Abstract
The increasing application of titanium dioxide nanoparticles (NTiO2) in life and the toxicity potential of these nanoparticles have raised concerns about their detrimental effects on human health. This study was conducted to investigate the hepatoprotective effects of vitamin E and vitamin A against hepatotoxicity induced by NTiO2 in rats. Thirty-six male Wistar rats were randomly divided into six groups of six rats each. Intoxicated group received 300 mg/kg NTiO2 for two weeks by gavage. Groups treated with vitamin E (100 IU/kg), vitamin A (100 IU/kg) and mixture of these vitamins were orally administered for 3 weeks (started 7 days before NTiO2 administration). In order to investigate the redox changes, total antioxidant capacity, total oxidant status, and lipid peroxidation were determined in liver tissue as well as activity of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and catalase. In addition, inflammatory responses were assessed by measuring the expression of NF-κB (mRNA) and TNF-α (mRNA and protein). Histopathological analysis and measurement of liver enzymes (ALP, ALT, AST, and LDH in serum) were also done to determine hepatic injury. In liver, NTiO2 caused hepatic injury, redox perturbation, and reduction of antioxidant enzymes and elevation of inflammatory mediators, significantly. However, treatment with vitamins was able to significantly ameliorate these alterations. This study highlights the antioxidant and anti-inflammatory properties of vitamins A and E against toxicity of NTiO2 and poses the use of these vitamins to mitigate the toxic effects of this nanoparticles in NTiO2-contained products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With progression in the field of nano-engineered materials, there is a growing demand on the use of these particles in different industries. Some physicochemical properties of nanomaterials such as small size, large surface area, redox potential, photocatalytic, and quantum properties make them a useful tool in the relevant industries [1,2,3,4]. Titanium dioxide nanoparticles is one of the most broadly applied compounds in the medical (photosensitizer for photodynamic therapy [2, 5] drug delivery [5, 6], biomedical ceramics [7], and implant biomaterials [8]), food [9, 10], cosmetics (sunscreen, toothpaste and personal care products [9, 11, 12]), sterilization [13, 14] and paint industry. Its prominent physical and chemical properties include the bright white color (which causes whitening of toothpaste, cosmetics and food), high resistance, photocatalytic property (which produces free radicals when exposed to UV light and is used in sterilization devices), redox potential etc. [9, 15].
Regarding the extensive application of NTiO2 in everyday life, the question arises as to whether this nanoparticles has detrimental effects on human health.
Human exposure to NTiO2 may occur through the skin, inhalation, and ingestion. Studies have revealed that pulmonary and digestive exposures are the predominant routes of toxicity [16]. Since NTiO2 penetrate into the body, they may find their way into the circulatory system and reach other organs, which, according to the concentrations determined, include the liver, spleen, lung, brain, and testis, respectively [17, 18]. It has been considered that liver is the target organ for the toxic effects of xenobiotics such as nanoparticles [19,20,21]. The results of previous studies indicate that oral administration of NTiO2 increases the content of liver damage biomarkers such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH), in the serum and also MDA as a marker of lipid peroxidation of liver in rodents [22, 23].
Previous studies showed that exposure to NTiO2 can lead to induction of toxicity which includes damage to membrane integrity [24], protein instability and oxidation [25, 26], nucleic acid damage [27, 28], cell toxicity through reactive oxygen species (ROS) generation [29, 30], and disturbance in the supply of energy and the electron transport chain [29].
According to the investigations of Cui et al. [80], the signaling cascade proposed for hepatic toxicity induced by NTiO2 is: TLRs → NIK → IκB kinase → NF-ΚB → TNF-α → inflammation → apoptosis → liver injury [31]. Similarly, Chen et al. [32] reported that NF-κB, which is a key transcription factor involved in transduction of signals that promote inflammation and cell death, is activated by TLRs and finally induces the expression of inflammatory cytokines like TNF-α, IL10, IL12A, and IL2 [32].
Although NTiO2 can also cause cellular damage without producing ROS, generation of ROS is the main activity of NTiO2 induced cellular damage. It has been recognized that NTiO2 is deposited on the cell surface or within the subcellular organelles and induces oxidative stress signaling cascades that eventually leads to cell oxidative damage [33]. The main factors of NPs involved in ROS generation include: (a) pro-oxidant groups on the reactive surface of NPs; (b) active redox cycling on the surface of NP due to transition metal-based NP; and (c) interactions of NP with cells [28, 34]. On the reactive surface of NPs, the electron donor or acceptor interact with O2 to form O2−· which in turn generates additional ROS via Fenton-type reactions [35]. These events also occur within an aqueous environment like body fluids. Study conducted by electron spin resonance methods showed that excited NTiO2 can produce radical species such as hydroxyl radical (OH·) by oxidation of H2O and superoxide radical (O2−·) by reduction of O2 [30]. It is well established that excessive accumulation of ROS by affecting the biological macromolecules causes mutation, cancer, aging, apoptosis, and necrosis. In addition, it triggers the activation of cytokines and pro-inflammatory mediators through the MAPK and NF-κB signaling pathway which controls the transcription of inflammatory genes such as IL-1β, IL-8, and TNF-α. Through this, it is associated with many disorders such as cancer, cardiovascular, and neurodegenerative diseases [36,37,38,39]. One strategy of the human body to overcome the surplus ROS response is to employ enzymatic or non-enzymatic antioxidant defense system. Major antioxidant enzymes include superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase which alleviates toxicological effects of ROS in living systems.
Alpha-tocopherol (AT) is an active form of vitamin E which has antioxidant and anti-inflammatory properties. Vitamin E acts as a strong scavenger of free radicals, especially radical peroxyl, which prevents peroxidation of unsaturated fatty acids in the cell membrane [40,41,42,43]. These functions of vitamin E were examined in many studies and the results indicate the effect of vitamin E on suppression of inflammatory and oxidative stress response by reduction in IL-1, TNF-α, IL-6, low density lipoprotein oxidation susceptibility, ROS production in monocyte, TGF-β1, expression of VEGF (Vascular endothelial growth factor), and HSP70 [44,45,46,47,48,49].
Vitamin A plays important roles in different cellular processes including growth, differentiation, vision, and reproduction [50, 51]. All-trans retinoic acid (AtRA) which is the most active form of vitamin A exhibits anti-cancer, anti-inflammatory, and anti-oxidant effects [52, 53]. It is able to reduce inflammatory cytokines (e.g. IL-6, TNF-α, TGF-β1), prevent collagen formation and induction of anti-oxidant enzyme like SOD through modulating the activation of transcription factors such as NF-κB, P38 MAPK, and Akt [54,55,56].
With regard to the toxicity of NTiO2 and its proposed mechanism in the liver, it is necessary to introduce effective antioxidant and anti-inflammatory compounds which can reduce the toxic effects induced by NTiO2. In this study, a toxic dose of NTiO2 (300 mg/kg body weight) was used to evaluate whether vitamin A, vitamin E, and the combination of these vitamins could restrain the toxicity of NTiO2 in the liver of rats.
Materials and methods
Treatment agents
AtRA (≤ 98%), α-tocopherol (≤ 96%), and olive oil were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). According to previous studies, Vitamin A and vitamin E were dissolved in olive oil to obtain the desired concentrations of 1000 IU/kg and 100 IU/kg body weights, respectively [49, 56].
TiO2 nano particles
Titanium dioxide nanoparticles (20 nm) was purchased from Iranian Nanomaterials Pioneers Company, NANOSANY (Mashhad, Iran) which contains 80 vol% anatase + 20 vol% rutile. Table 1 presents the characteristics of Titanium dioxide nanoparticles and the transmission electron micrograph (TEM) and crystal characteristics of TiO2 nanoparticles are shown in Figs. 1 and 2, respectively.
Preparation of TiO2 nanoparticles suspension
There is a lack of information about the exact duration and dose of human exposure to titanium dioxide nanoparticles. But what’s clear is that the amount of human exposure with this nanoparticle is very variable [57]. Take the example of paint industry staff, who expose to a high level of NTiO2 by the rout of respiratory system [58].On the other hand, according to a study by Rompelberg et al. [59], children (2–6 years) of Dutch population has been reported to intake 0.67 mg/kg bw/day [59]. Since human exposure to NTiO2 has inevitable consequences, we have to study the mechanism of toxicity as well as finding a way to tackle these detrimental effects.
According to previous studies the concentration of NTiO2 to induce acute toxicity in rodent has been reported from 50 [16, 60] to 500 mg/kg body weight [61]. Although some studies have shown that toxicity occurs at lower concentrations, but in such quantities, toxicity for a wide range of parameters cannot be generalized. Khorsandi et al. claimed that NTiO2 at the concentration of 300 mg/kg could alter biochemical and histological parameters in Wistar rats [23]. So that we decided to use 300 mg/kg of NTiO2 to ensure the induction of definite acute toxicity in rats.
A stock suspension at 2 g/ml concentration was prepared by suspending TiO2 nanoparticles in bi-distilled water. Before each administration, the stock solution was diluted in bi-distilled water to obtain the desired concentration (300 mg/kg body weight), and then placed in a sonication device for 10–15 min in order to disperse nanoparticles and prevent agglutination.
Experimental design and procedure
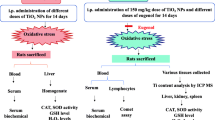
Thirty-six adult male Wistar rats aged 6–8 weeks, weighing 180–200 g were purchased from Pasteur Institute of Iran, IRAN. The animals were housed two rats per plastic cage and allowed to acclimatize under standard conditions (12 h light/dark cycles, relative humidity of 50 ± 5% and 22 ± 3 °C) for 1 week before the experiment. These conditions were maintained until the end of the experiment. The rats were given free access to distilled water and commercialized food throughout the experiment. The animals were divided into six groups of six animals each.
- Group A:
-
Control group 1, received 1 ml/day bi-distilled water by gavage for 14 days.
- Group B:
-
NTiO2 group, received 300 mg/kg body weight by gavage for 14 days.
- Group C:
-
NTiO2 + vitamin E, received 300 mg/kg NTiO2 and 100 IU/kg α-tocopherol by gavage.
- Group D:
-
NTiO2 + vitamin A, received 300 mg/kg NTiO2 and 1000 IU/kg AtRA by gavage.
- Group E:
-
NTiO2 + vitamins A and E, received the afore-mentioned concentration of NTiO2, vitamin A, and vitamin E.
- Group F:
-
Control group 2, received 1 ml/day olive oil by gavage for 14 days.
By using two solvents in this experiment, one used to prepare a titanium solution and the other used to dissolve fat-soluble vitamins, in this study two control groups of distilled water and olive oil were considered.
One the day after the last administration, after blood sampling, the rats were sacrificed by cervical dislocation under ether anesthesia, and the livers from each animal were quickly removed. Small pieces of liver (left lateral lobe) were stored separately in a deep freezer (− 80 °C) for the total antioxidant capacity (TAC), total oxidant status (TOS), lipid peroxidation (MDA), catalase, SOD, GPx, enzyme linked immunosorbent assay (ELISA), and reverse transcription polymerase chain reaction (RT-PCR) assay. Other pieces (from left lateral lobe) were fixed in 10% buffered formalin for histopathologic assay. Blood samples were collected by cardiac puncture, using 23 or 26 G needles. The whole blood was allowed to clot at room temperature and centrifuged at 1000 g for 10 min, and serum were separated and analyzed for ALT, AST, ALP, TAC, and LDH.
Measurement of liver enzymes in serum
Animal serum was analyzed for ALT, AST, ALP, and LDH using standard diagnostic Kit (Pars Azmun, Iran).
Measurement of TAC, TOS, and MDA
TAC was estimated according to the manufacturer’s instructions using (Kiazist, Iran). In this method (CUPRAC assay), the cupric (Cu+2) is reduced into cuprous (Cu+1) in the presence of antioxidants and produces color in the presence of chromogen. This color is absorbed at 450 nm. The advantage of this method is the measurement of antioxidants such as thiol, which cannot be identified in methods such as FRAP (which works with iron).
The oxidation of ferrous ion to ferric ion correlated with the number of oxidant species in acidic pH was used to evaluate the TOS in liver homogenate. The ferric ion content was determined by xylenol orange [62].
Malondialdehyde (MDA) as a lipid peroxidation indicator in liver homogenate was assessed fluorometrically using thiobarbituric acid method [63].
Measurement of catalase activity
Catalase enzyme catalyzes the neutralization of hydrogen peroxide to H2O. Catalase activity in liver homogenate was determined based on the manufacturer’s instruction using (Kiazist, Iran). In this experiment, the catalase progresses the reaction in presence of its substrate, and then stops by means of its inhibitor with a characteristic yellow color in an inverse manner that is absorbed at 405 nm. Specific activity of catalase is expressed as nmol/min/mg tissue or mU/mg protein.
Measurement of superoxide dismutase (SOD) activity
SOD catalyzes the dismutation of superoxide anion (O2−) to H2O2 and O2. The activity of SOD in liver homogenate was assessed using ZellBio GmbH kit (SOD assay kit, ZellBio GmbH, Germany). In this method, assay kit uses the superoxide anion for conversion to hydrogen peroxide and oxygen under enzymatic reaction conditions. Finally, the product forms a Chromogen to a color which is measured colorimetrically at 420 nm. Specific activity of SOD is expressed as U/mg protein.
Measurement of glutathione peroxidase (GPx) activity
GPx is a hydroperoxidase-reducing enzyme that contributes in reducing H2O2 and lipid hydroperoxides to H2O and lipid alcohols, respectively and also catalyzes GSH to GSSG simultaneously.
The activity of GPx in liver homogenate was estimated using ZellBio GmbH kit (GPx assay kit, ZellBio GmbH, Germany). Briefly, the enzyme regenerates the reduced form of the selenocysteine existing in active site of the enzyme by using glutathione as the ultimate electron donor. By adding excess GSH, GPX converts it to GSSG and the remaining GSH generates a yellow color by reducing DTNB (at 412 nm). The GPX activity is indirectly related to color formation. Specific activity of GPx is expressed as U/mg protein.
ELISA assay
TNF-α concentration in liver homogenate was determined using Rat TNF-α ELISA MAX™ Deluxe set kit (BioLegend, San Diego, USA), according to the manufacturer’s instructions.
Expression of TNF-α and NF-κB gene
Total RNA from liver tissue was extracted using Kiazol reagent (Kiazist, Iran) following the manufacturer’s protocol [64]. Nanodrop (One Thermo Scientific, USA) and 1% agarose gel electrophoresis were used to ensure the integrity of the RNA and the accuracy of the RNA extraction process. An aliquot (500 ng) of extracted total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara, Japan) according to the manufacturer’s procedure.
Synthesized cDNA was used for RT-PCR assay. Primers were designed using primer3 software and listed in Table 2. RT-PCR reactions contained 2 µl cDNA, 1.0 µl forward and reverse primers, and 10 µl SYBR Green PCR Master Mix (Roche, USA). RT-PCR was performed on Light Cycler®96 System (Roche, USA). Amplification with 40–45 cycles was performed according to the following steps: 95 °C 20 s for denaturation, 56 °C 30 s for renaturation, then 72 °C 30 s for elongation. The fluorescence signal was detected in the end of each cycle. β-actin was used as an internal control. The specificity of the primers was confirmed using melting curve. The expression level was calculated from the PCR cycle number (CT) where the increased fluorescence curve passes across a threshold value. The relative expression of target genes was achieved using comparative CT (∆∆CT) method. The ∆CT was determined by subtracting β-actin CT from that of the target gene, whereas ∆∆CT was calculated by subtracting the ∆CT of the calibrator sample (control 1) from that of the test sample. The amplified expression was calculated from the 2−∆∆CT formula [65].
Histopathological analysis
The formalin-fixed liver tissues were embedded in paraffin blocks, sectioned with a 5 µm thickness, placed on glass slide and then stained with hematoxylin and eosin (H&E). Slides were analyzed by light microscope (ProWay, China) for histopathological changes in all groups.
Statistical analysis
Statistical analyses were carried out using SPSS 24 software (IBM, USA). The data were expressed as mean ± standard deviation. Comparison of the differences of means among multi-group data was made using One-way analysis of variance (ANOVA). For statistical analysis of all data, p < 0.05 was assigned as the threshold for significance.
Results
Biochemical tests
Elevation of liver enzymes such as ALT, AST, ALP, and LDH in serum indicated liver injury. In this study, plasma levels of these indicators were significantly increased in NTiO2 group compared to control groups (p ≤ 0.001). In vitamin E and combination of vitamin A&E groups, a significant reduction in leakage of liver enzymes in plasma was observed in comparison to NTiO2-intoxicated group (p ≤ 0.01). Although the vitamin A-treated group could reduce these enzymes in serum, this reduction was not significant for ALT and LDH (p = 0.06, p = 0.193, respectively). Data are presented in Table 3.
Oxidant and antioxidant parameters in rat liver
According to the data shown in Table 4, in NTiO2 group a significant increase was observed in lipid peroxidation, TOS, as well as significant reduction in TAC, in comparison to the control groups (p ≤ 0.001). Unlike vitamin E-treated groups, groups orally administrated with vitamin A could not significantly alter TAC and TOS values compared to nano titanium dioxide intoxicated group. Treatment with vitamin A and vitamin E caused significant reduction in lipid peroxidation (MDA) level (p < 0.01), while no noticeable difference was observed between Vitamin A&E-treated group and control groups in terms of lipid peroxidation (p > 0.05).
Antioxidative enzymes activity in rat liver
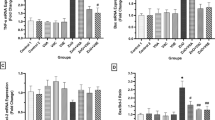
The most prominent enzymes involved in oxidative stress protection are SOD, GPX, and Catalase. Activities of these enzymes were measured after 2 weeks administration of nano-sized titanium dioxide (300 mg/kg body weight) and treatment with vitamin A, vitamin E and combination of these vitamins. The results are presented in Figs. 3, 4 and 5. There was noticeable decrease in SOD activity in the group administered NTiO compared to control groups (p < 0.05), while this activity was significantly increased in vitamin-treated groups compared to nano-titanium dioxide intoxicated group (p < 0.05). GPx activity in NTiO2 group was significantly lower than in the control groups (p < 0.01), whereas treatment with vitamins could overcome this reduction (p < 0.01), so that no significant differences were observed between the control groups and vitamin E&A-treated group (p > 0.05). In NTiO2 group, a remarkable reduction in catalase activity was observed in comparison to the control groups (p < 0.01). Treatment with vitamins could significantly prevent this reduction (p < 0.05).
SOD activity in liver of rats in vitamin-treated (NTiO2 + vit E, NTiO2 + vit A, and NTiO2 + vitE&A), NTiO2 intoxicated (NTiO2), control 1 (water), and Control 2 (olive oil) groups. Values expressed as mean ± standard deviation for six rats. *Represent significant different to control groups (P ≤ 0.001). #Represent significant different to NTiO2 intoxicated group (P < 0.001)
GPx activity in liver of rats in vitamin-treated, NTiO2 intoxicated, control 1 (water), and control 2 (olive oil) groups. Values expressed as mean ± standard deviation for six rats. *Represent significant different to control groups (P < 0.05). #Represent significant different to NTiO2 intoxicated group (P < 0.001)
Catalase activity in liver of rats in vitamin-treated (NTiO2 + vit E, NTiO2 + vit A, and NTiO2 + vitE&A), NTiO2 intoxicated (NTiO2), control 1 (water), and control 2 (olive oil) groups Values expressed as mean ± standard deviation for 6 rat. Groups having the same symbols (* or #) are not significantly different from each other (P > 0.05). aRepresent significant different to control groups (P < 0.001). bRepresent significant different to NTiO2 intoxicated group (P < 0.001)
ELISA result
Considering the ability of nano-titanium dioxide to induce inflammatory responses, we quantitatively examined TNF-α as an inflammatory cytokine in the liver of rat. Increase of TNF-α in the liver of NTiO2 group was conspicuous in comparison to the control groups (p < 0.01). In contrast, oral administration of vitamins significantly ameliorated this change (p < 0.05). It should be noted that this reduction was more effective in the vitamin E&A group. Data are presented in Table 5.
RT-PCR results
Studies have shown that up-regulation of NF-κB promoted the release of inflammatory cytokines such as TNF-α and caused further damage to organs. To further confirm the role of vitamin A and vitamin E in cellular protection against molecular mechanism-dependent toxicity of nano-titanium dioxide, the changes of the inflammation related genes were assessed using real time RT-PCR. The results showed that relative expression of NF-κB and TNF-α was almost 3- and twofold in NTiO2 group compared to the control groups, respectively (p < 0.01). Nevertheless, a significant down regulation in both genes was observed following treatment with vitamin A and vitamin E (p < 0.01). In this study, vitamin A exhibited greater efficacy in controlling the up-regulation of NF-κB and TNF-α gene expression induced by nano titanium dioxide than vitamin E. The results are presented in Fig. 6.
Effect of vitamin E (vit E), vitamin A (vit A), and their combination (vit E & A) on mRNA expression of NF-κB, TNF-α, and β-actin (as reference gene) after intoxication with titanium dioxide nanoparticles (NTiO2) in liver of rats. Except NF-κB mRNA in control 1 (water) and control 2 (olive oil) groups, other values have significant difference between each other (P < 0.05). *Represent significant different to control groups (P < 0.001). #Represent significant different to NTiO2 intoxicated group (P < 0.001)
Histopathological findings
The histological photomicrograph of liver sections is presented in Fig. 7. Histological examinations revealed that control groups showed normal architecture of liver tissue as well as normal monolayer of hepatocytes arranged radially around central vein and normal portal triad. Intragastric administration of NTiO2 caused extensive histopathological changes including dilatation of congested portal vein with red blood cell, hypertrophy of Kupffer cells, hydropic and vacuolar degeneration of hepatocytes along with edema and necrosis around dilated central vein, and inflammatory cell infiltration, while treatment with vitamin A and vitamin E could effectively mitigate these changes.
The histological photomicrograph of H & E stained liver sections. a control 1 (water), demonstrating normal port space and hepatocytes (100 ×); b control 2 (oil), demonstrating normal port triad and normal monolayer of hepatocytes arranged radially around central vein (100 ×); c NTiO2-intoxicated, demonstrating portal vein (star) and hyperplasia in the kupffer cells, (10 ×); d NTiO2-intoxicated, demonstrating degeneration of hepatocytes along with edema and congestion around dilated central vein (arrows)(4 ×); e NTiO2-intoxicated, demonstrating hepatocyte changes, inflammatory and central vein congestion (10 ×); f NTiO2 + Vitamin E, demonstrating attenuated hepatocyte changes compare to NTiO2 intoxicated group (10 ×); g NTiO2 + Vitamin E, demonstrating lower cellular damage severity in the vicinity of central vein (arrow) compared to NTiO2 group (4 ×); h NTiO2 + Vitamin A, demonstrating increase the number of kupffer cells with attenuated hepatocyte degradation compared to NTiO2 group (10 ×); i NTiO2 + Vits E & A, demonstrating normal hepatocytes and intact central vein (arrow) (10 ×)
Discussion
Due to some of the unique physicochemical properties of nanoparticles, the use of these materials in various industries is expanding. Despite all the benefits of nanomaterials, there is a growing number of studies that claim severe consequences of nanoparticle exposure [66]. Titanium dioxide nanoparticles is one of the most broadly applied compounds in the medical, food, cosmetics, sterilization and paint industry. It has been recognized that NTiO2 exposure may induce toxicity via different mechanism, most of which is related to the generation of ROS and subsequent induction of oxidative stress in living systems [29, 33]. It has been proven that among different forms of titanium dioxide nanoparticles, the anatase form exhibits more phototoxicity and cytotoxicity than other forms like rutile [67, 68]. The liver is the main target for metabolizing xenobiotics such as nanoparticles, and according to previous studies, the highest level of titanium dioxide nanoparticles have been proven to accumulate in the liver tissue [69]. This was the rationale for using liver tissue in the current study. This study aimed to introduce antioxidant and anti-inflammatory vitamins (vitamin A and vitamin E) to attenuate toxicity induced by NTiO2. AtRA is an active form of vitamin A which has been proven to act as an antioxidant and anti-inflammatory agent [52, 70, 71]. α-Tocopherol (AT) which is a functional form of vitamin E is reported to act as a striking anti-inflammatory and antioxidant of major importance for defense against diseases and degenerative processes induced by oxidative stress [40, 46]. Vitamin E does not act as a separate antioxidant, but forms part of an antioxidant network which includes enzymatic and non-enzymatic components that are water-soluble and fat-soluble [72]. In the transformation of the form of tocopheroxyl (caused by free radical contact) to α-tocopherol (reduced native state), vitamin C plays an important role, either directly or indirectly via thiol antioxidants such as lipoic acid and glutathione [73]. It has been claimed that α-tocopherol can affect cell signaling through alteration of protein kinase C (PKC) activity [74]. In this way, by inhibiting PKC, NF-κB also remains inactive and ultimately prevents inflammation.
In this study, redox status, antioxidant enzymes, and inflammatory responses were assessed after intoxication by NTiO2 (300 mg/kg body weight) for 2 weeks, and the protective effects of vitamin A, vitamin E, and the combination of these vitamins was evaluated in adult male Wistar rat at the same time. NTiO2 have been considered to accumulate in the liver and induce ROS generation and lipid peroxidation [69, 75, 76]. The potential of vitamin A and vitamin E to attenuate oxidative stress induced by NTiO2 was assessed by measuring the redox status (TAC, TOS, and lipid peroxidation) and the activity of antioxidant enzymes (SOD, GPx, and catalase). A significantly increased level of TOS and MDA, as well as reduction of TAC levels was observed in the liver homogenate of NTiO2 group, whereas vitamin treatment could prevent these alterations. These findings are comparable to the results of Meena et al. [75], who reported that administration of NTiO2 increased lipid peroxidation levels in the liver of Wistar rats [75]. Similarly, Shukla et al. [77] claimed that exposure to NTiO2 (50,100 mg/kg bw) could increase ROS generation and MDA level in addition to depletion of reduced GSH in mice [77]. In the study conducted by Abdelazim et al. [49], it was found that NTiO2 causes increase in hepatic TAC and NOx (Nitrite/Nitrate) levels in Wistar mice after 2 weeks of oral exposure, and vitamin E noticeably had an effective role in reducing these two oxidative factors. One of the most important strategies of the cells to overcome the surplus of free radicals and ROS is to engage the endogenous antioxidant enzyme defense system, in which the SOD, GPx, and catalase enzymes play a significant role in neutralization of ROS and prevent the propagation of oxidative stress-mediated damage [78]. In this study, the reduced activity of SOD, GPx, and Catalase followed by intragastric administration of NTiO2, was observed. Interestingly, treatment with vitamin E and A exhibited significant increase in the activity of these enzymes which points out the antioxidant properties of vitamin E and vitamin A. Earlier studies reported that exposure to NTiO2 could decrease the activity and protein level of antioxidant enzymes (SOD, GPx, and Catalase) in liver and kidney of rodents [75, 79]. Cui et al. [80] demonstrated that intragastric administration of NTiO2 (10 and 50 mg/kg bw) consecutively for 60 days significantly reduced the mRNA expression of SOD, GPX, and catalase [80]. Another cause of increased activity of the SOD enzyme by vitamin A includes its ability to induce the expression of the mRNA and protein of the enzyme through cellular signaling pathways. Rao et al. [55] demonstrated that AtRA increases the protein concentration and mRNA level of SOD by activation of P38 MAPK, and subsequently triggers the Akt which ultimately leads to the induction of SOD [55].
Increasing the level of free radicals in the cell, followed by peroxidation of the membrane lipids, leads to instability, and ultimately, collapse of the cell. Exposure to nanomaterials such as NTiO2 could lead to distinctive types of cell death including apoptosis, necrosis, and pyroptosis (cell death related to inflammation) through different mechanism of signaling pathways. It has been shown that the administration of NTiO2 can activate apoptotic pathways, which has been proven by increasing the expression of pro-apoptotic mRNA and proteins (Bax, P53, caspase-3, and caspase-9), reducing the expression of anti-apoptotic mRNA and proteins (Bcl-2), and increase in apoptotic index, which implies the activation of the intrinsic pathway [77, 81]. Another mechanism of cell death-induced by NTiO2 is through activation of cell death receptors which result in apoptosis. It has been proven that NTiO2 can activate FAS receptor [60]. According to the transmission electron microscope (TEM) images, accumulation of NTiO2 aggregates with sharp edges within cells clarify the physical damage to the lysosome and subsequently the release of lysosomal enzymes which leads to exacerbating induction of apoptosis [82]. On the other hand, several studies have confirmed that release of lysosomal enzyme or excessive production of ROS caused by NTiO2 would induce mitochondria-mediated apoptosis [60, 83, 84]. With regard to the described pathways of cell death and NTiO2-induced cellular collapse, liver cells are susceptible to decomposition and destruction as a result of exposure to NTiO2, which can be tracked by release of liver enzymes (ALT, AST, ALP, and LDH) into the plasma and measuring their concentrations, which directly reflects the liver injury. The current study showed the significant elevation of concentration of liver enzymes in the plasma of the rats after 2 weeks of oral administration of NTiO2. The reversal of increased plasma enzyme activity in NTiO2-induced hepatic injury by vitamin A and vitamin E could describe the protection against leakage of the liver enzymes by its membrane stabilizing and antioxidant activity. Since fat-soluble vitamins can penetrate the cell membrane, they could play their protective roles there, effectively inhibit the lipid peroxidation and sustain the membrane integrity [49, 85]. We concluded that vitamin E and the combination of vitamin E & A had a greater effect on prevention of liver enzymes leakage than vitamin A which could demonstrate the better efficiency of vitamin E to scavenge free radicals. These findings are comparable with our histopathological examination of liver tissue, which showed hepatocyte degeneration and dilatation of the congested central vein in NTiO2 intoxicated group as a consequence of perturbed permeability of hepatocyte membrane and endothelial cells of veins induced by NTiO2. There was also evidence of inflammatory cell infiltration indicating the necrotic potential of NTiO2, which is believed to be due to the induction of oxidative stress and activation of inflammatory pathways. Since apoptosis and necrosis are dependent processes [86], induction of apoptosis might be a guesstimate for this group. A vast majority of NTiO2 are taken up by kupffer cells in the liver [87]. It has been reported that NTiO2 caused phagocytosis impairment in murine liver macrophages [88] as manifested by kupffer cell hypertrophy in our result. On the other hand, ultrastructure of hepatocytes in vitamin-treated groups showed ameliorated damage compared to NTiO2 intoxicated group. So that, the portal structure and hepatocytes in vitamin E & A group were similar to the control groups. These results are due to the fact that the combination of these two vitamins not only inhibits oxidative stress but also prevents inflammatory responses. Alarifi et al. [89] demonstrated that different routes of exposure to NTiO2 leads to varied and extensive changes in the structure of liver cells which briefly includes lymphocytic infiltration, dilatation of central vein, necrosis and scattered hemorrhages, hepatocyte degeneration and vacuolization [89].
One of the ways to induce cell death by NTiO2 is to trigger inflammatory responses that include MAPK and NF-KB signaling pathways or receptor activation, which releases inflammatory cytokines [60, 90]. Studies have shown that exposure to NTiO2 induces IL-1β and MMP 1 secretion, which may be based on the performance of caspase-1 and cathepsin B to maturate pro IL-1β [91]. As already mentioned, NTiO2 causes damage to lysosomes and other Intracellular organelles, followed by the release of cathepsin B and caspase-1. According to the investigations by Cui et al. [80], the signaling cascade proposed for hepatic toxicity induced by NTiO2 is: TLRs → NIK → IκB kinase → NF-ΚB → TNF-α → inflammation → apoptosis → liver injury [31]. In this paper, the cytotoxicity and inflammation induced by NTiO2 and protective effects of vitamin A and vitamin E was addressed by analyzing the mRNA expression of NF-κB which is a critical intracellular mediator of the inflammatory response and TNF-α as a result of inflammatory cascade activation, as well as quantification of TNF-α protein in liver homogenate of Wistar rats. It is important to note that in many studies, the effect of NTiO2 on the enhancement of NF-κB and TNF-α gene expression has been confirmed [22, 92, 93]. The findings of the current study showed that NTiO2 caused the significant increase in the expression of NF-κB mRNA, TNF-α mRNA, and protein while vitamin treatment could hinder this elevation. This is in line with the results of Abdelazim et al. [49] who claimed that administration of NTiO2 significantly increased the mRNA expression of NF-κB as well increased the concentration of TNF-α protein in liver of mice; but vitamin E treatment had an effective role in reducing these changes [94]. Our results showed that although both vitamins significantly reduced the expression of NF-κB and TNF-α mRNA, the effect of vitamin A, in this case, was greater than that of vitamin E. This particular property of vitamin A to reduce the expression of inflammatory mediators and cytokines such as NF-KB and TNF-α has been shown in previous studies, as claimed in this paper [54, 56, 95]. Our results also showed that, in addition to a significant reduction in TNF-α mRNA expression by vitamin A and vitamin E, the concentration of TNF-α protein was also significantly decreased in the liver cells by treatment with these two vitamins, of which no significant difference was observed between the vitamin E & A group and control groups. During this experiment, no significant difference was detected between the control groups 1 and 2.
The protective effects of vitamin E and vitamin A were sufficient to remarkably reduce the NTiO2-induced toxicity in liver, but in some parameters these effects did not reach the normal levels (control group) because of the fact that in the design of this experiment, we chose toxic concentration of NTiO2 (300 mg/kg) to cause certain hepatotoxicity, while estimates of exposure to these nanoparticles are much lower than these values. In the study by Rompelberg et al. [59], it was shown that the maximum mean long term oral intake of NTiO2 was 0.67 mg/kg bw/day in the children of a Dutch population (2–6 years old) [59].
Conclusion
The results represent the antioxidant and anti-inflammatory effects of vitamins A and E against toxicity of NTiO2 and poses the use of these vitamins to reduce the toxic effects of NTiO2-contained products. Following the use of vitamins, A and C as antioxidant supplements, improvement of the oxidative stress status and the liver function parameters were observed. Therefore, it appears that the antioxidant vitamins act as scavenger of free radicals produced by nanoparticles-induced oxidative stress.
Data availability
All data used and analyzed during the current study are included in this manuscript and available from the corresponding author on reasonable request.
References
Brown SC et al (2013) Toward advancing nano-object count metrology: a best practice framework. Environ Health Perspect 121(11–12):1282
Ackroyd R et al (2001) The history of photodetection and photodynamic therapy. Photochem Photobiol 74(5):656–669
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21(10):1166
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107(7):2891–2959
Ren W et al (2013) Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv 3(43):20855–20861
Du Y et al (2015) The enhanced chemotherapeutic effects of doxorubicin loaded PEG coated TiO 2 nanocarriers in an orthotopic breast tumor bearing mouse model. J Mater Chem B 3(8):1518–1528
Haugen H et al (2004) Ceramic TiO2-foams: characterisation of a potential scaffold. J Eur Ceram Soc 24(4):661–668
Cui C et al (2005) Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater Lett 59(24–25):3144–3148
Weir A et al (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46(4):2242–2250
Peters RJ et al (2014) Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. J Agric Food Chem 62(27):6285–6293
Lorenz C et al (2010) Imaging and characterization of engineered nanoparticles in sunscreens by electron microscopy, under wet and dry conditions. Int J Occup Environ Health 16(4):406–428
Contado C, Pagnoni A (2010) TiO2 nano-and micro-particles in commercial foundation creams: field flow-fractionation techniques together with ICP-AES and SQW voltammetry for their characterization. Anal Methods 2(8):1112–1124
Mahmoud WM, Rastogi T, Kümmerer K (2017) Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr Opin Green Sustain Chem 6:1–10
Rezaei B, Mosaddeghi H (2006) Applications of titanium dioxdie nanocoating. in nano-technology in environments conference, Isfahan University of Technology, Isfahan, February 2006
Mahmoud WM, Rastogi T, Kuemmerer K (2017) Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr Opin Green Sustain Chem 6:1–10
Shakeel M et al (2016) Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: a review. Biol Trace Elem Res 172(1):1–36
Elgrabli D et al (2015) Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS ONE 10(4):e0124490
Disdier C et al (2015) Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part Fibre Toxicol 12(1):27
Fabian E et al (2008) Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol 82(3):151–157
Sturgill MG, Lambert GH (1997) Xenobiotic-induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem 43(8):1512–1526
Sugibayashi K, Todo H, Kimura E (2008) Safety evaluation of titanium dioxide nanoparticles by their absorption and elimination profiles. J Toxicol Sci 33(3):293–298
Ma L et al (2009) The acute liver injury in mice caused by nano-anatase TiO2. Nanoscale Res Lett 4(11):1275
Khorsandi L et al (2015) Glycyrrhizic acid attenuated lipid peroxidation induced by titanium dioxide nanoparticles in rat liver. Bratislavske Lekarske Listy 116(6):383–388
Xu X-HN et al (2004) Real-time probing of membrane transport in living microbial cells using single nanoparticle optics and living cell imaging. Biochemistry 43(32):10400–10413
Tsai Y-C, Chen S-Y, Liaw H-W (2007) Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sens Actuators B 125(2):474–481
Ranjan S et al (2018) Titanium dioxide nanoparticles–protein interaction explained by docking approach. Int J Nanomed 13:47
Trouiller B et al (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69(22):8784–8789
Knaapen AM et al (2004) Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer 109(6):799–809
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013:15
Li M et al (2014) Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J Food Drug Anal 22(1):76–85
Cui Y et al (2011) Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A 96(1):221–229
Chen P, Kanehira K, Taniguchi A (2013) Role of toll-like receptors 3, 4 and 7 in cellular uptake and response to titanium dioxide nanoparticles. Sci Technol Adv Mater 14(1):015008
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2(4):MR17–MR71
Risom L, Møller P, Loft S (2005) Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res/Fundam Mol Mech Mutagen 592(1):119–137
Nel A et al (2006) Toxic potential of materials at the nanolevel. science 311(5761):622–627
Kawanishi S et al (2002) The role of metals in site-specific DNA damage with reference to carcinogenesis1, 2. Free Radic Biol Med 32(9):822–832
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313(Pt 1):17
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol-Lung Cell Mol Physiol 279(6):L1005–L1028
Uttara B et al (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74
Ognjanovic B et al (2003) Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res 52(5):563–570
Zaidi SKR, Banu N (2004) Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta 340(1–2):229–233
Traber MG, Atkinson J (2007) Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43(1):4–15
Niki E (2014) Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med 66:3–12
Mol MJ et al (1997) Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: effects of vitamin E treatment. Atherosclerosis 129(2):169–176
Devaraj S, Chan AVC, Jialal I (2002) α-Tocopherol supplementation decreases plasminogen activator inhibitor-1 and P-selectin levels in type 2 diabetic patients. Diabetes Care 25(3):524–529
Shirpoor A et al (2015) Protective effect of vitamin E against diabetes-induced oxidized LDL and aorta cell wall proliferation in rat. Iran Biomed J 19(2):117
Singh U, Devaraj S, Jialal I (2005) Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 25:151–174
Calvisi DF et al (2004) Vitamin E down-modulates iNOS and NADPH oxidase in c-Myc/TGF-α transgenic mouse model of liver cancer. J Hepatol 41(5):815–822
Abdelazim SA et al (2015) Potential antifibrotic and angiostatic impact of idebenone, carnosine and vitamin E in nano-sized titanium dioxide-induced liver injury. Cell Physiol Biochem 35(6):2402–2411
De Luca LM (1991) Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J 5(14):2924–2933
Leid M, Kastner P, Chambon P (1992) Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci 17(10):427–433
Kawakami S et al (2006) Induction of apoptosis in A549 human lung cancer cells by all-trans retinoic acid incorporated in DOTAP/cholesterol liposomes. J Control Release 110(3):514–521
Di C et al (2005) Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res 65(3):919–924
Rao J et al (2013) All-trans retinoic acid preconditioning protects against liver ischemia/reperfusion injury by inhibiting the nuclear factor kappa B signaling pathway. J Surg Res 180(2):e99–e106
Rao J et al (2010) All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull 33(5):869–875
Hisamori S et al (2008) All-trans-retinoic acid ameliorates carbon tetrachloride-induced liver fibrosis in mice through modulating cytokine production. Liver Int 28(9):1217–1225
Jia X et al (2017) The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett 12(1):478
Boffetta P et al (2004) Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 15(7):697–706
Rompelberg C et al (2016) Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 10(10):1404–1414
Shi H et al (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10(1):15
Rizk MZ et al (2017) Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed Pharmacother 90:466–472
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38(12):1103–1111
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15(3):532–534
Livak KJ, Schmittgen TD, (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ∆∆CT method. Methods 25(4):402–408
Papp T et al (2008) Human health implications of nanomaterial exposure. Nanotoxicology 2(1):9–27
Xue C et al (2010) Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J Nanosci Nanotechnol 10(12):8500–8507
Sanders K et al (2012) In vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicol Appl Pharmacol 258(2):226–236
Wang J et al (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2):176–185
Palace VP et al (1999) Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med 26(5–6):746–761
Mehta K et al (1994) Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol 55(3):336–342
Constantinescu A, Han D, Packer L (1993) Vitamin E recycling in human erythrocyte membranes. J Biol Chem 268(15):10906–10913
Packer L, Weber SU, Rimbach G (2001) Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J Nutr 131(2):369S–373S
Tasinato A et al (1995) d-alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci 92(26):12190–12194
Meena R, Paulraj R (2012) Oxidative stress mediated cytotoxicity of TiO2 nano anatase in liver and kidney of Wistar rat. Toxicol Environ Chem 94(1):146–163
Hong J, Zhang Y-Q (2016) Murine liver damage caused by exposure to nano-titanium dioxide. Nanotechnology 27(11):112001
Shukla RK et al (2014) Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 9(9):1423–1434
Ighodaro O, Akinloye O, First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 54:287–293 2017
Liu H et al (2010) Toxicity of nano-anatase TiO2 to mice: liver injury, oxidative stress. Toxicol Environ Chem 92(1):175–186
Cui Y et al (2010) Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J Hazard Mater 183(1–3):874–880
Orazizadeh M et al (2014) Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chemico-Biol Interact 220:214–221
Hussain S et al (2009) Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology 260(1–3):142–149
Stern ST, Adiseshaiah PP, Crist RM (2012) Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol 9(1):20
Tang Y et al (2013) Mitochondrial injury induced by nanosized titanium dioxide in A549 cells and rats. Environ Toxicol Pharmacol 36(1):66–72
Lucy J, Dingle J (1964) Fat-soluble vitamins and biological membranes. Nature 204:156–160
Lemasters JJ (1999) V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol-Gastrointest Liver Physiol 276(1):G1–G6
Sadauskas E et al (2007) Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol 4(1):1
Möller W et al (2002) Ultrafine particles cause cytoskeletal dysfunctions in macrophages. Toxicol Applied Pharmacol 182(3):197–207
Alarifi S et al (2013) Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int J Nanomed 8:3937
Donaldson K et al (2005) Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol 2(1):10
Armand L et al (2013) Titanium dioxide nanoparticles induce matrix metalloprotease 1 in human pulmonary fibroblasts partly via an interleukin-1β–dependent mechanism. Am J Respir Cell Mol Biol 48(3):354–363
Song B et al (2016) Unraveling the neurotoxicity of titanium dioxide nanoparticles: focusing on molecular mechanisms. Beilstein J Nanotechnol 7:645
Sun Q et al (2012) Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J Hazard Mater 235:47–53
Azim SAA et al (2015) Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol 67(4):305–314
Charoensit P et al (2008) Incorporation of all-trans retinoic acid into lipoplexes inhibits nuclear factor κB activation mediated liver injury induced by lipoplexes in mice. J Gene Med 10(1):61–69
Acknowledgements
This study has been adapted from MSc. Thesis at Hamadan University of Medical Sciences.
Funding
The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (Grant No. 963021433).
Author information
Authors and Affiliations
Contributions
AM analyzed and interpreted the lab test. AG performed the histological examination of the liver. NZ was a major contributor in writing the manuscript. All authors read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no competing interest.
Ethics approval and consent to participate
The experimental procedure was approved at the Faculty of Medicine, at Hamadan University of Medical Sciences (UMSHA), and the research was conducted according to the guidelines for the care and use of laboratory animals of UMSHA (IR.UMSHA > REC.1396.29).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, A., Ziamajidi, N., Ghafourikhosroshahi, A. et al. Effects of vitamin A and vitamin E on attenuation of titanium dioxide nanoparticles-induced toxicity in the liver of male Wistar rats. Mol Biol Rep 46, 2919–2932 (2019). https://doi.org/10.1007/s11033-019-04752-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04752-4