The corrosion properties of normalized steel 45 are studied after a combined hardening of its surface layer, which consists of electromechanical treatment and surface plastic deformation (EMT + SPD). The effect of different aggressive environments on the structure, microhardness and corrosion rate of the hardened surface layer is determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The possibility of fabrication of materials with a wide spectrum of specified physical, mechanical and operating properties widens the range of their application. This concerns not only the traditional characteristics of strength, ductility, wear resistance etc. but also the properties related to specific operating conditions of the part, for example, the resistance to corrosion impact or the capacity to dissipate energy under vibration of mechanical systems.
The recent advanced hardening processes involving the action of a high-energy flux on the surface make it possible to raise purposefully some specific mechanical characteristics of the material by creating a specific high-strength state of the surface layer [1,2,3]. Surface plastic deformation is an efficient final hardening operation, which makes it possible to improve the required properties not worsening the characteristics obtained in the preceding treatment [2, 3].
The results of earlier studies show that the surface layer of a steel subjected to a combined action of electromechanical treatment (EMT) and surface plastic deformation (SPD) acquires a specific martensitic structure (a white layer). This structure possesses a set of unique properties such as high fineness of the martensite, elevated content of carbon and other alloying elements in the martensite, distortion of the crystal structure, and high level of residual microstresses [4, 5]. Despite the variety of opinions on the properties of “white layers,” we may state that they have common qualities of reduced susceptibility to etching and enhanced hardness as compared to conventionally hardened martensite. The known studies of corrosion resistance chiefly concern some particular problems of corrosion of surface layers obtained by electromechanical hardening. However, detailed studies of the corrosion behavior of a material after reinforcing its surface by a combined method of EMT + SPD are still scarce.
The aim of the present work was to study the transformation of the strength properties of the surface bainitic layer of steel 45 and its resistance to corrosion in aggressive environments after the treatment involving EMT + SPD.
Methods of Study
We studied carbon steel 45 (0.45% C, 0.2 – 0.3% Si, 0.5 – 0.8% Mn, 0.04% P, 0.25% Ni, 0.25% Cr, 0.25% Cu) in normalized condition after combined hardening by the EMT + SPD technique.
The electromechanical treatment consisted in simultaneous action of pulse electric current with density j = 400 A/mm and mechanical force P = 200 N through a hard-alloy tool electrode. A specific structure of fine acicular martensite (a white layer) with a thickness of up to 200 μm and a hardness HV = 8.5 GPa formed as a result of the temperature and force impact and intense removal of heat into the depth of the material in the zone of contact between the roll and the surface of the specimen. Due to the longitudinal feeding of the roll S = 1.0 mm/rev the surface of the cylindrical specimen acquired a regular macrostructure in the form of spiral-like continuous bands (tracks) of hardened metal with width a = 0.65 mm; the bands were separated by zones of metal preserving the initial condition.
The finishing operation of surface plastic deformation was conducted in a three-roll facility at force P = 1.2 kN on the roll and feed S = 0.25 mm/rev; the speed of rotation of the spindle was 100 mm/rev; the number of passes n = 1.
When studying the effect of active environments on the properties of the steel with surface layer hardened by EMT + SPD, we used a conventional commercial electrolyte (35% solution of H2SO4 in distilled water) and road reagents, i.e., MgCl2 · 6H2O (bischofite brine) and a 26%-solution of sodium chloride (NaCl) in water.
The corrosion resistance of hardened steel 45 was determined by the following method. Cylindrical specimens (10 mm long and 10 mm in diameter) were placed into a vessel with an active environment and held for 12 h. The total duration of the test was 72 h. After the exposure to the corrosive environment, the specimens were cleaned and dried. Then we measured their microhardness and weighed them. We calculated the loss in the mass in grams and the relative mass by an equation Δm = m i /mo , where mo is the original mass of the specimen and m i is the mass of the specimen after holding in the aggressive environment. The corrosion rate was calculated as the ratio of the decrease in the mass of the specimen to the duration of the test (i.e., we determined the mean loss in the mass in 72 h). The corrosion resistance was determined without applying an external load to the specimens.
The structural state of the surface before the test and after the action of the aggressive media was studied under a “Neophot-21” microscope with magnification × 16.
The microhardness of the hardened surface layer was evaluated using a PMT-3 device at a load of 0.5 N. The distance between the indents was 30 μm.
Results and Discussion
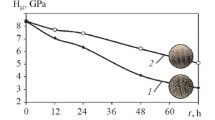
Analyzing the structures of specimens with hardened surface layer we established that the EMT produced what is known as structureless martensite on the surface [4, 5]. Measurement of the microhardness of a specimen subjected to EMT showed the presence of zones with elevated and reduced hardness over its generatrix (curve 1 in Fig. 1). Analysis of the transition zone between the hardened and original layers showed formation of a well-manifested interface; the “resolution of the hardening” for white layer was limited by the contours of one grain. This made it possible to detect different regular structures [6] on the surface. The finishing operation of surface plastic deformation virtually did not change the hardness of the surface (curve 2 in Fig. 1). The structure and the properties of the white layer and the typical features of the distribution of microhardness both on the surface and in the depth of the hardened layer of the specimens after the EMT were “inherited” under the combined treatment [7]. The additional SPD caused partial relaxation and redistribution of the residual compressive stresses.
Structure and microhardness (HV ) of the surface of hardened layer on normalized steel 45 over the axis of a cylindrical specimen [7]: 1 ) after electromechanical treatment (EMT) at tool feed S = 1.25 mm/rev; 2 ) after electromechanical treatment and surface plastic deformation (EMT + SPD).
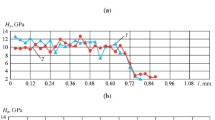
According the results of the corrosion tests, the microhardness of the surface of the hardened specimens decreases noticeably with intensification of the aggressive effect of the environment. We compared the microhardness of the surfaces of hardened specimens placed in different aggressive media. The loss in their hardness was 400 MPa in the salt solution, 2900 MPa in bischofite, and 5300 MPa in the electrolyte after 72 h of residence in the solutions (Fig. 2).
The hardness of the hardened specimens after holding in the electrolyte decreased 2 and 13 times more intensely than after holding in bischofite and in the solution of NaCl respectively. The hardness varied with the time of the hold in the same manner both in the electrolyte and in the bischofite. In the first 20 h of the exposure, the white layer played the role of a barrier for corrosion of the specimen as a whole. When the time of holding in the aggressive environment was increased, the latter “attacked” the white layer and reached the initial structure raising the corrosion rate and lowering the hardness.
In the case of exposure to the salt solution the state of the hardened surface layer changed in another manner. The microhardness of the surface layer and the appearance of the surface did not change substantially after testing for 72 h, corrosion damage on the surface was absent and it exhibited traces of mechanical treatment (Fig. 3a ).
The macrostructure after typical corrosion damage of surface-hardened steel 45 after an exposure to different aggressive environments for 72 h is presented in Fig. 3. The thin surface layer of the matrix of the specimens held in the salt solution (Fig. 3a ) acquires a lusterless gray deposit; corrosion damage is virtually absent; the shallow pits of the initial metal are traces of selective corrosion; the tracks of the white layer are not damaged by corrosion.
The appearance of the surface after 72 h of holding in bischofite is given in Fig. 3b. The general nonuniform corrosion observable of the surface has an ordered nature. The corrosion damage has the form of parallel wavy grooves oriented over the longitudinal axis of the specimen. Bands of the matrix normalized steel 45 are present between the tracks; the tracks are colored differently. The matrix is corroded to a higher degree.
The specimens subjected to the electrolyte are corroded all over the surface. The damage in the form of shallow cavities and pits is combined with wavy grooves parallel to the longitudinal axis of the specimen and has the form of typical pitting corrosion. Weak traces of the tracks can be observed.
Table 1 presents the results of the corrosion tests of the steel in the original condition and after hardening of the surface layer. The change in the mass fraction of the specimens is evaluated in terms of the relative mass Δm = m i /mo . The specimens with hardened surface exhibit elevated resistance to all the tested aggressive environments as compared to the untreated specimens, despite the fact that the hardened layer takes an inconsiderable volume. For example, after the hardening of the surface layer, the corrosion resistance increases by 20% in the electrolyte and by 6% in the bischofite.
Analysis of the variation of the mass fraction of the hardened specimens with the time of the action of the corrosive media has shown that the loss in the mass, like the decrease in the surface hardness, depends on the kind of the aggressive environment. Figure 4 presents the loss in the mass fraction as a function of the duration of the test. It can be seen that after a 72-h exposure to the electrolyte the loss in the relative mass is 7.6 and 1.5 times higher than in the salt solution and in the bischofite, respectively. In the salt the solution the mass remains virtually unchanged.
The corrosion behavior of the hardened specimens is explainable not only by formation of a white layer with specific structure but also by the very complex distribution of residual stresses in the surface layer as a result of the combined treatment.
The specific structure of the white layer is characterized by higher fineness, considerable distortions of the crystal lattice, concentration inhomogeneity, presence of carbides, nitrides and oxides, and changes in the electron structure and chemical bonding of the individual phases due to the high temperatures and pressures arising in the zone of the action of a highly concentrated energy source [4, 5]. A very complex distribution of stresses in the surface layer arises in the hardened specimens is a result of the heat and force effects under the combined treatment. It is known that the presence of compressive stresses in a metal may turn it into a cathode with respect to the original metal, and the rate of corrosion in this case decreases considerably. If the surface bears compressive stresses, they may be responsible for the start of corrosion process at these very places.
Conclusions
-
1.
A specific hardened surface layer has been obtained on steel 45 by a combination of electromechanical treatment and surface plastic deformation (EMT + SPD).
-
2.
The hardness of the hardened surface layer after holding in the electrolyte (an aqueous solution of H2SO4) decreases 2 and 13 times more intensely than after holding in the bischofite (MgCl2 · 6H2O) and in the solution of NaCl respectively, i.e., the strength properties of the surface layer, and hence the lives of the hardened steel parts, decrease intensely upon intensification of the aggressive action of the environments.
-
3.
Under the action of all the corrosive environments, the hardened tracks are corroded less than the matrix of normalized steel 45.
-
4.
Steel 45 surface-hardened by the method of EMT + SPD possesses a higher resistance to corrosion in all kinds of aggressive environments than the untreated steel; the losses in the mass after testing in the electrolyte and in the bischofite are 20 and 6% lower than after normalizing; in the salt solution, losses in the mass are virtually absent.
References
Yu. I. Babey, Physical Foundations of Pulsed Hardening of Steel and Cast Iron [in Russian], Naukova Dumka, Kiev (1988), 238 p.
B. M. Askinazi, Hardening and Reconditioning of Machine Parts by Electromechanical Treatment [in Russian], Mashinostroenie, Moscow (1989), 200 p.
V. P. Bagmutov, S. N. Parshev, N. G. Dudkina, and I. N. Zakharov, Electromechanical Treatment: Process and Physical Foundations, Properties, Implementation [in Russian], Nauka, Novosibirsk (2003), 318 p.
V. P. Bagmutov, N. G. Dudkina, and I. N. Zakharov, “A study of the structure of the surface layer of medium-carbon steel reinforced by electromechanical treatment,” Metalloved. Term. Obrab. Met., No. 12, 18 – 21 (2002).
N. G. Dudkina, L. N. Burminskaya, and A. Yu. Svitachev, “Effect of the thermal processes under EMT on formation of the structure of surface layers of quenched carbon steels,” Fiz.-Khim. Mekhan. Mater., No. 5, 113 – 115 (1995).
A. V. Gur’ev, N. G. Dudkina, and A. V. Fedorov, “Effect of electromechanical hardening on the mechanical properties of carbon steel,” Fiz.-Khim. Mekhan. Mater., No. 3, 26 – 29 (1990).
M. M. Matlin, P. G. Dudkina, and A. P. Boldov, “Special features of plastic deformation of steel parts hardened by combined EMT + SPD treatment,” Uproch. Tekhnol. Pokr., No. 8, 44 – 48 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 9, pp. 41 – 44, September, 2017.
Rights and permissions
About this article
Cite this article
Dudkina, N.G. Corrosion Resistance of Steel 45 Subjected to Electromechanical Treatment and Surface Plastic Deformation. Met Sci Heat Treat 59, 584–587 (2018). https://doi.org/10.1007/s11041-018-0194-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-018-0194-5