Abstract
Background
The rapidly increasing applications of zinc oxide nanoparticles (ZnO NPs) in various industries have led to growing concerns about their damaging influence on human health. The present research was designed to determine the protective action of vitamins (Vits) A, C and E on the heart toxicity induced by ZnO NPs.
Methods
Fifty-four male Wistar rats were allocated into 9 groups of 6 and then exposed to ZnO NPs (200 mg/kg), water (Control1), olive oil (Control2), Vit A (1000 IU/kg), Vit C (200 mg/kg), Vit E (100 IU/kg) and three groups were co-treated with ZnO and one of the Vits A, C or E. The oxidative stress situation was evaluated by measuring oxidative stress markers and the tissue antioxidant enzyme activity. Besides, the mRNA expression of Bcl-2 and Bax and caspase 3,7 activity were assessed. A histopathological examination was also performed to determine the rate of cardiac injury.
Results
The results indicated that co-administration of ZnO NPs and the aforementioned Vits significantly reduced the total oxidant status and lipid peroxidation relative to the ZnO group (P < 0.05). Furthermore, the supplementation of vitamins, notably Vit E, decreased the ZnO NPs-induced oxidative damage by enhancing the activity of antioxidant enzymes compared to the ZnO NPs-fed rats (P < 0.05). Data also showed the mitigating effects of Vits against ZnO NPs-mediated apoptosis by suppressing the ratio of Bax/Bcl-2 expression and caspase 3,7 activity.
Conclusion
This study highlights the protective role of Vits A, C and E against ZnO NPs cardiotoxicity, though at different levels of effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has many applications in medicine including pharmacy, implants, surgery, diagnostic tools and cancer treatment. Nanoparticles are defined as those smaller than 100 nanometers (between 1 and 100 nanometers). Due to their unique properties, nanoparticles are used in various industries [1]. Despite many applications, harmful biological effects of the nanoparticles have been reported [2, 3]. Among various metal oxides nanoparticles, zinc oxide nanoparticles (ZnO NPs) are found in a variety of commercial products, such as agriculture and the food industry. Several studies have investigated the toxic effects of ZnO NPs inducing cell damage, oxidative stress, DNA damage and apoptosis [4, 5]. Furthermore, their harmful effects have been reported on cardiac tissue in different species of animals where ZnO NPs induce oxidative stress [6, 7].
The human body neutralizes elevated oxidative stress by employing antioxidant defense mechanisms including enzymatic or non-enzymatic responses. The antioxidant enzymes consist of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) which can mitigate the adverse effects of reactive oxygen species (ROS) in living cells [8]. It is demonstrated that oxidative damage may lead to cell death by apoptosis or necrosis in the presence of nanoparticles [9].
Retinoic acid is an active form of Vit A which is allocated to the category of fat-soluble Vits and Vit C is an organic compound with antioxidant properties [10].
Vit E is known as a powerful antioxidant as it can scavenge free radicals, especially proxy radicals, and protect the cell membrane from oxidative damage [11].
There are many reports on the protective potential of Vits E and C in some toxicant-induced organ injuries via relieving oxidative stress and apoptosis [12, 13]. In this research, the anti-apoptotic and antioxidant properties of Vits A, C and E were investigated in the heart tissue of rats that were exposed to ZnO NPs.
Materials and methods
Chemicals
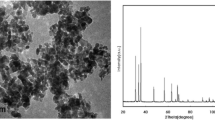
Vit A (all-trans retinoic acid), Vit E (α-tocopherol), Vit C (L-Ascorbic acid) as well as olive oil were obtained from Sigma-Aldrich (St. LOUIS). The purity of mentioned Vits was ≤ 98%, ≤ 96% and 99%, respectively. The ZnO NPs (20 nm) were procured from Iranian Nanomaterial’s Pioneers Company NANOSANY (Mashhad, Iran). Figure 1 A and B show transmission electron micrograph (TEM) and X-Ray Diffraction (XRD) pattern of ZnO NPs. Furthermore, size distribution of the ZnO NPs was shown in a previous publication [10].
Commercial test kits including (Total antioxidant capacity) TAC, TOS (Total oxidant species), MDA, GPx, SOD, CAT and caspase 3,7 as well as protease inhibitor cocktail were obtained from Kiazist Company (Hamedan, Iran).
Preparation of vitamins and ZnO NPs solutions
The stock solution of ZnO NPs (2 g/ml) was dispersed in distilled water for 10 min with the help of a sonicator (James ultra 5060D-H, UK). We prepared the desired concentration of NPs (200 mg/kg body weight) [14] before each use. According to the previous investigations, Vits A and E were suspended in olive oil to achieve the desired doses of Vits [15, 16]. The needed concentration of Vit C was dissolved in distilled water [17].
Experimental design and procedure
In this research work, 54 male Wistar rats (7–8 week of old and weighing approximately 150–200 g) were provided from Hamadan University of Medical Sciences (Hamadan, Iran), and housed in standard plastic cages. Standard food and drinking water were provided to them throughout the study. Standard conditions of temperature (23 ± 2ºC), normal humidity (50 ± 5%) and light (12 h of light and 12 h of darkness) were also preserved until the end of experiment and no rats died during treatment. The animals were randomly divided into 9 groups of 6. It should be mentioned that the groups receiving Vits were pre-treated with Vits one week before administration of ZnO NPs (by gavage). Administration of treatments were performed by oral gavage for 3 weeks. It is noteworthy that the water and olive oil groups were selected as controls due to the solvent of nanoparticles and Vits.
The grouping of experimental animals is provided below.
-
1.
Control 1: The rats were gavaged with 1 ml/day bi-distilled water for 3 weeks.
-
2.
Control 2: The rats were gavaged with 1 ml/day olive oil for 3 weeks.
-
3.
Vit A: The rats were gavaged with Vit A (1000 IU/kg) for 3 weeks.
-
4.
Vit C: The rats were gavaged with Vit C (200 mg/kg) for 3 weeks.
-
5.
Vit E: The rats were gavaged with Vit E (100 IU/kg) for 3 weeks.
-
6.
ZnO: The rats were gavaged with ZnO NPs (200 mg/kg) for 2 weeks.
-
7.
ZnO + Vit A: The rats were gavaged with ZnO NPs (200 mg/kg) and Vit A (1000 IU/kg) for 2 weeks.
-
8.
ZnO + Vit C: The rats were gavaged with ZnO NPs (200 mg/kg) and Vit C (200 mg/kg) for 2 weeks.
-
9.
ZnO + Vit E: The rats were gavaged with ZnO NPs (200 mg/kg) and Vit E (100 IU/kg) for 2 weeks.
Eventually, the rats were anesthetized and heart tissue was immediately isolated after sacrificing the rats. A part of tissue was separated, frozen in liquid nitrogen and then kept at -70 °C until use. Also, a portion of cardiac tissue was placed in 10% formalin and maintained for histopathological analysis.
Preparation of heart tissue lysate
First, recommended amount of tissue was separated. Then, the tissues were homogenized in PBS or the recommended buffer supplemented by protease inhibitor using a homogenizer. Next, the samples were placed on a shaker-incubator for 20 min at 4 °C and then centrifuged at 12,000 rpm, for 15 min at 4°C. The resultant supernatants were transferred to new tubes and kept at -80 °C for further experiments.
Determination of oxidative stress parameters
TAC level of cardiac lysates was measured following the protocol of company. In this method, cupric (Cu2+) is converted to copper (Cu+) in the presence of antioxidants. Finally, the produced color was measured at a wavelength of 450 nm using an ELISA reader.
TOS level was determined according to the procedure specified in the kit. In this experiment, oxidants of samples lead to convert ferrous (Fe2+) to ferric (Fe3 +) and produces color with the use of a chromogen. This color was recorded at 560 nm.
Oxidative stress index (OSI) was evaluated as the ratio of TOS to TAC and reported in arbitrary scale unit.
For determination of lipid peroxidation, malondialdehyde (MDA) content was assessed by colorimetric assay so that MDA reacts with thiobarbituric acid and the absorbance was recorded at 532 nm.
Determination of SOD, GPx and CAT activities
SOD activity of cardiac tissue was examined based on an enzymatic method according to kit manual. The activity of GPx was determined by a colorimetric assay using a coupling reaction along with the glutathione reductase enzyme and its coenzyme, NADPH. The activity of CAT was also measured using a colorimetric reaction following the instruction of company.
Determination of Bcl-2 and Bax genes expression by quantitative reverse transcription-PCR (qRT-PCR)
Total RNA from heart tissue was isolated using Kiazol reagent (KiaZist, Iran) as regards the manufacturer’s manual. Nano drop (One Thermo Scientific, USA) and 1% agarose gel electrophoresis were used to assess the concentration and integrity of the RNAs, respectively. Extracted RNAs were reverse transcribed into cDNA using the PrimeScriptTM RT reagent kit (Takara, Japan) following the kit’s protocol. Primers were designed with the aid of primer 3 software and are given in Table 1. The qRT-PCR reaction was carried out on a LightCycler®96 System (Roche, Germany). The expression level was calculated based on 2−ΔΔCT method [18] using β-actin as a reference gene.
Determination of caspase 3,7 activity
The measurement of caspase 3,7 activity was carried out by using p-nitroaniline (pNA) as a standard according to the protocol of commercial kit.
Histopathology
A full thickness piece of approximately 0.5 × 0.5 cm from left ventricle of heart of all experimental rats were cut and placed in 10% neutral buffered formalin. After fixation, the samples were dehydrated in increasing ethanol concentrations, cleared in xylene, and infiltrated and embedded in paraffin. A rotary microtome (Leica RM2255, Germany) was used to section the blocks at 5–6 μm thick. The sections were then stained with hematoxylin and eosin (H&E) and examined independently by a veterinary pathologist using a light microscope (Olympus CX41, Japan) equipped with a digital camera (Olympus DP25, Germany).
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 9 software (IBM, USA). The values were displayed as mean ± standard deviation (SD). The differences of means among multi-group data were analyzed using One-way analysis of variance (ANOVA). Any P value less than 0.05 was assigned as the threshold for significance.
Results
Oxidative stress parameters in cardiac tissue
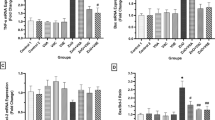
In the ZnO group, a significant enhancement of TAC was observed in comparison to the control (P < 0.001), However, the amount of TAC expressed a remarkable increase after consumption of Vits E, C and E relative to the ZnO NPs-exposed animals (P < 0.01) (Fig. 2A).
Oxidative stress markers in heart tissue of rats. (A) Total antioxidant capacity (TAC), (B) Total oxidative status (TOS), (C) Oxidative stress index (OSI) (D) Malondialdehyde (MDA) in the studied groups. Results are shown as mean ± SD. Asterisks show significant differences compared to the controls (Water and olive oil groups) (*P < 0.05) and number sign show significant differences compared to the ZnO group (# P < 0.05)
The content of TOS and MDA was meaningfully increased in the ZnO NPs-fed animals in comparison with the controls (P < 0.0001). Administration of Vits A, C and C could noteworthy prevent the enhancement of TOS and MDA compared to the ZnO NPs group (P < 0.01) (Fig. 2B and D).
OSI level showed a significant increase in the cardiac tissue of rats that were only treated with ZnO NPs compared to the controls (P < 0.001) though it was meaningfully decreased in the groups that were co-treated with ZnO NPs and Vits C or E compared to the animals that were only given ZnO NPs (P < 0.001) (Fig. 2 C).
Antioxidant enzyme activities in cardiac tissue
As it is shown in Fig. 3 A and B, the activity of CAT and GPx enzymes was declined after exposure to the ZnO NPs compared to the control groups (P < 0.001). Administration of Vits C and E remarkably increased the CAT and GPx activities in rats receiving Vits A, C and E + ZnO NPs as compared to the ZnO NPs group (P < 0.05). By contrast, supplementation of Vit A could not significantly increase the activity of GPx compared to the ZnO group (P > 0.05).
Activities of (A) glutathione peroxidase (GPx) (B) catalase (CAT) and (C) Superoxide dismutase in heart tissue of control and experimental rats. Results are shown as mean ± SD. Asterisks show significant differences compared to the controls (Water and olive oil groups) (*P < 0.05) and number sign show significant differences compared to the ZnO group (# P < 0.05)
The SOD activity was diminished in the ZnO group with respect to the control group (P < 0.001). Meanwhile, mitigating effects of Vits A, C and E increased the activity of SOD compared to the ZnO NPs-fed rats (P < 0.001) (Fig. 3 C).
According to the oxidative stress parameters, supplementation of Vit E has the most ameliorative effect on ZnO NPs-induced cardiotoxicity.
Changes in apoptotic gene expression
As shown in Fig. 4, a significant reduction of Bcl-2 expression along with up-regulation of Bax was observed in ZnO group with respect to the control (P < 0.005). In comparison to the ZnO NPs-treated rats, consumption of Vits A, C and especially E markedly compensate for the gene expression changes though the Bax and Bcl-2 expression is not completely restored to the control values.
Gene expression of (A) Bcl-2 associated X protein (Bax) (B) B-cell lymphoma 2 (Bcl-2), (C) Ratio of Bax to Bcl-2 expression and (D) Caspase 3,7 activity in the heart tissue of control and experimental rats. Results are shown as mean ± SD. Asterisks show significant differences compared to the controls (Water and olive oil groups) (*P < 0.05) and number sign show significant differences compared to the ZnO group (# P < 0.05)
Caspase 3,7 activity in cardiac tissue
The activity of caspase 3,7 was increased in ZnO group compared with the controls (P < 0.001). Treatment with Vits A, C and E could prevent the increased activity of caspase 3,7 relative to the ZnO NPs-fed rats (P < 0.05) (Fig. 4D).
Histopathology
Microscopic examination of the heart tissue of control and vitamin receiving groups showed typical histology of the organ (Fig. 5A-E). However, disorganization of tissue structure, sarcoplasmic degeneration and necrosis, the presence of hypercontracted segments and fragmentation of myocardiocytes as well as tissue congestion and inflammation along with leukocyte infiltration were pathologic features of the group administered with ZnO NPs (Fig. 5F). While vitamins A and C had little if any ameliorating effect on the damage caused by ZnO NPs (Fig. 5G H), the administration of Vit E was found to have remarkable preservative effects against the nanoparticles (Fig. 5I).
Light micrographs of heart tissue from normal and treated rats. (A) Normal histology of cardiac tissue showing the typical morphology of myocardial cells with striation pattern (large arrow) and normal nuclei (small arrow) in the middle of myocardiocytes of the control group. (B-E) The cardiac histology of rats administered with olive oil or Vits A; C and E is normal. (F) Disarrangement of tissue, degeneration and fragmentation of myocardiocytes (large arrow), the presence of hypercontracted segments (asterisk), fading of the myofibrillar striations, microvascular congestion (small arrow), inflammation and leukocyte infiltration (arrowhead) are the main pathologic changes in the rats received ZnO NPs. (G, H) The groups administered with ZnO NPs and Vit A/or Vit C show tissue damage in the form of mycardiocyte degeneration (large arrow), hypercontracted segments (asterisks), disappearance of the striation, microvascular congestion (small arrow) and infiltration of inflammatory cells (arrowhead). (I) Microscopic features of tissue damage such as cellular degeneration, vascular congestion and the presence of leukocytes in the cardiac tissue are substantially decreased in the group administered with ZnO NPs and Vit E. Scale bar = 20 μm, 400× magnification, Hematoxylin & Eosin
Discussion
Our results revealed that cardiac oxidative stress is elevated following administration of ZnO NPs as the level of TOS, MDA and OSI were augmented in rats that were only received ZnO NPs. In line with this, it is widely reported that ZnO NPs cause oxidative damage in the tissues of rats [19, 20]. In the present work, the observations highlight that pretreatment with Vits A, C and E might attenuate the heightened level of oxidative stress caused by ZnO NPs in the heart tissue of rats. Recently, the administration of Vits or other antioxidants has been proven to have prevented NPs-induced toxicity [21,22,23].
Moreover, it was reported that the supplementation of Vit E prevents nephrotoxicity and lipid peroxidation induced by gold nanoparticles [24]. Consistent with this report, our findings indicated strong antioxidant properties of Vits A, C and E against oxidative stress through the enhancement of SOD, GPx, CAT activities and TAC levels in the rats receiving Vit and ZnO NPs. Furthermore, we found Vit E as the most effective Vit compared with Vit C and A in oxidative stress conditions.
In addition to oxidative damage, ZnO NPs induce apoptosis in the heart tissue of rats. According to our results, ZnO NPs cause an upregulation of Bax and reduce the expression of Bcl-2. Besides, a marked elevation was observed in caspase 3,7 activity in the animals intoxicated with ZnO NPs. These findings are based on previous studies [4, 25] that revealed the involvement of Bax/Bcl2 in ZnO NPs-induced apoptosis. Furthermore, this and earlier research have found that apoptotic pathways are activated following exposure to nanoparticles [26, 27]. Considering the mentioned issues, we evaluated the anti-apoptotic effects of Vits A, C and E in rats receiving ZnO NPs. Our results showed that the apoptosis rate is mitigated by co-administration of ZnO NPs and Vits A, C, and notably E which is in agreement with previous studies [28,29,30,31].
Generally, Bax and Bcl-2 as pro- and anti-apoptotic proteins are involved in apoptosis signaling pathways. In normal conditions, the balance between Bax and Bcl-2 expression prevents cell death or apoptosis [32]. However, mitochondria rapidly lose their normal function under damage conditions and the membrane potential (ΔΨm) is collapsed leading to the release of cytochrome c, which activates the caspases to trigger apoptosis [26].
In the present study, histopathological features of heart sections were correlated with biochemical findings, which showed that ZnO NPs exposure exerts negative alterations in the heart tissue of rats. In contrast, the structure modifications caused by ZnO NPs were mitigated by Vit’s treatment, suggesting that Vits can protect the heart from ZnO NPs-induced damage. Previous data also demonstrated that Vit E administration can ameliorate cardiotoxicity [33]. Moreover, the antioxidant and anti-apoptotic properties of Vit C were previously reported, which can modulate cardiac damage [34].
Importantly, previous studies indicated that nutrient molecules can interact with nanoparticles and affect their toxicity though it somewhat depends on the types of nanoparticles as well as nutrient molecules. Additionally, nutrient molecules might alter the release of Zn ions which affect the toxicity of ZnO NPs [35, 36].
Accordingly, the current work suggests that oral exposure to Vits A, C and E has modulatory effects on cardiotoxicity induced by ZnO NPs.
Conclusion
The present research showed that ZnO NPs cause oxidative stress and apoptosis in cardiac tissue, as the level of oxidative markers including TOS, OSI, MDA was augmented in ZnO NPs group. Moreover, the activity of antioxidant enzymes was lowered in rats receiving ZnO NPs. We found that ZnO NPs trigger apoptosis in a cell via the activation of caspase 3,7 and increasing Bax to Bcl-2 ratio. Importantly, the cardiac injury was restrained following the administration of Vits A, C and mainly E by alleviating oxidative stress and apoptosis. Since industries such as cosmetics have made extensive use of nanoparticles, in this research, we found that the consumption of Vits can prevent ZnO NPs-induced toxicity. Thus, it seems that taking Vit’s supplementation along with additives such as ZnO NPs can prevent the side effects caused by nanoparticles.
References
Brown SC et al (2013) Toward advancing nano-object count metrology: a best practice framework. Environ Health Perspect 121(11–12):1282–1291
Cao Y et al (2018) A review of cardiovascular toxicity of TiO2, ZnO and Ag nanoparticles (NPs). Biometals 31(4):457–476
Sengul AB, Asmatulu E (2020) Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett 18(5):1659–1683
Daei S et al (2022) The Alleviative Efficacy of Vitamins A, C, and E Against Zinc Oxide Nanoparticles-Induced Hepatic Damage by Reducing Apoptosis in Rats.Biological Trace Element Research, : p.1–9
Subramaniam VD et al (2019) Health hazards of nanoparticles: understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem Toxicol 42(1):84–93
Nagarajan M et al (2022) Exposure to zinc oxide nanoparticles (ZnO-NPs) induces cardiovascular toxicity and exacerbates pathogenesis–Role of oxidative stress and MAPK signaling. Chemico-Biol Interact 351:109719
Baky NA et al (2013) Induction of inflammation, DNA damage and apoptosis in rat heart after oral exposure to zinc oxide nanoparticles and the cardioprotective role of α-lipoic acid and vitamin E. Drug Res 63(05):228–236
Daei S et al (2022) Effects of gold nanoparticles on oxidative stress status in bladder cancer 5637 cells. Folia medica 64(4):641–648
Sardaro N et al (2019) Oxidative stress and oral mucosal diseases: an overview in vivo. 33:289–2962
Bayat M et al (2021) The protective effects of vitamins A, C, and E on zinc oxide nanoparticles (ZnO NPs)-induced liver oxidative stress in male Wistar rats. Drug and Chemical Toxicology, pp 1–10
Afshari-Kaveh M et al (2021) The protective effects of vitamins A and E on titanium dioxide nanoparticles (nTiO2)-induced oxidative stress in the spleen tissues of male Wistar rats. Biol Trace Elem Res 199(10):3677–3687
Xie D et al (2022) Vitamin Supplementation Protects against Nanomaterial-Induced Oxidative Stress and Inflammation Damages: A Meta-Analysis of In Vitro and In Vivo Studies. Nutrients 14(11):2214
Abdelhalim MAK et al (2018) Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int J Nanomed 13:7931
Abbasalipourkabir R et al (2015) Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem Toxicol 84:154–160
Hisamori S et al (2008) All-trans‐retinoic acid ameliorates carbon tetrachloride‐induced liver fibrosis in mice through modulating cytokine production. Liver Int 28(9):1217–1225
Abdelazim SA et al (2015) Potential antifibrotic and angiostatic impact of idebenone, carnosine and vitamin E in nano-sized titanium dioxide-induced liver injury. Cell Physiol Biochem 35(6):2402–2411
Aly N et al (2010) Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pestic Biochem Physiol 97(1):7–12
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25(4):402–408
Xiao L et al (2016) Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem Toxicol 90:76–83
Farokhcheh M et al (2021) Geraniol improved memory impairment and neurotoxicity induced by zinc oxide nanoparticles in male wistar rats through its antioxidant effect. Life Sci 282:119823
Wani MR, Maheshwari N, Shadab G (2021) Eugenol attenuates TiO2 nanoparticles-induced oxidative damage, biochemical toxicity and DNA damage in Wistar rats: an in vivo study, vol 28. Environmental Science and Pollution Research, pp 22664–22678. 18
Shotop YM, Al-Suwiti IN (2021) The possible role of vitamins E and C in reducing the toxicity of copper nanoparticles in the kidney and liver of the rats (Rattus norvegicus). J King Saud University-Science 33(2):101357
Kumar M et al (2022) Toxicity ameliorative effect of vitamin E against super-paramagnetic iron oxide nanoparticles on haemato-immunological responses, antioxidant capacity, oxidative stress, and metabolic enzymes activity during exposure and recovery in Labeo rohita fingerlings Aquaculture International, : p. 1–29
Abdelhalim MAK et al (2020) The protective roles of vitamin E and α-lipoic acid against nephrotoxicity, lipid peroxidation, and inflammatory damage induced by gold Nanoparticles. Int J Nanomed 15:729
Efendic F et al (2022) Histological and biochemical apoptosis changes of female rats′ ovary by Zinc oxide nanoparticles and potential protective effects of l-arginine: An experimental study. Annals of Medicine and Surgery 74:103290
Aouey B et al (2022) Silica Nanoparticles Induce Hepatotoxicity by Triggering Oxidative Damage, Apoptosis, and Bax-Bcl2 Signaling Pathway. Biol Trace Elem Res 200(4):1688–1698
Meng X et al (2021) Lycopene Alleviates Titanium Dioxide Nanoparticle-Induced Testicular Toxicity by Inhibiting Oxidative Stress and Apoptosis in Mice.Biological Trace Element Research, : p.1–13
Fang J et al (2021) Protective effect of vitamin e on cadmium-induced renal oxidative damage and apoptosis in rats. Biol Trace Elem Res 199(12):4675–4687
Abdel-Daim MM, Abdeen A (2018) Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem Toxicol 114:69–77
Kong L et al (2019) Mechanisms underlying nickel nanoparticle induced reproductive toxicity and chemo-protective effects of vitamin C in male rats. Chemosphere 218:259–265
Navidhamidi M et al (2022) Therapeutic Potential of Combined Therapy of Vitamin A and Vitamin C in the Experimental Autoimmune Encephalomyelitis (EAE) in Lewis Rats Molecular Neurobiology, : p. 1–20
Haddadi P, Mahdavi M, Rahbarghazi R (2020) Down-regulation of Bcl2 and Survivin, and up-regulation of Bax involved in copper (II) phenylthiosemicarbazone complex-induced apoptosis in leukemia stem-like KG1a cells. Process Biochem 92:190–196
Aboubakr M et al (2020) L-Carnitine and vitamin E ameliorate cardiotoxicity induced by tilmicosin in rats. Environ Sci Pollut Res 27(18):23026–23034
Aşcı H et al (2016) Protective effects of aspirin and vitamin C against corn syrup consumption-induced cardiac damage through sirtuin-1 and HIF-1a pathway. Anatol J Cardiol 16(9):648
Moradi M et al (2022) Interactions between nanoparticle-based food additives and other food ingredients: A review of current knowledge. Trends in Food Science & Technology
Cao Y (2022) Nutrient molecule corona: an update for nanomaterial-food component interactions.Toxicology, : p.153253
Funding
This study has been derived from a MSc thesis at Bandar Abbas University of Medical Sciences, Iran. The study was funded by Vice-chancellor for Research and Technology, Hormozgan University of Medical Sciences (No. 980343).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedure was approved at the Faculty of Medicine, at Hormozgan University of Medical Sciences (HUMS), Bandar Abbas, Iran. The research was conducted according to the guidelines for the care and use of laboratory animals of HUMS IR.HUMS.REC.1398.425.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekhlasian, A., Eftekhar, E., Daei, S. et al. The antioxidant and anti-apoptotic properties of vitamins A, C and E in heart tissue of rats exposed to zinc oxide nanoparticles. Mol Biol Rep 50, 2357–2365 (2023). https://doi.org/10.1007/s11033-022-08103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08103-8