Abstract
Matrix metalloproteinases (MMPs) or matrixins, are members of a zinc-dependent endopeptidase family. They cause remodeling of the extracellular matrix (ECM) leading to numerous diseases. MMPs subfamilies possess: collagenases, gelatinases, stromelysins and membrane-type MMPs (MT-MMP). They consist of several domains; pro-peptide, catalytic, linker peptide and the hemopexin (Hpx) domains. MMPs are involved in initiation, proliferation and metastasis of cancer through the breakdown of ECM physical barriers. Overexpression of MMPs is associated with poor prognosis of cancer. This review will discuss both types of MMPs and current inhibitors, which target them in different aspects, including, biosynthesis, activation, secretion and catalytic activity. Several synthetic and natural inhibitors of MMPs (MMPIs) that can bind the catalytic domain of MMPs have been designed including; peptidomimetic, non-peptidomimetic, tetracycline derivatives, off-target MMPI, natural products, microRNAs and monoclonal antibodies.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs) belong to a zinc dependent endopeptidases family. They are involved in many vital biological functions through the proteolysis of different protein targets [1, 2]. The substrates of MMPs include gelatins, collagens, elastin, proteoglycans and many other proteins [3, 4]. The alteration in MMPs function is involved in numerous diseases, which may lead to high mortality rates. Among these diseases are cancer, autoimmune diseases, cardiovascular diseases, inflammation and neurodegenerative disease states [5, 6]. Several clinical and experimental studies demonstrate the role of MMPs in tumor invasion, metastasis and neo-angiogenesis, which make them promising targets for cancer therapy [7, 8].
There are about 28 MMP members have been identified till now; 23 MMPs are expressed in humans, as well as, 15 members are present in vasculature [9]. There are different subfamilies of MMPs including: gelatinases which include two enzymes: gelatinase A (MMP-2) and gelatinase B (MMP-9) [10, 11].
It possesses also collagenases (MMP-1, MMP-8, MMP-13 and MMP-18), matrilysins (MMP-7 and MMP-26), membrane-type MMPs (MT-MMPs), as transmembrane type MMP-14, MMP-15, MMP-16, and MMP-24, and glycosylphosphatidylinositol, or glycophosphatidylinositol, or GPI-anchored MMP-17 (as GPI is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification) and MMP-25 [9, 15].
Other family possesses stromelysins (MMP-3, MMP-10 and MMP-11) and other MMPs (MMP-4, MMP-5, MMP-6, MMP-12, MMP19, MMP-20, MMP-21, MMP-22, MMP-23, MMP-27, and MMP-28) [12].
There are other two new families of membrane-anchored metalloproteinases that have a disintegrin domain: the ADAMs (a disintegrin and metalloproteinases) and ADAMTs (a disintegrin and metalloproteinases with thrombospondin motifs) [13]. Different classes and common members of MMPs are shown in Table 1.
Structure of MMPs
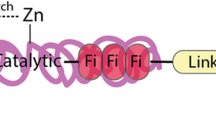
The MMP typically consists of several domains; the pro-peptide domain contains about 80 amino acids, the catalytic domain contains about 170 amino acids and a variable lengths of linker peptide (hinge region)[14]. The hemopexin (Hpx) domain, which is involved in the interaction with other MMP, has about 200 amino acids, Fig. 1 [15]. MT-MMPs lack the pro-domain, while MMP-7 (matrilysin 1), MMP-26 (matrilysin 2) and MMP-23 lack the Hpx domain and the linker peptide. The MMP-23 also contains an immunoglobulin-like domain and a unique cysteine-rich domain after the metalloproteinase domain. Three repeats of a fibronectin are present in the metalloproteinase domain of gelatinase A (MMP-2) and gelatinase B (MMP-9). The catalytic domain of MMP contains zinc binding motif HEXXHXXGXXH, while the pro-peptide contains “cysteine switch” motif PRCGXPD. The pro-MMPs remain inactive through the coordination of three histidines in the zinc binding motif and the coordination between the cysteine in the pro-peptide domain and the catalytic zinc ion. This coordination prevents the binding of a water molecule to the zinc ion, which is necessary for activation. A conserved methionine is also present in the catalytic domain creating a “Met-turn” eight residues after the zinc binding motif. This supports the structure around the catalytic zinc [16].
Structural domains of matrix metalloproteinases MMP. A schematic representation of the general structure of the inactive and active forms of MMPs (only the membrane-type group has the extra membrane binding domain). The pro-domain, catalytic domain, hemopexin domain and fibronectin (c-Fib) domains (only present in MMP-2 and MMP-9) are shown in red, turquoise, yellow and green, respectively. Both prodomain and catalytic domain (domain) together represents the enzyme motif. Zn ions are indicated in red [15]
Other members also contain the zinc binding motif and the Met-turn, which are called “metzincins, including; the ADAMTS (ADAM with thrombospondin motifs) family, members of the ADAM (a disintegrin and metalloproteinase) family, a protozoan proteinase leishmanolysin, the bacterial serralysin family, the astacin family and 2 pregnancy associated plasma proteins [17]. MMPS are classified according to domain organization and substrate preference into gelatinases, collagenases, matrilysins, stromelysins, membrane-type (MT)-MMPs and others [18].
Regulation of MMPs activity
The activity of MMPs is highly regulated, otherwise they can negatively affect the biological system [19]. MMPs activity is regulated at several levels involving, mRNA expression, pro-enzyme activation and the action reversal of endogenous tissue inhibitors of metalloproteinases (TIMPs) [20, 21]. The transcription of MMPs is affected by several factors including, inflammatory cytokines, cell–cell, cell–matrix interactions, chemokines, growth factors and oncogenes. On the level of posttranslational modification, MMPs are formed in inactive pre-pro-MMPs. During translation, the signal peptide is removed to form the pro-MMPs. The cysteine forms the PRCGXPD “cysteine-switch” motif in the pro-MMPs, which coordinates with the zinc ion in the catalytic site keeping the pro-MMPs inactive. In order to obtain the active MMPs, the cleavage of the cysteine switch by the help of other proteolytic enzymes including, the endopeptidase furin, is required, or by serine proteases, plasmin, or other MMPs [2, 22]. Urea and 4-aminophenylmercuric acetate are other non-proteolytic substances that can activate the pro-MMPs [23]. The MMPs are also regulated by the tissue inhibitors of MMPs (TIMPs), which is important for maintaining the extracellular matrix. The alteration in the balance between TIMPs and MMPs activities leads to several diseases as cancer. There are 4 distinct TIMPs that can bind to MMPs catalytic site causing their inactivation [16, 20].
MMPs and cancer
The pathological changes that happen during carcinogenesis and cell transformation require the interaction between cells and the ECM. The phenotype of the tumor is affected by some proteins of the ECM, which affect angiogenesis or cell migration including, fibronectin, laminin, thrombospondin-1 and osteopontin. MMPs are involved in metastasis through the breakdown of ECM physical barriers. They are also involved in all stages of cancer starting from cancer initiation to proliferation up to metastasis [2, 24].
Cancer cells synthesize MMPs, which are involved in cancer cell expansion and survival in a very small amount. Cancer cells stimulate the neighboring cells to generate the needed MMPs by secreting interferon, interleukin, extracellular MMP inductor and growth factors. The generated MMPs can be bounded on the surface of the cancer cell and used in all stages of cancer [25]
MMPs can regulate the growth of tumor cells through diverse mechanisms such as the regulation of proliferative signals by integrins, the release of some growth factor precursors that bound to the cell membrane and the change in the growth factor bioavailability. The growth of tumor cells can be also inhibited by MMPs through different mechanisms as they can stimulate the proapoptotic production (TNF-α and Fas ligand) or they can activate transforming growth factor-β (TGF-β) [26].
TGF-β is one of the important factors that stimulate the growth of the tumor cells. MMP-9 can degrade fibronectin, which binds to CD44, leading to the generation of active TGF-β. MMP-1,2,3,7 and 9 also can stimulate the production of TGF-β after the degradation of its reservoir (decorin) [27]. MMPs play an important role in the activation of many growth factors, which bind to the cell surface in an inactive form. MMP-7, when connects to CD44, leads to the generation of the active form of heparin epidermal growth factor through its proteolytic activation. MMPs also have a vital role in the production of TNF-α, which stimulates the tumor cells' survival through an NF-ĸb dependent manner [28, 29].
MMPs have both apoptotic and anti-apoptotic actions. They exhibit anti-apoptotic activity through different mechanisms including; the activation of serine/threonine kinase AKT/ Protein kinase B, the cleaving of the Fas ligand and the proteolytic shedding of tumor-associated major histocompatibility complex (MHC) complex class I related protein. MMPs retain pro-apoptotic action by changing the composition of ECM and cleaving adhesion molecules. The over-expression of MMP-3 in the epithelial cells causes degradation of the laminin and apoptosis induction. As a result of such phenomena, the selection of resistant cells occurs and the activity of MMPs may lead to tumorigenic cell survival with reduced sensitivity to apoptotic stimuli [30, 31].
MMPs play a vital role in angiogenesis by the degradation of ECM and the basement membrane. The basement membrane degradation causes the endothelial cells to migrate from the existing vessels to the newly created ones. MMP-9 plays a vital role in angiogenesis through generating the factors bound to ECM and elevating their bioavailability. Vascular endothelial growth factor (VEGF) is an important factor that promotes angiogenesis. It can be released by MMPs. MMPs are considered as specific endothelial cell mitogenic factors, which promote the new blood vessel formation and increase their permeability. They also stimulate the intracellular signaling of integrin [32]. MMPs can also release angiostatin which inhibits angiogenesis through the cleavage of plasminogen and collagen XVIII that stimulates the production of endostatin. MMP-2,,9 and 12 can digest plasminogen and release angiostatin, which promotes cancer cell apoptosis. MMP-3,7,9,12,13 and 20 stimulate the production endostatin, which inhibits the activation of pro-MMP-9 and 13 by forming a stable complex with them. It also binds to a5b1 integrin and blocks the phosphorylation of focal adhesion kinase (FAK), thus inhibiting the capillary formation [33].
Involvement of MMPs in different cancers
The contribution of MMPs in human cancers was frequently reported. Thus, MMP-1 over-expression participated in the proliferation, invasion, metastasis, and stem-like properties of osteosarcoma cells [34]. In addition, MMP-9 is the most involved in the development of melanoma [35], and generally MMP expression patterns change in different stages of liver diseases [36] and pancreatic cancer [37].
On the other hand, MMPs may exert a positive role against cancer by favoring lymphocyte tumor infiltration. By using the immunohistochemistry staining, a reported significant increase of MMP-9 protein had been correlated to tumor-infiltrating CD3 lymphocytes in the close vicinity of the endometrial cancer milieu [38]. Interestingly, It has been emphasized that the proteolytic action of MMPs is not confined to degradation of ECM components, but they can play an immunomodulating role [39, 40].
MMPs role in invasion and metastasis
Tumor invasion is a multistep process involving the migration of cells, which is associated with proteolysis and cell-ECM interaction [41]. The degradation of ECM and the basement membrane is necessary for metastasis and invasion [42, 43]. MMPs can increase the infiltration and the migration of the tumor cell by promoting the epithelial-to-mesenchymal transition (EMT) process [44]. In this process, the epithelial cells lose the epithelial phenotype and acquire mesenchymal phenotype. This leads to loss of the integrity of epithelial cells, increasing the migration, invasion and eventually metastasis [45]. Collagen IV of the basement membrane can be degraded by MMP-2 and 9, which stimulate the invasion [46, 47].
Cadherin, the cell adhesion molecules, can maintain the integrity of epithelial cells by mediating the adhesion between cells in normal mucosal cells [48]. The deregulation of cadherin is usually associated with the progression of tumor cells [49]. The invasion and metastasis can be increased by decreasing the E-cadherin expression, which leads to the loss of cell adhesion and the increase of cell dissociation [50, 51]. MMP-3 and 7 cause cleavage of E-cadherin, which stimulates the EMT process [52]. TGF-β, a strong stimulator of EMT, can be activated by MMP-28 [53]. MMPs can induce cell migration through different mechanisms such as cleaving cell–matrix or cell–cell receptors, removing sites of adhesion, exposing new building sites and releasing chemoattractants from ECM [54]. Laminin 5 can be degraded by MMP-2 and 14, which exposes a cryptic site and stimulates the motility [14, 55].
Invadopodia is a specialized surface protrusion where MMPs can localize and stimulate the invasion. MMP 2, 9 and 14 are examples of these MMPs, which promote basement membrane degradation [56]. The interstitial collagen degradation is essential for further cancer cells spread after the cleavage of their basement membrane. The interstitial collagen degradation can be promoted by MMP-1, which has an essential role in metastasis [57]. Tumor cells can cross different barriers of ECM during metastasis, including, basement membrane, surrounding stroma, blood vessels or lymphatics and finally after extravasation they can create new colonies [55]. MMP-9 plays an important role in intravasation [58]. Several studies also showed the role of MMPs in extravasation, the process in which cancer cells can exit the blood vessel. MMPs also have a vital role in different steps involved in metastasis such as local migration, angiogenesis and the creation of a microenvironment required for metastatic growth [59].
Innate and adaptive immunity can destroy cancer cells when they reach the circulation. The immune system can recognize and attack tumor cells, which can establish different ways to escape from this attack and maintain their survival [60]. They are attacked by different inflammatory cells such as neutrophils, macrophages, natural killer cells and tumor-specific cytotoxic T cells [55]. Cytokines are locally acting proteins that can be secreted by T cells when activated by antigen. They can stimulate T- cells proliferation as interleukin 2 (IL-2). IL-2Rα can be cleaved by MMPs, which inhibit their proliferation [46]. The immune responses can be down-regulated by TGF-β cytokine through its effect on lymphocyte activation, growth, and differentiation. TGF-β can be activated by MMPs, which can indirectly control the function of T lymphocyte [61]. MMPs can regulate the action of chemokines; small proteins function as chemo-attractants for certain kinds of leucocytes, and affect their action [62].
MMPs as targets for therapy
It is well known that MMPs have an essential role in cancer progression through the remodeling of the ECM. The overexpression of MMPs is associated with the poor prognosis of diverse kinds of cancer, so it is necessary to develop new agents that can target MMPs. They can be targeted through different ways including, their biosynthesis, activation, secretion and enzymatic activity [63, 64].
The expression and the activity of MMPs can be regulated through four molecular levels. The first level is the transcriptional level through targeting transcription factors such as activator protein-1 (AP-1), hypoxia-inducible factor 1 (HIF-1), nuclear factor κB (NF-κB), extracellular factors like epidermal growth factor (EGF), TGF-β and signaling pathways like extracellular signal–regulated kinase (ERK) and mitogen-activated protein kinase (MAPK) pathways. The second level is the translation level through developing antisense strategies as small interfering RNA (siRNA) that can inhibit the translation of a specific MMP. The third level is pro-MMPs activation through specific antibodies against a particular MMP. For instance; the activation of pro-MMP-2 can be inhibited by the anti-MMP-14 monoclonal antibody. The fourth level is the inhibition of the proteolytic and non-proteolytic MMPs activities [65, 66].
MMP inhibitors (MMPIs) could be divided into two major categories: synthetic and natural inhibitors. Some synthetic inhibitors are still in clinical trials on humans, as synthetic peptides, non-peptide molecules, chemically modified tetracyclines, and bisphosphonates. As well as, natural MMP inhibitors are mostly isoflavonoids and shark cartilage [67]. Several synthetic and natural inhibitors of MMPs (MMPIs) that can bind to the catalytic domain of MMPs have been designed, which made their way to clinical trials. Some of them are mentioned in Table 2. The synthetic MMPIs include; the chemically modified tetracycline derivatives and the synthetic peptidomimetic and non-peptidomimetic inhibitors. Next-generation of MMPIs includes; specific microRNAs that can block the transcription of a specific MMP and monoclonal antibodies that can inhibit the catalytic domain of a particular MMP [63].
The antiproliferative and proapoptotic properties of flavonols in head and neck cancer were reported during various processes associated with cancer progression. These compounds could modulate signal transduction pathways that contribute to cancer development [68].
Due to toxicity limitation of MMPIs, recently, nanomaterials were extensively designed, showing promising outcomes through screening of antibodies to target the terminal region located outside the zinc catalytic site. In this model, the antibody might directly act on the specific MMP, and the nanomaterials could inhibit its activity. This way showed the best safety margin [69].
Peptidomimetic MMPIs
The peptidomimetic MMPIs are derivatives of pseudopeptides. They mimic the MMP substrates cleaving site and act as competitive inhibitors. They interact with the Zn2+ in catalytic sites of MMPs and inhibit their action [60, 70]. Different classes of peptidomimetic MMPIs have been identified, including; hydro-carboxylates, sulfhydryls, phosphoric acid derivatives, hydroxamates and carboxylates [71]. Hydroxamates, the first generation of peptidomimetic MMPIs, include batimastat (BB-94) and marimastat (BB-2516). Batimastat has a broad spectrum of inhibition and it can inhibit the activity of MMP-1, MMP-2, MMP-3, MMP-7, and MMP-9. It is administrated intraperitoneally due to its poor water solubility [72]. Marimastat inhibits the activity of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 and MMP-12. It can be administrated orally as it is a major water-soluble peptidomimetic MMPI [73]. Among the side effects of marimastat are inflammation and musculoskeletal pain. These adverse effects are due to the ability of marimastat to block the activity of TNF-α converting enzyme (TACE) and it can remove the TNF-α receptor II [2, 74].
Non- peptidomimetic MMPIs
The design of non-peptidomimetic MMPIs is based on 3D X-ray crystallographic confirmation of the Zn binding site making them more specific than peptidomimetic MMPIs [75]. The non-peptidomimetic MMPIs have better oral bioavailability than the peptidomimetic MMPIs [76]. Tanomastat (BAY 12-9566) and prinomastat (AG3340) are examples of non-peptidomimetic MMPIs. Tanomastat can block MMP-2, MMP-3, MMP-9, MMP-13 and MMP-14 activities. prinomastat inhibits the enzymatic activity of MMP-2, MMP-3, MMP-7, MMP-9, MMP-13 and MMP-14 [73, 77]. Prinomastat has dose-dependent side effects like joint and musculoskeletal symptoms as stiffness, arthralgias and swelling [78].
Chemically modified tetracyclines
Chemically modified tetracyclines are derivatives of tetracyclines that lake their antibiotic activity. The removal of the dimethylamino group from this class is necessary for lacking their antibiotic activity and limiting their systemic toxicity [74]. They can block the enzymatic activity of MMPs through different ways, including; interfering with pro-MMPs activation, binding to Zn+2 and Ca+2 ions and reducing the transcription of MMPs [73, 79]. This group includes metastat (CMT-3, COL-3), minocycline and doxycycline. Doxycycline is involved in the prevention of periodontitis by blocking the activities of MMP-7 and MMP-9 and it had been approved by the Food and Drug Administration [71]. Doxycycline can also inhibit the enzymatic activity of MMP-2 and MMP-9 and inhibit their secretion. Metastat can block the activities of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 and MMP-12 [73]. It is helpful in the treatment of Kaposi's sarcoma, which is associated with a 40% overall response rate and reduction in the serum level of MMP-2 [80]. It has dose-dependent toxicities like headache, anorexia, nausea, vomiting, cutaneous phototoxicity. elevation of the activities of liver enzymes and anemia [71].
Off-target inhibitors of MMPs
Off-target inhibitors of MMPs can decrease the enzymatic activities of MMPs without targeting MMPs themselves. Bisphosphonates, pyrophosphate (PPi) analogs are examples of this group. They are designed to inhibit bone resorption and treat osteoporosis and they can block the activities of MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MMP-13, MMP-14, and MMP-15. This drug can also inhibit the secretion of MMP-2 by targeting TIMP-2 [60]. For instance, zoledronic acid can decrease the expression of MMP-2 and MMP-9 in nasopharyngeal carcinoma cells and MMP-2, MMP-9, MMP-14 and MMP-15 in breast cancer cells [81, 82]. The treatment with zoledronic acid can improve the outcome of patients with advanced breast and prostate cancer as it not only can prevent bone metastasis but also it can interfere with the growth and the invasion of tumor cells [83]. Letrozole is a non-steroidal agent that can be used in breast cancer but it has also inhibitory effects on MMPs. It can block the activities of MMP-2 and MMP-9 in breast cancer cells and diminishes the invasion potential of tumor cells in a dose-dependent manner [84].
Natural MMPIs
A lot of bioactive molecules contained in natural products can allow researchers to investigate numerous biochemical pathways that facilitate the development of new therapeutic interventions. Many natural products have been approved as drugs for the treatment of several diseases or they act as a starting point for the synthesis of helpful new derivatives [85,86,87]. In this review, we discuss several natural products that have an inhibitory action on MMPs. Sinulariolide is a marine diterpene that was isolated from the soft coral Sinularia flexibilis and belongs to the cembranoid family [1]. Numerous derivatives of sinulariolide have been identified and have different biological activities [88]. Sinulariolide can inhibit the migration and invasion of tumor cells in human bladder cancer cells (TSGH-8301) and human hepatocellular carcinoma cells (HA22T). Sinulariolide and 11-epi-sinulariolide acetate can reduce the expression of MMP-2 and MMP-9 and increase TIMP-1 and TIMP-2 expressions. This can reduce the phosphorylation of serine/threonine-specific protein kinase (AKT) and mammalian target of rapamycin (mTOR) [89, 90].
Genipin is an iridoid natural product that has been used as an anti-inflammatory agent in oriental medicine. It can be isolated from Gardenia jasminoides Ellis fruit [91]. Genipin at non-toxic doses can decrease the motility and invasion of tumor cells in human hepatocellular carcinoma cells (HepG2) and MHCC97L [92]. This effect is due to its ability to up-regulate TIMP-1 and it also can inhibit the activities of MMPs released from TNF-α-stimulated cells like MMP-1 and MMP-3 [93]. Aeroplysinin-1 is a metabolite of bromotyrosine that have diverse biological activities such as anti-angiogenic, antibiotic and anticancer effects [94]. Both enantiomers of aeroplysinin-1 can be isolated from diverse sponge species. The more studied enantiomer is ( +)-aeroplysinin-1 that was isolated from the yellow tube sponge Aplysina aerophoba. It can inhibit the bovine aortic endothelial (BAE) cell growth and decrease the expression of MMPs, particularly MMP-2 and urokinase expression [95]. It can also reduce the expression of MMP-1, MMP-2 and interleukin 1 alpha (Il-1α) in other endothelial cell types [94, 96].
The Aplysina aerophoba aqueous extract showed the ability to decrease the expression and the activity of MMP-2 and MMP-9 in rat astrocyte cultures. This indicates that the more polar compounds of this extract may participate in the chemical defenses of this marine organism [97]. Epigallocatechin—Gallate (EGCG) is another natural product that can bind to MMPs [98]. EGCG can block MMP-2 and MMP-9 enzymatic activity in lung carcinoma cells via direct binding with the proteins of MMP, which approved by affinity gel chromatography experiments [99]. A preliminary in silico analysis; performed by Chowdhury et al. in 2017, showed that there is a strong interaction between the galloyl group of ECG and EGCG and pro-MMP-2 in pulmonary artery smooth muscle cell culture supernatant. They demonstrated that ECG and EGCG are better inhibitors of proMMP-2, in contrast to MMP-2 [100]. Another study showed the interactions of green tea catechins with pro-MMP-9 via computational methods. This study showed a strong interaction between EGCG/ECG and pro-MMP-9 [101].
Spirulina platensis is a cyanobacterium that contains numerous important bioactive molecules [102]. These molecules have different biological activities such as anticancer, antioxidant [103], anti-inflammatory [104], neuroprotective [105, 106], hypolipidemic [107], antiviral [108] and hepatoprotective effects [109]. C-phycocyanin containing protein extract (C-PC extract) of Spirulina platensis can interfere with the activity of MMPs such as MMP-2 and MMP-9 and tissue inhibitors of MMPs (TIMP-2). C-PC extract can block the activity and the expression of MMP-2 and MMP-9 and it can also inhibit the expression of TIMP-2 in HepG2 cells [110]. Other natural products that inhibit MMPs include Neovastat (AE 941) and Genistein. Neovastat (AE 941) was extracted from shark cartilage. It showed antiangiogenic and antimetastatic effects by blocking MMP-2, MMP-9, MMP-12, MMP-13 and VEGF [111]. Genistein, a soy isoflavonoid similar to estradiol; can interfere with diverse MMPs and TIMPs expression [112, 113].
Flavonoids showed diverse mechanisms on the stages of initiation and promotion of carcinogenesis. The principal molecular mechanisms of their activity are; repression of mutant p53 protein, stimulation of apoptosis, hindering of the cell cycle, inhibition of the heat shock protein, and inhibition of Ras protein expression [114]. Several members have been proved to be useful in controlling metastasis in head and neck cancers as fisetin (3,7,3−,4− -tetrahydroxyflavone), kaempferol 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, quercetin is 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one [68].
Targeting MMP gene expression using microRNAs
MicroRNAs (miRNAs) are small noncoding RNAs, which contain 17–25 nucleotides. They participate in the posttranscriptional regulation of gene expression and control the stability and translation of mRNA by base-pairing with complementary 3′ untranslated region of mRNA. miRNAs can target and regulate the activity of MMPs, which can be therapeutic targets [115, 116].
Generally, the mechanism of action of these miRNAs in sustaining MMP expression includes the aberrant production of MMP protein, and other proteins contributing for the activation or inhibition of MMP, as osteopontin [35, 117]. The exact mechanism of action of miRNAs in this respect is still unclear, although modest observations reported loss of invasion, metastasis, and angiogenesis to be contributory mechanisms [118].
Numerous malignancies were involved during deregulation of MMPs by miRNAs, like glioblastoma [119], osteoarthritis [120], endometrial cancer [121], lung cancer [122], and bladder cancer [123].
miR-146 b was found to downregulate the activity of MMP-16 in U 373 glioma cells, affecting the migration and invasion of the tumor cells [124]. miR-93-5p also has a suppressive effect in glioma as it can target MMP-2. It was found that the expression of miR-93-5p was reduced in glioma by targeting MMP-2. The upregulation of miR-93-5p results in decreased expression of MMP-2, which affects the migration and invasion of U87-MG cells [125]. Osteosarcoma tissues and cell lines showed upregulation of miRNA-130b-5p and its overexpression is related to the poor prognosis of osteosarcoma patients. The upregulation of microRNA-130b-5p increases the invasion and the migration ability of osteosarcoma cells by negatively targeting TIMP-2 [126].
miR-21 participates in glioblastoma through the regulation of apoptosis, proliferation and invasion of glioma cells. It also can increase the aggressiveness of glioma cells through the activation of MMPs by targeting their inhibitors. The use of specific antisense oligonucleotides that can inhibit miR-21, causes an increase in the expression of reversion-inducing-cysteine-rich protein with kazal motifs (RECK) and TIMP-3 gene and protein levels. These lead to inhibiting the enzymatic activities of MMPs in vivo and in vitro, which can serve as a new anticancer therapy [127, 128]. In the highly metastatic brain-trophic metastatic MDA-MB-435-LvBr2 breast cancer cells, the induced upregulation of miR-146a can decrease the migration and the invasion ability of these cells by inducing β-catenin and downregulating MMP-1, urokinase-type plasminogen activator (uPA) and its receptor (uPAR) [129]. The expression of different MMPs can be regulated by a single miRNA due to sequence homology in MMP structure. For example, MMP-2 and MMP-9 can be downregulated by miR-143 at the protein and gene levels in pancreatic cancer cells [130]. miR-143 can target MMP-13 in osteosarcoma in vivo models [131]. The upregulation of miR-143 also can inhibit EGFR-dependent cell invasion by indirect mediating the expression of MMP-9 in osteosarcoma [132]. miR-146b can downregulate the expression of MMP-16 in U373 glioma cells [124]. From the previous data, it is clear that understanding the mechanism of miRNA in the regulation of MMPs expression will be helpful to use them as diagnostic or prognostic markers or serving as novel therapeutic targets to prevent the aggressive and metastatic malignancies.
Besides miRNAs, different studies have highlighted how DNA methylation is strongly associated with the alteration of MMPs expression levels. It was indicated that abnormal hypermethylation of many MMP gene promoters is a another indirectly functional event in breast carcinogenesis [133], which was previously exemplified by hypermethylation of MMP-9 gene in melanoma [134] and breast cancer [135].
Mono-clonal antibodies and MMPs
The broad range MMPIs have diverse side effects and failed in clinical trials so there is a great interest in using therapy that can target a specific MMP. Several monoclonal antibodies (mAbs) have been developed that can target the catalytic domain of a single MMP, which can be used in diverse primary and metastatic cancers [16]. DX-2400 is an antibody fragment (Fab) that can selectively inhibit the enzymatic activity of MT1. It can inhibit angiogenesis, tumor growth, invasion and metastasis in numerous preclinical models [136, 137]. Full-length mAb REGA-3G12 can target the catalytic domain of MMP-9 [138, 139] more selectively when compared to MMP-2 [140]. Another mAb is a humanized full-length allosteric mAb GS-5745, which can selectively inhibit the enzymatic activity of MMP-9. GS-5745 can inhibit tumor growth, invasion, and metastasis in a colorectal carcinoma model without exhibiting serious adverse effects [141]. Sela-Passwell et al. have developed inhibitory antibodies that have similar binding mechanisms as the endogenous TIMPs that can block gelatinase activities. Monoclonal antibodies SDS3 and SDS4 that can inhibit the enzymatic activities of MMP-9 and the closely related MMP-2 while showing, by an order of magnitude, lower inhibition of MMP-14 and sparing MMP-1, MMP-7, and MMP-12. These antibodies can bind their target MMPs through protein–protein interactions concerning the metal–protein motif, as well as to the enzyme surface. Further selectivity towards a single MMP may be achieved by classical protein engineering procedures that refine protein surface interactions between the antibody and the target enzyme [142]. Although the use of mAb is limited due to undesired effector functions, high production costs and selectivity restrictions, which result from the high homology of the catalytic sites of several MMPs, the treatment with mAb either alone or combined with chemotherapy might exhibit promising efficacy [63].
Conclusion
Matrix metalloproteinases (MMPs) are members of a zinc-dependent endopeptidase family. Being the most frequent cause of death worldwide; cancer gains a great interest in finding new and more effective anticancer therapies with fewer side effects [143]. Diverse and aggressive metastasis to vital body organs is always responsible for survival periods and quality of life among cancer patients. MMPs have a vital role in cancer progression by remodeling the ECM, so the development of new agents that can target MMPs or their inhibitors might help find newly synthesized anticancer therapies. The Selective inhibition of MMPs enzymatic activities in a specific location may present a more potential anticancer activity with lower adverse effects and more efficacy. MMPs can be investigated for their role in different processes like angiogenesis, cell migration, apoptosis and cell proliferation, which allow them to be used as tumor markers. Elevated activity and expression of MMPs in both patients' blood and tissues with numerous types of cancer was observed. MMPs might have a vital value as a diagnostic invasiveness marker to predict the risk of distant metastasis. The co-therapy of MMPIs with chemo, radio, surgical and hormonal therapy of cancer will certainly introduce great outcomes in survival among cancer patients.
Data availability
Data were obtained from cancer registries and published information. The journal after publication is authorized to make all data available.
Abbreviations
- ADAMs:
-
A disintegrin and metalloproteinase
- ADAMTs:
-
A disintegrin and metalloproteinases with thrombospondin motifs
- AKT:
-
Serine/threonine-specific protein kinase
- AP-1:
-
Activator protein-1
- BAE:
-
Bovine aortic endothelial
- ECM:
-
Extracellular matrix
- EGCG:
-
Epigallocatechin—Gallate
- EGF:
-
Epidermal growth factor
- EMT:
-
Epithelial-to-mesenchymal transition
- ERK:
-
Extracellular signal–regulated kinase
- Fab:
-
Antibody fragment
- FAK:
-
Focal adhesion kinase
- HIF-1:
-
Hypoxia-inducible factor 1
- Hpx:
-
Hemopexin
- MAPK:
-
Mitogen-activated protein kinase
- MHC:
-
Major histocompatibility complex
- mAbs:
-
Monoclonal antibodies
- MMPs:
-
Matrix metalloproteinases
- MMPIs:
-
MMP inhibitors
- MT-MMP:
-
Membrane-type MMPs
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor κB
- siRNA:
-
Small interfering RNA
- TACE:
-
TNF-α converting enzyme
- TGF-β:
-
Transforming growth factor-β
- TIMPs:
-
Tissue inhibitors of metalloproteinases
- uPA:
-
Urokinase-type plasminogen activator
- uPAR:
-
Urokinase-type plasminogen activator receptor
References
Kumar GB, Nair BG, Perry JJP, Martin DB (2019) Recent insights into natural product inhibitors of matrix metalloproteinases. MedChemComm 10:2024–2037
Lenci E, Cosottini L, Trabocchi A (2021) Novel matrix metalloproteinase inhibitors: an updated patent review (2014–2020). Expert Opin Ther Patents 31:509–523
Wen D, Chen Z, Zhang Z, Jia Q (2020) The expression, purification, and substrate analysis of matrix metalloproteinases in Drosophila melanogaster. Protein Exp Purif 171:105629
Kapoor C, Vaidya S, Wadhwan V, Kaur G, Pathak A (2016) Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther 12:28
Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665
Nguyen TT, Ding D, Wolter WR, Pérez RL, Champion MM, Mahasenan KV, Hesek D, Lee M, Schroeder VA, Jones JI, Lastochkin E, Rose MK, Peterson CE, Suckow MA, Mobashery S, Chang M (2018) Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J Med Chem 61:8825–8837
Winer A, Adams S, Mignatti P (2018) Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther 17:1147–1155
Lian G-Y, Wang Q-M, Mak TS-K, Huang X-R, Yu X-Q, Lan H-Y (2021) Inhibition of tumor invasion and metastasis by targeting TGF-β-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol Ther 20:277–289
Puente XS, Sánchez LM, Overall CM, López-Otín C (2003) Human and mouse proteases: a comparative genomic approach. Nat Rev Genet 4:544–558
Dufour A, Overall CM (2013) Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci 34:233–242
Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR, Ilbeigi S, Alahari SK (2021) Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 40:1043–1063
Zhong Y, Lu Y-T, Sun Y, Shi Z-H, Li N-G, Tang Y-P, Duan J-A (2018) Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin Drug Discov 13:75–87
Klein T, Bischoff R (2010) Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: biological function and structure. J Proteome Res 10:17–33
Xu I, Thériault M, Brunette I, Rochette PJ, Proulx S (2021) Matrix metalloproteinases and their inhibitors in Fuchs endothelial corneal dystrophy. Exp Eye Res 205:108500
Javaid MA, Abdallah M-N, Ahmed AS, Sheikh Z (2013) Matrix metalloproteinases and their pathological upregulation in multiple sclerosis: an overview. Acta Neurol Belg 113:381–390
Fischer T, Riedl R (2021) Challenges with matrix metalloproteinase inhibition and future drug discovery avenues. Expert Opin Drug Discov 16:75–88
Bode W, Gomis-Rüth F-X, Stöckler W (1993) Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins.’ FEBS Lett 331:134–140
Wolak D, Sechman A, Hrabia A (2021) Effect of eCG treatment on gene expression of selected matrix metalloproteinases (MMP-2, MMP-7, MMP-9, MMP-10, and MMP-13) and the tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary. Anim Reprod Sci 224:106666
Makowski GS, Ramsby ML (1998) Binding of matrix metalloproteinase 9 to fibrin is mediated by amorphous calcium-phosphate. Inflammation 22:599–617
Cui N, Hu M, Khalil RA (2017) Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 147:1–73
Öztürk VÖ, Meriç P, Sorsa T, Tervahartiala T, Bostanci N, Nwhator SO, Emingil G (2021) Regulation of matrix metalloproteinases-8,-9 and endogenous tissue inhibitor-1 in oral biofluids during pregnancy and postpartum. Arch Oral Biol 124:105065
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573
Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, Salvesen G, Nagase H (1990) Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem 194:721–730
Mannello F, Tonti G, Papa S (2005) Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets 5:285–298
Noël A, Jost M, Maquoi E (2008) Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol 19:52–60
Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA 102:9182–9187
Krzyzanowska-Gołab D, Lemańska-Perek A, Katnik-Prastowska I (2007) Fibronectin as an active component of the extracellular matrix. Postepy Hig Med Dosw(Online) 61:655–663
Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R (2011) Interaction of tumor cells with the microenvironment. Cell Commun Signal 9:18–18
Lu YE, Chen YJ (2021) Resveratrol inhibits matrix metalloproteinase-1 and-3 expression by suppressing of p300/NFκB acetylation in TNF-α-treated human dermal fibroblasts. Chem-Biol Interact 337:109395
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochem Biophys Acta 1833:3481–3498
Fouzder C, Mukhuty A, Kundu R (2021) Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch Biochem Biophys 697:108700
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer—roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Deryugina EI, Quigley JP (2010) Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochem Biophys Acta 1803:103–120
Tang M-L, Bai X-J, Li Y, Dai X-J, Yang F (2018) MMP-1 over-expression promotes malignancy and stem-like properties of human osteosarcoma MG-63 cells in vitro. Curr Med Sci 38:809–817
Napoli S, Scuderi C, Gattuso G, Di Bella V, Candido S, Basile MS, Libra M, Falzone L (2020) Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 9:1151
Geervliet E, Bansal R (2020) Matrix metalloproteinases as potential biomarkers and therapeutic targets in liver diseases. Cells 9:1212
Knapinska AM, Estrada C-A, Fields GB (2017) The roles of matrix metalloproteinases in pancreatic cancer. Prog Mol Biol Transl Sci 148:339–354
Jedryka M, Chrobak A, Chelmonska-Soyta A, Gawron D, Halbersztadt A, Wojnar A, Kornafel J (2012) Matrix metalloproteinase (MMP)-2 and MMP-9 expression in tumor infiltrating CD3 lymphocytes from women with endometrial cancer. Int J Gynecol Cancer 22:1303–1309
Edsparr K, Basse PH, Goldfarb RH, Albertsson P (2011) Matrix metalloproteinases in cytotoxic lymphocytes impact on tumour infiltration and immunomodulation. Cancer Microenviron 4:351–360
Chiou S-H, Sheu B-C, Chang W-C, Huang S-C, Hong-Nerng HJ (2005) Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol 67:35–50
Zhang Y-Y, Chen B, Ding Y-Q (2012) Metastasis-associated factors facilitating the progression of colorectal cancer. Asian Pac J Cancer Prev 13:2437–2444
Shen Z, Wang X, Yu X, Zhang Y, Qin L (2017) MMP16 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 8:72197
Garde A, Sherwood DR (2021) Fueling cell invasion through extracellular matrix. Trends Cell Biol 31:445–456
Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, Constantin C, Neagu M (2019) The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol 2019:10
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer: roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Chen H, He S, Sa G (2021) Podosome formation in the murine palatal mucosae: its proteolytic role in rete peg formation. Ann Anat 235:151703
Choi S, Myers JN (2008) Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res 87:14–32
Birchmeier C, Birchmeier W, Brand-Saberi B (1996) Epithelial-mesenchymal transitions in cancer progression. Acta Anat 156:217–226
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455
Han L, Zhou W, Wu F (2021) Long non-coding RNA LOC284454 promotes hepatocellular carcinoma cell invasion and migration by inhibiting E-cadherin expression. J Oncol Rep 45:1–1
Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118
Illman SA, Lehti K, Keski-Oja J, Lohi J (2006) Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119:3856–3865
McCawley LJ, Matrisian LM (2001) Tumor progression: defining the soil round the tumor seed. Curr Biol 11:R25–R27
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Luo Y, Hu J, Liu Y, Li L, Li Y, Sun B, Kong R (2021) Invadopodia: a potential target for pancreatic cancer therapy. Crit Rev Oncol/Hematol 159:103236
Jin Y-J, Ji Y, Jang Y-P, Choung S-Y (2021) Acer tataricum subsp. ginnala inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and TGFβ/Smad signaling in UVB-irradiated human dermal fibroblasts. Molecules 26:662
Kim J, Yu W, Kovalski K, Ossowski L (1998) Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 94:353–362
Park JY, Shin M-S (2021) Inhibitory effects of pectic polysaccharide isolated from Diospyros kaki leaves on tumor cell angiogenesis via VEGF and MMP-9 regulation. Polymers 13:64
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278:16–27
Gorelik L, Flavell RA (2001) Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 7:1118–1122
Willcockson H, Ozkan H, Chubinskaya S, Loeser RF, Longobardi L (2021) CCL2 induces articular chondrocyte MMP expression through ERK and p38 signaling pathways. Osteoarthr Cartilage Open 3:100136
Piperigkou Z, Manou D, Karamanou K, Theocharis AD (2018) Strategies to target matrix metalloproteinases as therapeutic approach in cancer. In: Cal S, Obaya AJ (eds) Proteases and cancer: methods and protocols. Springer, New York, pp 325–348
Wang XY, Wang YH, Song Z, Hu XY, Wei JP, Zhang J, Wang HS (2021) Recent progress in functional peptides designed for tumor-targeted imaging and therapy. J Mater Chem C 9(11):3749–3772
Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R (2019) Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol 137:57–83
Shi Y, Ma X, Fang G, Tian X, Ge C (2021) Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: recent progress and current challenges. NanoImpact 21:100293
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhibit Med Chem 31:177–183
Kubina R, Iriti M, Kabała-Dzik A (2021) Anticancer potential of selected flavonols: fisetin, kaempferol, and quercetin on head and neck cancers. Nutrients 13:845
Shi Y, Ma X, Fang G, Tian X, Ge C (2021) Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: recent progress and current challenges. NanoImpact. 21:100293
Das N, Benko C, Gill SE, Dufour A (2021) The pharmacological TAILS of matrix metalloproteinases and their inhibitors. Pharmaceuticals 14:31
Li W, Saji S, Sato F, Noda M, Toi M (2013) Potential clinical applications of matrix metalloproteinase inhibitors and their future prospects. Int J Biol Markers 28:117–130
Steward WP, Thomas AL (2000) Marimastat: the clinical development of a matrix metalloproteinase inhibitor. Expert Opin Investig Drugs 9:2913–2922
Yang J-S, Lin C-W, Su S-C, Yang S-F (2016) Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol 12:191–200
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer-roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Broccoli A, Zinzani PL (2021) Emerging new small molecules in peripheral T-cell lymphomas. Wiley, New York, pp 343–349
Vihinen P, Kähäri V-M (2002) Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 99:157–166
Hidalgo M, Eckhardt SG (2001) Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 93:178–193
Rudzińska M, Daglioglu C, Savvateeva LV, Kaci FN, Antoine R, Zamyatnin A Jr (2021) Current status and perspectives of protease inhibitors and their combination with nanosized drug delivery systems for targeted cancer therapy. Drug Des Dev Ther 15:9
Kanagaraj AS, Kumar Patel VM (2020) Host modulation therapy: a mini review. Arch Oral Biol 105:72–50
Sapadin AN, Fleischmajer R (2006) Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol 54:258–265
Dedes P, Kanakis I, Gialeli C, Theocharis A, Tsegenidis T, Kletsas D, Tzanakakis G, Karamanos N (2013) Preclinical evaluation of zoledronate using an in vitro mimetic cellular model for breast cancer metastatic bone disease. Biochim Biophys Acta 1830:3625–3634
Li X-Y, Lin Y-C, Huang W-L, Hong C-Q, Chen J-Y, You Y-J, Li W-B (2012) Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngeal carcinoma cells. Med Oncol 29:714–720
Coleman R, Cook R, Hirsh V, Major P, Lipton A (2011) Zoledronic acid use in cancer patients: more than just supportive care? Cancer 117:11–23
Moses AS, Demessie AA, Taratula O, Korzun T, Slayden OD, Taratula O (2021) Nanomedicines for endometriosis: lessons learned from cancer research. Small 17(7):2004975
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Abdel-Hamid NM, Nazmy MH, Abdel-Bakey AI (2011) Polyol profile as an early diagnostic and prognostic marker in natural product chemoprevention of hepatocellular carcinoma in diabetic rats. Diabetes Res Clin Pract 92:228–237
Elmosallamy A, Abdel-Hamid N, Srour L, Hussein SA (2020) Identification of polyphenolic compounds and hepatoprotective activity of artichoke (Cynara scolymus L.) edible part extracts in rats. Egypt J Chem 63(6):2273–2285
Kamel HN, Slattery M (2005) Terpenoids of sinularia.: chemistry and biomedical applications. Pharm Biol 43:253–269
Wu Y-J, Neoh C-A, Tsao C-Y, Su J-H, Li H-H (2015) Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci 16:16469–16482
Cheng T-C, Din Z-H, Su J-H, Wu Y-J, Liu C-I (2017) Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar Drugs 15:238
Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A, Sethi G (2018) Potential role of genipin in cancer therapy. Pharmacol Res 133:195–200
Wang N, Zhu M, Tsao S-W, Man K, Zhang Z, Feng Y (2012) Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS ONE 7:e46318–e46318
Shindo S, Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T (2014) Genipin inhibits MMP-1 and MMP-3 release from TNF-a-stimulated human periodontal ligament cells. Biochimie 107:391–395
García-Vilas JA, Martínez-Poveda B, Quesada AR, Medina MÁ (2015) Aeroplysinin-1, a sponge-derived multi-targeted bioactive marine drug. Mar Drugs 14:1–1
Martínez-Poveda B, García-Vilas JA, Cárdenas C, Melgarejo E, Quesada AR, Medina MA (2013) The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro- inflammatory molecules in human endothelial and monocyte cells. PLoS ONE 8:e55203–e55203
Ciccone L, Vandooren J, Nencetti S, Orlandini E (2021) Natural marine and terrestrial compounds as modulators of matrix metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s disease. Pharmaceuticals 14:86
Di Bari G, Gentile E, Latronico T, Corriero G, Fasano A, Nonnis Marzano C, Liuzzi GM (2015) Inhibitory effect of aqueous extracts from marine sponges on the activity and expression of gelatinases A (MMP-2) and B (MMP-9) in rat astrocyte cultures. PLoS ONE 10:e0129322–e0129322
Negri A, Naponelli V, Rizzi F, Bettuzzi S (2018) molecular targets of epigallocatechin-gallate (EGCG): a special focus on signal transduction and cancer. Nutrients 10:1936
Sazuka M, Imazawa H, Shoji Y, Mita T, Hara Y, Isemura M (1997) Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci Biotechnol Biochem 61:1504–1506
Chowdhury A, Nandy SK, Sarkar J, Chakraborti T, Chakraborti S (2017) Inhibition of pro-/active MMP-2 by green tea catechins and prediction of their interaction by molecular docking studies. Mol Cell Biochem 427:111–122
Sarkar J, Nandy SK, Chowdhury A, Chakraborti T, Chakraborti S (2016) Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed Pharmacother 84:340–347
Desai K, Sivakami S (2004) Spirulina: the wonder food of the 21st Century. Asia-Pacific Biotech News 8:1298–1302
Miranda M, Cintra R, Barros SBDM, Mancini-Filho J (1998) Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res 31:1075–1079
Abdel-Daim MM, Farouk SM, Madkour FF, Azab SS (2015) Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol 37:126–139
Pérez-Juárez A, Chamorro G, Alva-Sánchez C, Paniagua-Castro N, Pacheco-Rosado J (2016) Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm Biol 54:1408–1412
Salama AF, Abdel-Hamid NM, El-Sheekh M, Tosson E, Gabr AM (2017) Spirulina platensis microalgae protects against diethyl nitrosamine carcinogenic effect on female albino rats. Alex J Vet Sci 53:167–179
Samuels R, Mani U, Iyer U, Nayak U (2002) Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food 5:91–96
Chen Y-H, Chang G-K, Kuo S-M, Huang S-Y, Hu I-C, Lo Y-L, Shih S-R (2016) Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality. Sci Rep 6:24253
Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem 141:1972–1979
Kunte M, Desai K (2017) The inhibitory effect of C-phycocyanin containing protein extract (C-PC Extract) on human matrix metalloproteinases (MMP-2 and MMP-9) in hepatocellular cancer cell line (HepG2). Protein J 36:186–195
Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Mehrotra R (2010) Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci 17:10–10
Kousidou OC, Mitropoulou T, Roussidis A, Kletsas D, Theocharis A, Karamanos N (2005) Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol 26:1101–1109
Ramkita N, Falamy R, Farishal A (2021) Potential of genistein isoflavones as supportive therapy in prostate cancer. Cancer 2:63–70
Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M (2016) Anticancer efficacy of polyphenols and their combinations. Nutrients 8:552
Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T (2011) Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res 1411:108–115
Wang H, Qi C, Wan D (2021) MicroRNA-377–3p targeting MMP-16 inhibits ovarian cancer cell growth, invasion, and interstitial transition. Ann Transl Med 9:124
Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, Wang H, Chen Y, Liu K, Shao Z, Shang Z (2018) Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 37:1–15
Abba M, Patil N, Allgayer H (2014) MicroRNAs in the regulation of MMPs and metastasis. Cancers (Basel) 6:625–645
Li L, Li H (2013) Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol Ther 14:796–805
Xu B, Li Y-Y, Ma J, Pei F-X (2016) Roles of microRNA and signaling pathway in osteoarthritis pathogenesis. J Zhejiang Univ 17:200–208
Ruan H, Liang X, Zhao W, Ma L, Zhao Y (2017) The effects of microRNA-183 promots cell proliferation and invasion by targeting MMP-9 in endometrial cancer. Biomed Pharmacother 89:812–818
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang L, Zhang H, Chen X, Yang Y, Liu G (2013) miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin β1 and matrix metalloproteinase2 (MMP2). PLoS ONE 8:e70192
Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M (2016) Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget 7:72758–72766
Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He M-L, Kung H-F, Lai L, Lin MC (2009) microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res 1269:158–165
Wu H, Liu L, Zhu JM (2019) MiR-93-5p inhibited proliferation and metastasis of glioma cells by targeting MMP2. Eur Rev Med Pharmacol Sci 23:9517–9524
Cheng ZH, Luo C, Guo ZL (2019) MicroRNA-130b-5p accelerates the migration and invasion of osteosarcoma via binding to TIMP2. Eur Rev Med Pharmacol Sci 23:9267–9276
Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28:5369–5380
Costa PM, Cardoso AL, Custódia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC (2015) MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release 207:31–39
Hwang SJ, Seol HJ, Park YM, Kim KH, Gorospe M, Nam D-H, Kim HH (2012) MicroRNA-146a suppresses metastatic activity in brain metastasis. Mol Cells 34:329–334
Hu Y, Ou Y, Wu K, Chen Y, Sun W (2012) miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour Biol 33:1863–1870
Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T (2011) MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther 19:1123–1130
Wang Q, Cai J, Wang J, Xiong C, Zhao J (2014) MiR-143 inhibits EGFR-signaling-dependent osteosarcoma invasion. Tumour Biol 35:12743–12748
Simonova OA, Kuznetsova EB, Tanas AS, Rudenko VV, Poddubskaya EV, Kekeeva TV, Trotsenko ID, Larin SS, Kutsev SI, Zaletaev DV (2020) Abnormal hypermethylation of CpG dinucleotides in promoter regions of matrix metalloproteinases genes in breast cancer and its relation to epigenomic subtypes and HER2 overexpression. Biomedicines 8:116
Falzone L, Salemi R, Travali S, Scalisi A, McCubrey JA, Candido S, Libra M (2016) MMP-9 overexpression is associated with intragenic hypermethylation of MMP9 gene in melanoma. Aging (Albany NY) 8:933
Klassen LM, Chequin A, Manica GC, Biembengut IV, Toledo MB, Baura VA, Pedrosa FDO, Ramos EA, Costa FF, De Souza EM (2018) MMP9 gene expression regulation by intragenic epigenetic modifications in breast cancer. Gene 642:461–466
Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, van Gool R, Sexton DJ, Kuang G, Rank D, Hogan S, Pazmany C, Ma YL, Schoonbroodt S, Nixon AE, Ladner RC, Hoet R, Henderikx P, TenHoor C, Rabbani SA, Valentino ML, Wood CR, Dransfield DT (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res 69:1517–1526
Lemaître V, D’Armiento J (2006) Matrix metalloproteinases in development and disease. Birth defects research. Part C. Embryo Today 78:1–10
Paemen L, Martens E, Masure S, Opdenakker G (1995) Monoclonal antibodies specific for natural human neutrophil gelatinase B used for affinity purification, quantitation by two-site ELISA and inhibition of enzymatic activity. Eur J Biochem 234:759–765
Martens E, Leyssen A, Van Aelst I, Fiten P, Piccard H, Hu J, Descamps FJ, Van den Steen PE, Proost P, Van Damme J, Liuzzi GM, Riccio P, Polverini E, Opdenakker G (2007) A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochem Biophys Acta 1770:178–186
Hu J, Van den Steen PE, Houde M, Ilenchuk TT, Opdenakker G (2004) Inhibitors of gelatinase B/matrix metalloproteinase-9 activity comparison of a peptidomimetic and polyhistidine with single-chain derivatives of a neutralizing monoclonal antibody. Biochem Pharmacol 67:1001–1009
Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, Lee M, O’Sullivan C, Barry-Hamilton V, Ghermazien H, Mikels-Vigdal A, Garcia CA, Jorgensen B, Velayo AC, Wang R, Adamkewicz JI, Smith V (2015) Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS ONE 10:e0127063
Sela-Passwell N, Kikkeri R, Dym O, Rozenberg H, Margalit R, Arad-Yellin R, Eisenstein M, Brenner O, Shoham T, Danon T, Shanzer A, Sagi I (2011) Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat Med 18:143–147
Abdel-Hamid NM, Abass SA, Mohamed AA, Muneam Hamid D (2018) Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed Pharmacother 107:1246–2125
Mitropoulou TN, Tzanakakis GN, Kletsas D, Kalofonos HP, Karamanos NK (2003) Letrozole as a potent inhibitor of cell proliferation and expression of metalloproteinases (MMP-2 and MMP-9) by human epithelial breast cancer cells. Int J Cancer 104:155–160
Falardeau P, Champagne P, Poyet P, Hariton C, Dupont É (2001) Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Seminars in oncology. Elsevier, Amsterdam, pp 620–625
Author information
Authors and Affiliations
Contributions
SA: Collection of data and manuscript drafting. NM: Suggestion of the article outline and title, revision of the article, preparation to publication. Both authors equally contributed to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
KFS University Committee of Scientific Research approved the work.
Consent for publication
Accept.
Consent for participation
NOt applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Hamid, N.M., Abass, S.A. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol Biol Rep 48, 6525–6538 (2021). https://doi.org/10.1007/s11033-021-06635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06635-z