly by SDBS anionic surfactant. Figure 2b shows effect of ultrasonic time on the transmittance of modified S1-SWCNT dispersion at 550 nm. Compared with the pristine SWCNT, the SDBS modified SWCNT showed good dispersibility in water, especially the S1-SWCNT-2 h (35.2%). It was suggested that the dispersing effect of SWCNT by using different ultrasonic time in water can be improved after being modified with SDBS anionic surfactant. Because of the new π-π conjugation non-covalent modification between SWCNT and SDBS anionic surfactant, the agglomeration was controlled effectively. With the increasing ultrasonic time, the optical transmittance of S1-SWCNT decreased gradually. This may because when the ultrasonic time is insufficient, the uncoated SWCNT will be likely to agglomerate together.
Transmittance spectra of dispersion of pristine SWCNT and water-based S1-SWCNT with incident light wavelength ranging from 200 to 780 nm. The data in the figure indicates the optical transmittance with incident 550 nm visible light (a); effect of ultrasonic time on the transmittance (wavelength, 550 nm) of water-based S1-SWCNT dispersion (b); comparison of transmittance spectra of dispersion of pristine SWCNT, water-based S1-SWCNT-2 h, and S2-SWCNT (c). Pristine SWCNT dispersion prepared using similar technology in the “Modification of SWCNT with anionic surfactant” section has the same solid content with modified S1/S2-SWCNT dispersion
For preparing water-based SWCNT dispersion with good dispersibility, it is important to disperse SWCNT by using quick permeability of SDBS via ultrasonication [18]. However, it is hard to obtain stable SWCNT dispersion with long storage stability due to weak π-π conjugation non-covalent surface modification between SWCNT and SDBS anionic surfactant. Considering the long storage stability, S1-SWCNT dispersion has been modified by using SPS anionic surfactant with strong π-π conjugation non-covalent interaction. Compared to 35.2% at 550 nm of optimal S1-SWCNT-2 h dispersion, the optical transmittance of modified water-based S2-SWCNT dispersion is 32.8% in Fig. 2c, indicating that the dispersibility of S1-SWCNT has been improved by SPS anionic surfactant owing to strong π-π conjugation non-covalent interaction between SWCNT and SPS anionic surfactant displayed in Fig. 1.
Absorption spectroscopy
Comparing the absorbance intensity of nanotubes remaining after a centrifugation is a good way to prove the modification effect of SWCNT [20]. Figure 3 shows the absorbance spectra of diluted SWCNT and water-based SWCNT dispersion taken after centrifugation for 15 min. In Fig. 3, it is seen that the water-based SWCNT dispersion shows upward shift of the baseline. The shorter the wavelength is, the larger the baseline shift is. The phenomenon is attributed to wavelength dependence of light scattering by SWCNT in water. In addition, SWCNT shows peak at around 200–300 nm in UV region, and its tail extends to visible region as seen in Fig. 3. Furthermore, it is seen from Fig. 3 that the absorbance of SWCNT dispersion taken after centrifugation is the lowest owing to heavy sedimentation. Compared to that of SWCNT dispersion, the absorbance intensity of water-based S1-SWCNT dispersion is enhanced due to a little sedimentation and that of S2-SWCNT dispersion having red shift without sedimentation is the highest, proving that π-π stacking interactions has happened between SWCNT and benzene backbone of SPS anionic surfactant.
Dispersibility and stability
The vital parameter defining surface properties of electrostatically stabilized nanomaterials in aqueous solutions is the zeta potential value [17]. The relation between the zeta potential and ultrasonic time for the SWCNT dispersion is shown in Fig. 4. This plot shows that the zeta potential decreases with increasing ultrasonic time. The plot seems to keep balance when the ultrasonic time was 2 h; then the zeta potential of S2-SWCNT reaches the maximal absolute value at 52.66.3 mV. Therefore, it can be presumed that there are more sulfonic acid groups of SDBS and SPS on the surface of the nanotubes when the absolute value of the zeta potential is high. In other words, the stability of S2-SWCNT dispersion has been improved due to high zeta potential.
The simplest test defining dispersibility and storage stability of SWCNT in aqueous solution is sedimentation analysis [19]. Comparison photos of water-based S1-SWCNT dispersions stored for 3 months are shown in Fig. 5. The dispersibility of the SDBS modified SWCNT is much better than that of their totally physical mixture without dispersant in water. The sample of physical mixture has obvious black sediment in water. In contrast to pristine SWCNT and S1-SWCNT modified with different sonication time, the pristine SWCNT is insoluble, while three kinds of S1-SWCNT dispersion in Fig. 5A can be easily well-dispersed in water, and no sedimentation is observed more than 3 months. This suggests that S1-SWCNT dispersions have good solubility in water. However, the S1-SWCNT dispersions diluted 100 times have heavy sediment in Fig. 5B, indicating that SDBS anionic surfactant is not enough to bring mutual exclusion and steric hindrance effect.
Figure 6 shows the dispersibility comparison photos of pristine SWCNT, optimal S1-SWCNT-2 h, and S2-SWCNT modified by SDBS and SPS anionic surfactants in water. The dispersibility of S2-SWCNT is also much better than that of their totally physical mixture. Compared with pristine SWCNT and S1-SWCNT-2 h in Fig. 6A, the SDBS and SPS modified S2-SWCNT dispersion diluted 100 times can be easily well-dispersed in water, and no sedimentation is observed more than 3 months in Fig. 6B. The above results demonstrate that the S2-SWCNT dispersion is a physically stable system.
TGA measurements
Supporting evidence for the π-π conjugation non-covalent attachment of SDBS and SPS anionic surfactants on the pristine SWCNT surfaces comes from the thermogravimetry analysis (TGA) [17]. Figure 7 shows TGA curves for the analysis of pristine SWCNT, S1-SWCNT-2 h, and S2-SWCNT at a temperature rate of 10 °C/min, respectively. The TGA plot of SWCNT indicates a gradual mass loss of around 1.5% as the temperature reaches 600 °C. However, there is a distinct mass loss region between 300 and 600 °C for modified S1-SWCNT-2 h and S2-SWCNT. Compared with pristine SWCNT, the mass loss around 66.86% of S1-SWCNT-2 h terminates at approximately 600 °C, which was due to the pyrolysis of SDBS modifier. Furthermore, we noted that S2-SWCNT showed a significant weight loss around 43.40% corresponding to the pyrolysis of SDBS and SPS modifier. The results indicate that two kinds of anionic surfactants can be adsorbed on the surface of carbon nanotubes.
Surface morphology
The surface morphologies of pristine SWCNT, the best S1-SWCNT-2 h, and S2-SWCNT modified by SDBS and SPS anionic surfactants in water are checked by SEM, as shown in Fig. 8. The pristine SWCNT dispersed in water at the Si substrate shows tight agglomeration [20], which is attributed to the poor dispersion of SWCNT nanotubes in Fig. 8A. In comparison, we find small carbon bundles in the images of modified S1-SWCNT-2 h and S2-SWCNT, which is due to the modification of SDBS and SPS anionic surfactants. After using anionic surfactant, the evolvement of the film morphology is clearly observed.
Furthermore, TEM results confirmed the dispersibility of pristine SWCNT, S1-SWCNT-2 h, and optimal S2-SWCNT with the best dispersed stability in Fig. 8A’–C’. The pristine SWCNT dispersed in water at the carbon membrane also shows tight agglomeration and exists large carbon bundles, which is due to the poor dispersion (marked as red circles in Fig. 8A’) of SWCNT. After the modification with SDBS and SPS modifiers, the S1-SWCNT-2 h and S2-SWCNT nanotubes are all well dispersed in water and show good dispersibility and exist small bundles in Fig. 8B’–C’. It is obvious to find that the diameter of pristine SWCNT is reduced. This observation is consistent with SWCNT functionalization with noncovalent π-π interactions, where the organic chain may bleach the strong interactions between individual SWCNT. Thus, it can be inferred from the dispersion morphologies that S2-SWCNT with good dispersibility and long storage stability is easy to disperse in waterborne coatings and build good physical properties.
Conclusion
In summary, an easy, mixed anionic surfactants modified S2-SWCNT dispersion was successfully prepared in aqueous solution by two-step π-π conjugation non-covalent surface functionalization technique between SWCNT and benzene backbone. The results show that the best modified S2-SWCNT with the lowest optical transmittance (32.8%) at 550 nm can be easily well-dispersed in water and has good storage stability. The prepared S2-SWCNT dispersion with no sedimentation more than 3 months has the potential application as a functional antistatic or performance additive for waterborne coatings, which provided a new strategy for the high value-added use of SWCNT.
References
Ata S, Kobashi K, Yumura M, Hata K (2012) Mechanically durable and highly conductive elastomeric composites from long single-walled carbon nanotubes mimicking the chain structure of polymers. Nano Lett 12(6):2710–2716
Green AA, Hersam MC (2016) Colored semitransparent conductive coatings consisting of monodisperse metallic single-walled carbon nanotubes. Nano Lett 8(5):1417–1422
Simmons TJ, Hashim D, Vajtai R, Ajayan PM (2007) Large area-aligned arrays from direct deposition of single-wall carbon nanotube inks. J Am Chem Soc 129:10088–10089
Krause B, Pötschke P, Ilin E, Predtechenskiy M (2016) Melt mixed SWCNT-polypropylene composites with very low electrical percolation. Polymer 98:45–50
Li S, Wang K, Feng M, Yang H, Liu X, He Y, Zhang C, Wang J, Fu J (2020) Preparation of light-transmissive conductive film by free arc dispersed carbon nanotubes and thermos compression bonding. Carbon Lett 30:651–656
Doganci E (2021) Improving adhesion between polyester cord and rubber by using glycidyl-POSS. J Appl Polym Sci 138:49681
Basat MB, Lachman N (2021) Development of quality control methods for dispersibility and stability of single-wall carbon nanotubes in aqueous medium. Nanomaterials 11:2618
Li MH, Xu ZY, Chen JY, Zhu SE (2018) Covalent functionalization of multiwalled carbon nanotubes with super hydrophobic property. J Polym Eng 38(6):537–543
Fernando S, Lin Y, Sun YP (2004) High aqueous solubility of functionalized single-walled carbon nanotubes. Langmuir 20:4777–4778
Sohrabi B, Poorgholami-Bejarpasi N, Nayeri N (2014) Dispersion of carbon nanotubes using mixed surfactants: experimental and molecular dynamics simulation studies. J Phys Chem B 118:3094–3103
Peng S, Cho K (2000) Chemical control of nanotube electronics. Nanotechnology 11:57–60
Liu G, Liu N, López-Moreno A, Zhao P, Dai WB (2018) Efficient production of single-walled carbon nanotube aqueous dispersion using hexahydroxytriphenylene as a dispersant and stabilizer. ChemistrySelect 3:6081–6086
Zelikman E, Alperstein D, Mechrez G, Suckeveriene R, Narkis M (2013) Study of interactions between single-wall carbon nanotubes and surfactant using molecular simulations. Polym Bull 70:1195–1204
Sharma B, Sharma SK, Gupta SM, Kumar A (2018) Modified two-step method to prepare long-term stable CNT nanofluids for heat transfer applications. Arab J Sci Eng 43:6155–6163
Peter WM, Pawel W, David LO, Dirk MG (2018) Use of alkylated, amphiphilic zinc porphyrins to disperse individualized SWCNTs. J Porphyrins Phthalocyanines 22:1–8
Kim Y, Hong JS, Moon SY, Hong JY, Lee JU (2021) Evaluation of carbon nanotubes dispersion in aqueous solution with various dispersing agents. Carbon Lett 31:1327–1337
Li MH, Lu XY, Jiang JJ, Gao L, Gao J, Jiang DM (2022) Green modification of graphene dispersion with high nanosheets content and good dispersibility and long storage stability. RSC Adv 12:6037
Gu ZZ, Jia SL, Li GF, Li CQ, Wu YQ, Geng HZ (2019) Mechanism of surface treatments on carbon nanotube transparent conductive films by three different reagents. RSC Adv 9:3162
Babita, Sharma SK, Gupta SM (2018) Synergic effect of SDBS and GA to prepare stable in water for industrial heat transfer application. Mater Res Express 5:055511
Li MH, Xu P, Yang JG, Ying H, Haubner K, Dunsch L, Yang SF (2011) Synthesis of pyrene-substituted poly(3-hexylthiophene) via postpolymerization and its noncovalent interactions with single-walled carbon nanotubes. J Phys Chem C 115:4584–4593
Funding
This work was mainly supported by Anhui Provincial Natural Science Foundation (No. 1808085ME144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Jiang, D., Du, Z. et al. Green modification of single-walled carbon nanotubes dispersion with good dispersibility and long storage stability. J Nanopart Res 25, 85 (2023). https://doi.org/10.1007/s11051-023-05737-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05737-y