The processes and process parameters affecting the structure and properties of coatings are reviewed. Microstructures obtained at various deposition parameters are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The wear resistance and endurance of tools are raised by various methods of surface hardening. One of the methods is deposition of a pyrolytic chromium carbide coating (PCCC) from a vapor–gas phase of an organometallic compound (OMC).
PCCC have a high hardness, adhesive strength, corrosion resistance, heat resistance, adaptability to manufacture, and a low friction factor.
The deposition of PCCC may be represented by several successive stages, but each process has distinctive features. We have analyzed and compared the results obtained in [1–15] for deposition of PCCC with preliminarily specified operating properties and differentiated the following operating parameters:
-
(1).
the temperature of the deposition from 420 to 530°C;
-
(2).
the temperature of the evaporator from 190 to 320°C;
-
(3).
the rate of feeding of the initial chemical compound from 0.8 to 1.3 ml/min;
-
(4).
the rate of evacuation of the decomposition products from the deposition zone (under vacuum);
-
(5).
the pressure (vacuum, atmospheric pressure);
-
(6).
the carrier gas of the OMC vapors (argon);
-
(7).
the partial pressure of the carrier gas (growth in the partial pressure of argon promotes the occurrence of the process at lower temperatures);
-
(8).
the protective atmosphere (ammonia, argon, helium);
-
(9).
the source of heating of the substrate (direct or indirect heating);
-
(10).
the material of the substrate (aluminum and its alloys, copper and its alloys, nickel, titanium and its alloys, niobium, zirconium, molybdenum, silicon, tungsten, cast iron, steels of grades St3, 20, 45, 40Kh, 50KhG, 12Kh18N10T, 3Kh2V8F, 6M5 etc.);
-
(11).
the size of the substrate (part) (the substrate may be an article of any configuration and size limited only by size of the reaction chamber);
-
(12).
the use of microadditives [4, 5, 9, 16–26]; the additives may be of the following kinds:
-
catalyzing [polycyclic aromatic hydrocarbons, aromatic esters, chlorine- and sulfur-containing hydrocarbons, tin-organic additives, (tin, lead or germanium) tetraalkyl, benzyl ether, halogen (chlorex), tin tetrachloride, iodine, carbon tetrachloride, diiodo-phenyl vapors, benzyl benzoate vapors], which elevate the rate of the deposition of the coating;
-
inhibiting (aromatic ketones, loricata, nitrogen-bearing hydrocarbons), which suppress the action of the synthesis byproducts;
-
-
(13)
the heat treatment (HT) for additional enhancement of the hardness and wear-resistance of the coating (annealing) [3, 6, 9, 12, 19, 22].
The authors of [2–6, 11–13] describe the following types of microstructure of the coatings: structureless precipitates, horizontally layered structures, pyramidal structures, columnar structures.
An optimum combination of plastic properties and microhardness is obtained in coatings with horizontally layered structure.
Thus, the deposition of coatings by thermal decomposition of OMC is a field of science and engineering involving processes of fabrication of coatings and semiconductor materials and aspects of the chemical kinetics, catalysis, structure and reaction capacity of OMC [27].
The primary initial material for fabricating PCCC is the “Barkhos” organochromium liquid, i.e., an organometallic mixture of bis-arene organochromium compounds.
The aim of the present work was to study the effect of the substrate temperature, of the action of HFC, and of heat treatment on the structure and properties of PCCC.
METHODS OF STUDY
The substrates were specimens from steel R6M5, the carrier gas and the protective atmosphere was argon.
The facility for depositing coatings has a simple design. It consists of a rector (reaction chamber), a source for heating the substrate, an evaporator of the OMC, a system for feeding the carrier gas (argon), a system for arresting the decomposition products, and a system for automatic control of the deposition process.
The deposition occurs under the following conditions: the space of the reactor is filled with argon to remove the air atmosphere, the substrate temperature is (435 ± 15)°C, the temperature of the evaporator is 320°C; the OMC is fed next.
When depositing the coatings we used induction and radiation heating. The induction heating gave the best parameters. The substrate was heated faster, which shortened the time of the deposition process, reduced the cost of the products, and raised the efficiency of the utilization of the OMC.
RESULTS AND DISCUSSION
The electromagnetic field of HFC promotes localization of the OMC vapors near the coated surface and deposition of the coating only on the substrate. The path of a metal atom in a vapor–gas mixture is much shorter than in vacuum. We determined the energy action of HFC on a single atom in the OMC vapor. The microstructures of the coatings deposited under radiation heating and HFC heating are presented in Fig. 1. We can see that the HFC produces a more homogeneous coating.
Using a transparent reactor of a vertical flow type and arranging the evaporator below the level of the substrate we have a number of advantages, namely,
-
no need for additional devices for directing the OMC vapors to the substrate, because the vapors of the evaporating OMC liquid go upward and are corrected by the direction of the flow of the inert gas;
-
possibility of simultaneous deposition of a coating with homogeneous thickness on the external and internal surfaces of the substrate;
-
elimination side reactions; having reacted, the OMC vapors condense on the cold walls of the reactor;
-
the temperature difference on the substrate and on the reactor walls prevents deposition of coating on the walls and raises the efficiency of the utilization of the initial OMC;
-
elimination of unfavorable penetration of the liquid phase of the OMC into the deposition zone; the condensate flows to the bottom part of the reactor below the evaporator; the accumulated decomposition products are removed through the drain valve.
Our experiments show that the mechanism of formation of PCCC is influenced by the temperature of the substrate; the operating capacity of the coating on a cutting tool depends substantially on its structure.
The microstructure of the PCCC formed under different deposition temperatures is presented in Fig. 2. The coatings with horizontally layered structures exhibit the best properties (Fig. 2b ).
According to our results, the microhardness of the coatings depends on the substrate temperature. The maximum microhardness (14,000 – 16,000 MPa) is exhibited by the coatings deposited at 470 – 530°C (Fig. 2c and d ), but such coatings have a layered columnar structure, in which the microstresses are elevated.
Amedium microhardness (7000 – 12,000 MPa) and optimum properties are exhibited by the coatings with horizontally layered structure deposited at 430 – 450°C (Fig. 2b ).
When the coatings are deposited at 390°C (Fig. 2a ), the microhardness decreases due to formation of fully amorphous precipitates.
Deposition of PCCC onto the surface of end milling cutters 8 mm in diameter from steel R6M5 shows that the maximum endurance in treatment of steel 45 at a cutting rate of 20 m/min is attained when the coating has a thickness of from 3 to 4 μm. Thicker coatings lower the endurance of the milling cutters due to brittle fracture in operation. The authors of [3, 5, 6, 11, 12], who deposited PCCC in vacuum, have obtained the same dependence. The thickness of a coating depends on the duration of the deposition process.
We also tested drill bits 4 mm in diameter from steel R6M5. The thickness of the deposited layer was from 4 to 5 μm. The endurance of the drill bits with PCCC coatings was more than doubled.
Thus, the PCCC have a favorable effect on the quality of the surfaces of tools. Specifically, they “cure” the microflaws in the material (Fig. 3) without changing the initial geometry of the cutting part of the tool even when this geometry is complex. This is confirmed by the authors of [3, 6, 8, 12–14].
The advantages of the process of deposition of PCCC in an atmosphere of an inert gas over the traditional vacuum processes are as follows:
-
simplicity of the equipment;
-
no need for the expensive vacuum equipment;
-
elevation of the efficiency of utilization of the initial OMC due to heating the substrate by HFC, which eliminates deposition of the coating on the walls of the reactor;
-
high homogeneity of the coating under the action of HFC.
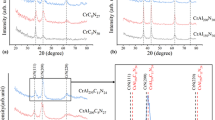
Subsequent annealing at 700°C for 40 – 50 min changes the microstructure of the coating.
Figure 4 presents the microstructure of PCCC prior to and after annealing in vacuum at 700°C for 45 min. After the annealing the microhardness increases and the layered pattern of the structure of the coating disappears (start of transition from an amorphous condition to a crystalline one). The diffraction patterns exhibit crystalline chromium and chromium carbides (Cr7C3, Cr23C6, Cr2C3).
The study of the phase transformations occurring under the heat treatment has shown that the growth in the microhardness is accompanied by considerable elevation of the internal stresses, which causes cracking of the coating.
The cracking develops in horizontal and vertical directions when the coated tools age for three months. The morphology of the surface after an additional heat treatment remains virtually unchanged. Propagation of cracks over the surface of a coating is presented in Fig. 5. The cracking reflects high internal stresses.
The data obtained allow us to infer that the microhardness of PCCC can be increased by the following measures:
-
annealing in vacuum right before using the coated tool;
-
choosing the cutting modes under which the temperature on the cutting edges does not exceed the red hardness of the tool. Then the microhardness of the tool will be increased in the cutting process due to “self-quenching” of the coating on the tool. Long-term breaks in operation (over 30 days) are undesirable due to the appearance of enhanced internal stresses in the coating, which cause cracking and degradation of the positive properties of the deposited coating.
The parameters mentioned can be controlled by using a lubricating-cooling liquid or by the cutting speed.
CONCLUSIONS
Deposition of PCCC is a controllable process, i.e., the properties of the coating can be specified by choosing the regime of the deposition by varying the conditions of the process and of the subsequent heat treatment. Additives of organic compounds may be used to control the cooling rate, the chemical and phase compositions, and the structure of the formed layers. Deposition of PCCC in an inert gas and heating of the substrate by HFC makes it possible to avoid the use of expensive vacuum equipment and to shorten the duration of the process.
References
V. A. Il’in and A. V. Panarin, “Pyrolytic chromium carbide coating (production process, equipment, properties),” Izv. Samarsk. Nauch. Tsentra Ross. Akad. Nauk, 13[4(2)], 357 – 360 (2013).
A. P. Gorovoy and P. M. Cherkasov, “Heterophase nanocrystalline coatings deposited by thermal decomposition of vapors of bis-arene chromium compounds,” Uproch. Tekhnol. Pokr., No. 9, 19 – 23 (2005).
L. L. Ivanov, Advancement of the Process of Depositing Pyrolytic Chromium Coatings for Hardening Tools, Author’s Abstract of Candidate’s Thesis [in Russian], Moscow (1988), 21 p.
A. S. Luzin, V. B. Polikarpov, N. V. Feldman, and L. S. Shustova, “Morphology and microstructure of coatings obtained by pyrolysis of metallization compositions based on the “Barkhos” organochromium liquid,” in: Mater. IV All-Union Workshop on Application of Organometallic Compounds for Depositing Inorganic Coatings and Materials [in Russian], Institute for Organometallic Chemistry of the USSR Academy of Sciences, Gorky (1989), pp. 53 – 61.
S. V. Boilo, Development of the Process of Deposition of Pyrolytic Chromium Coatings in Ammonia Atmosphere, Author’s Abstract of Candidate’s Thesis [in Russian], Moscow (1990), 21 p.
V. I. Yurshev, Development of the Process of Deposition of Pyrolytic Chromium Coatings and Equipment under Pulsed Action of Glow Discharge, Author’s Abstract of Candidate’s Thesis [in Russian], Moscow (1991), 205 p.
V. A. Kostenkov, “Reaction equipment for depositing coatings on articles of various configurations and sizes by pyrolysis of organometallic compounds,” in: Application of Organometallic Compounds for Creating Inorganic Coatings and Materials [in Russian], Nauka, Moscow (1986), pp. 180 – 201.
V. A. Kostenkov and V. N. Krasheninnikov, “Operating properties of pyrolytic chromium carbide coatings,” in: Application of Organometallic Compounds for Creating Inorganic Coatings and Materials [in Russian], Nauka, Moscow (1986), pp. 234 – 243.
V. A. Vasin, V. A. Nevrovskii, V. F. Sokolov, and A. D. Yurchenko, A Method for Depositing Protective Coating on the External Surface of Long-Length Metallic Articles, RF Patent 2169793, MPK7 C23C16/18, No. 99120982/02 [in Russian], Appl. 01.10.1999, Publ. 27.06.2001.
V. A. Vasin, L. A. Shabolinskaya, O. V. Somov, V. A. Pashkin, Horst Linn, A Method for Depositing Pyrolytic Chromium Carbide Coatings on the Surface of Cast Iron Parts, RF Patent 2188877, MPK7 C23C16/02, C23C16/32, No. 2000112356 [in Russian], Appl. 18.05.2000, Publ. 10.09.2002.
V. D. Aleksandrov, Surface Hardening of Aluminum Alloys, Author’s Abstract of Doctoral’s Thesis [in Russian], Moscow (2002).
I. S. Belashova, Surface Hardening Of Tool Steels, Author’s Abstract of Doctoral’s Thesis [in Russian], Moscow (2004), 384 p.
V. S. Repyakh, Development of a Process of Deposition of Pyrolytic Chromium Coatings at Atmospheric Pressure, Author’s Abstract of Candidate’s Thesis [in Russian], Orenburg, OGU (2005) 143 p.
S. A. Krokhmal’ and T. N. Zueva, “Deposition of pyrolytic chromium carbide coatings on internal surfaces of extended channels,” Vestn. Natsion. Tekh. Univ. “Kharkov Politekh. Inst.” Khim., Khim. Tekhnol., Ekologiya, No. 41, 29 – 38 (2008).
A. M. Slushkov and A. P. Timofeev, A Method for Depositing Protective Coatings on the Aluminum Flange of Aircraft Fuselage Antenna, RF Patent 2433210, MPK7 C23C16/18, C23C4/08 [in Russian], Appl. 29.06.2009, Publ. 10.01.2011.
Yu. G. Pokrovskii, A. A. Mikhailov, and A. I. Kostylev, “Deposition of pyrolytic coatings from ‘Barkhos’ liquid using additives of tin OMC,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Nizhny Novgorod (1991), Part 1, p. 97.
A. I. Kostylev, Yu. G. Pokrovskii, A. A. Mikhailov, and V. G. Shumkov, Vapor–gas Mixture for Pyrolytic Deposition of Chromium-Base Protective Coatings, SSSR Inv. Certif. 1453950, MKI3 C23C16/18, No. 4177315/02 [in Russian], Appl. 07.01.1987, Publ. 30.06.1994.
V. A. Il’in, V. V. Semenychev, E. V. Tyurikov, and A. V. Panarin, A Method for Depositing a Wear-Resistant Coating on Titanium Alloys, RF Patent 2449053C1, MPK7 C23C16/32, No. 2011112953 [in Russian], Appl. 05.04.2011, Publ. 27.04.20012.
B. I. Petrov, S. A. Vysotskii, and A. V. Lysenkov, “Effect oxygen-containing organic additives on thermal decomposition of OMC,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Inst. of Organometallic Chemistry of the USSR Academy of Sciences, Nizhny Novgorod, (1991), Part 1, pp. 94 – 95.
P. N. Akol’zin, A. S. Luzin, V. B. Polikarpov, and E. B. Raikova, “Hardening the surface of press molds with pyrolytic chromium carbide coatings,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Inst. of Organometallic Chemistry of the USSR Academy of Sciences, Nizhny Novgorod (1991), Part 1, pp. 107 – 108.
V. A. Varyukhin, G. M. Vlasov, N. M. Semenov, et al., “Novel ether-based metallization activators,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Inst. of Organometallic Chemistry of the USSR Academy of Sciences, Nizhny Novgorod, (1991), Part 1, p. 96.
V. F. Sokolov, A. D. Yurchenko, A. S. Luzin, et al., “Composite coatings from chromium and molybdenum OMC,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Inst. of Organometallic Chemistry of the USSR Academy of Sciences, Nizhny Novgorod, (1991), Part 1, pp. 101 – 103.
V. A. Pashkin, V. N. Krasheninnikov, V. A. Kostenkov, and N. V. Markin, A Method for Depositing Pyrolytic Chromium Carbide Coating, USSR Inv. Certif. 1798378, MKI3 C23C16/32, No. 4805053 [in Russian], Appl. 25.01.1990, Publ. 28.02.1993.
V. N. Krasheninnikov, V. A. Pashkin, V. A. Kostenkov, et al., “Possibilities of deposition of chromium pyrocarbide on parts from aluminum alloy AK-4-1-T1,” in: Abs. Rep. VI All-Union Conf. on Application of Organometallic Compounds for Creation of Inorganic Coatings and Materials [in Russian], Inst. of Organometallic Chemistry of the USSR Academy of Sciences, Nizhny Novgorod, (1991), Part 1, pp. 101 – 103.
L. M. Treiger, A Method for Removing Flaws in the Masking Coating of Photoplate, RF Patent 2017190C1, MPK7, G03F1/00, No. 5017487/21 [in Russian], Appl. 20.12.1991, Publ. 30.07.1994.
A. P. Gorovoy, V. M. Strulev, A. Al-Avar, “Modification of structure and phase composition of pyrolytic chromium coatings,” in: Resource-Saving Technology of Surface Hardening of Machine Parts, Coll. Works [in Russian], MADI, Moscow (1987), pp. 76 – 83.
B. G. Gribov, G. A. Domrachev, B. V. Zhuk, et al., Deposition of Films and Coatings by Decomposition of Organometallic Compounds [in Russian], Nauka, Moscow (1986), 322 p.
N. P. Kuz’mina and O. V. Kotova, Chemical Deposition of Films of Ordinary and Compound Oxides from Vapors of Organometallic Compounds (MOCVD) [in Russian], Izd. MGU Im. M. V. Lomonosova, Moscow (2011), 40 p.
V. V. Afonin and I. N. Akulinin, “Device for raising the wear resistance of contact materials of electrical apparatuses,” Vestn. TGTU, 18(2), 467 – 470 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 2, pp. 48 – 52, February, 2015.
Rights and permissions
About this article
Cite this article
Yurshev, V.I., Mukatdarov, R.I. & Yurshev, I.V. Surface Hardening of Tools by Depositing a Pyrolytic Chromium Carbide Coating. Met Sci Heat Treat 57, 107–111 (2015). https://doi.org/10.1007/s11041-015-9845-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-015-9845-y