Abstract
Atherosclerosis involves both innate and adaptive immunity. Here, we provide an overview of the role of regulatory T (Treg) cells in atherosclerotic diseases. Treg cells and their inhibitory cytokines, IL-10 and TGF-β, have been identified in atherosclerotic lesions and to inhibit progression through lipoprotein metabolism modulation. Treg cells have also been found to convert to T follicular helper (Tfh) cells and promote atherosclerosis progression. Treg cell involvement in different stages of atherosclerotic progression and Treg cell-mediated modulation of plaque development occurs via inflammation suppression and atheroma formation has been focused. Moreover, existing knowledge suggests that Treg cells are likely involved in the pathology of other specific circumstances including in-stent restenosis, neointimal hyperplasia, vessel graft failure, and ischemic arterial injury; however, there remain gaps regarding their specific contribution. Hence, advancements in the knowledge regarding Treg cells in diverse aspects of atherosclerosis offer translational significance for the management of atherosclerosis and associated diseases.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is characterized by the accumulation of plaque (vascular lesions which contain fat, cholesterol, calcium, and other substances) in arteries [1]. Rupture of the plaque may result in a thrombus that blocks blood flow and leads to in coronary heart disease, angina, carotid artery disease, peripheral artery disease, or many other complications [2]. According to a recent survey, heart diseases kill about 655,000 people in the United States every year, with coronary heart disease being the most common type [3]. Atherosclerosis is also characterized as a slow, progressive, chronic inflammatory disease and the understanding of the disease has shifted with advancements in medical sciences [1, 2]. Previously, atherosclerosis was thought to result solely from passive lipid accumulation in the vessel wall [3]. However, current understanding regarding the connection between immune response and lipid metabolism in the context of atherosclerosis has grown over time and is now known to be associated with elevated low-density lipoprotein (LDL) cholesterol and the corresponding autoimmune response to LDL [1, 3]. Immune cells, in addition to cap thickness, influence the plaque stability, which is important because unstable and ruptured atherosclerotic plaques lead to major adverse cardiovascular events (MACE) [1].
Immunology of atherosclerosis
Inflammation has been demonstrated to be crucial in the development of atherosclerotic plaques [4]. The immune response relating to atherosclerosis involves both innate and adaptive immunity. Cells involved in these responses include macrophages, T lymphocytes, B lymphocytes, endothelial cells, and vascular smooth muscles cells [4]. The immune response starts as innate immunity, playing an important role both for the maintenance of a healthy arterial wall and for atherosclerotic development [5].
Endothelial cell dysfunction with subendothelial deposition and lipoprotein modification can serve as damage-associated molecular patterns (DAMPs) to activate immune receptors and recruit macrophages [4]. In particular, when LDLs are oxidized, they establish immunogenic properties and contribute to the development of atherosclerotic plaques through its accumulation in the internal vascular walls [6]. Oxidized low-density lipoproteins (ox-LDLs) activate plaque macrophages and couple macrophage activation with foam cell formation [4]. Fatty streaks consisting of these foam cells are characteristic of early stages of atherosclerosis and serve to further promote inflammatory responses which occur throughout plaque development [7, 8]. After the foam cells, the debris and cholesterol accumulate to form stable extracellular deposits [8]. Additionally, the phagocytosis by macrophages produce aggregated cholesterol complexes or crystals [9]. Resulting cholesterol burden causes inflammasome activation as well as myeloid cell response including secretion of pro-inflammatory cytokines resulting in further proliferation of macrophages [9].
The death of lipid-loaded foam cells resembles necrosis than apoptosis [10]. Necrotic plaque cells release cytoplasmic content into the necrotic core, which contains a mixture of lipids, mostly consisting of cholesterol and cholesterol esters, and cell debris [10]. Furthermore, the necrosis stimulates inflammation through the loss of membrane integrity and leakage of intracellular components that act as danger signals [10]. Necrotic core originates early in lesion and is commonly associated with advanced atherosclerosis [10]. Besides oxidative stress, necrosis is triggered by depletion of ATP, increased intracellular calcium (which is an important signaling molecule from numerous cell responses), and impairment in phagocytic clearance of apoptotic cells by macrophages [10].
Myeloid cells are recruited and also accompanied with the infiltration of the cells of adaptive immunity including B and T cells [9]. B and T cells are both part of the cellular composition of human atherosclerotic plaques [9]. B cells play a role in systemic and local immune responses in atherosclerosis via direct cell contact, antigen presentation, and cytokine production [6]. Studies on passive and active immunization support that B cell-derived antibodies, especially IgM, play an atheroprotective role [11]. In particular, the IgM antibodies that bind to oxidation motifs in LDLs from B-1 cells and have been shown to be atheroprotective [11]. On the other hand, B-2 cells, which are conventional B cells that participate in adaptive immune responses, have been shown to promote atherosclerosis through the manipulation of the B cell activating factor (BAFF) receptor pathway, in which BAFF binds to its receptor for B cell survival, activation, and differentiation [11].
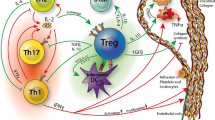
Evidence shows T cells to be critical drivers and modifiers of atherosclerosis [12]. About 70% of T cells present in atherosclerotic plaques are CD4+ T cells [13, 14]. T cell subsets, including CD4+ T helper 1 (Th1) cells and natural killer (NK) T cells, are pro-atherogenic while others, including Treg cells are anti-atherogenic [12]. Of the CD4 T cells, most are CD8+ T cells [14]. In fact, CD4 deficiency seems to result in compensatory changes through CD8+ T cells [14]. CD8+ T cells have been shown to produce inflammatory cytokines and have cytotoxic activity toward endothelial cells driving atherosclerosis [15]. However, Treg cells exhibit cytotoxic activity toward antigen-presenting cells to dampen immunity [15]. Because of their function in modulating immunity, Treg cells is an important cell type to understand in the context of inflammatory diseases including atherosclerosis. They may be able to explain differences in disease progression and presentation. Additionally, they serve as a potential target for disease interventions. The major class of immune cells and mediators associated with atherosclerosis are depicted in Fig. 1. In this review, we explore current understanding regarding Treg cells, especially in the context of atherosclerosis.
Role of immune cells at site of atherosclerotic lesions: Macrophages are recruited to sites of arterial lesions by damage-associated molecular patterns (DAMPs) which activate its pattern recognition receptors (PRRs). These macrophages engulf oxidized low-density lipoprotein (ox-LDL) deposits and transform into foam cells. Fatty streaks of foam cells further promote inflammatory responses throughout plaque development. Death of foam cells creates extracellular pools of cell debris and cholesterol unable to be removed. Dendritic cells also accumulate at these sites and can present antigens to T cells, which act as critical drivers and modifiers of atherosclerosis disease. B cells at the site play a role through oxLDL-binding antibodies especially IgM that can be atheroprotective
Regulatory T cells
Naïve CD4+ T cells differentiate into distinct T cell sub-populations, including Treg cells, based on specific antigen and cytokine stimulation [16]. These CD4+ CD25+ FOXP3+ Treg cells, a subset that comprises 5–10% of peripheral CD4+ T cells, play an important role in immune system homeostasis regulating autoimmunity through the regulation of other immune cells including effector T cells [16, 17]. This characteristic feature has motivated research regarding the role of Treg cells in cardiovascular diseases such as atherosclerosis [16]. Within regulatory T cells, there exists different subsets based on the origin [18]. Thymus-derived Treg cells are CD28-dependent and peripherally-derived Treg cells are CD28-independent [18]. CD28 does play an important role in rapid proliferation of both the Treg cell subsets [18]. Generally, Treg cells are generated during the positive selection of CD4+ T cells via a two-step process [19]. The first step involves TCR stimulation in developing CD4+ Thymocytes which upregulates CD25 among others to generate CD25+ FOXP3- Treg cell progenitors (TregP), which are then converted into mature Treg cells via the upregulation of FOXP3 in a cytokine-dependent second step [19]. Moreover, the Treg cells are able to suppress other T cells and antigen presenting cells (APCs) through mechanisms such as inhibitory cytokines including transforming growth factor (TGF)-β and interleukin (IL)-10 [16, 20]. Treg cells can be classified according to their cytokine production with TGF-β production from Th3 cells, IL-10 production from Tr10 cells, and IL-35 production from Tr35 cells [20]. TGF-β suppresses effector T cell differentiation, promotes differentiation of naïve T cells into regulatory T cells, inhibits T and B cell proliferation, and inhibits activity of macrophages, dendritic cells, and NK cells [20]. IL-10 inhibits inflammatory cytokine production as well as tyrosine phosphorylation in the costimulatory molecule CD28, which is involved in the effector cell and APC interaction [20]. IL-35 is an immunosuppressive cytokine that suppresses proliferation of T helper cells and promotes naïve T cell differentiation into Treg cells [20].
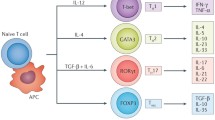
Other mechanisms by which Treg cells act include inhibition of effector cells by producing granzyme and perforin, metabolic interruption, and dendritic cell modulation [20]. Treg cells secrete granzyme and perforin during Treg-effector cell interaction [20]. Perforin molecules, which insert into cellular membrane to form pores, facilitate granzyme entry into target cells to induce apoptosis [20]. For metabolic interruption, Treg cells constitutively express high levels of IL-2 alpha chain, part of the IL-2 receptor, and thus competes with proliferating effector cells for IL-2 [20]. Treg cells interact with dendritic cells through cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), competing with effector cells to bind CD80 and CD86 on dendritic-cells [20]. The mediators and receptors of Treg cells are shown in Fig. 2.
Treg cell receptors and mediators: Treg cells act to suppress other T cells and APCs through production of inhibitory cytokines including IL-10 (from Tr10 cells), TGF-β (from Th3 cells), and IL-35 (from Tr35 cells). They also prevent effector cell action by competing for IL-2 with Treg cells’ high expression of IL-2 alpha chain (ILR2A) as well as for dendritic cell interaction through CTLA-4 and CD80/CD86 interaction. Lastly, they incite apoptosis of target cells through granzyme and perforin production
Regulatory T cells in atherosclerosis
Regulatory T cells are found in lesions of patients with atherosclerosis and are crucial in atherosclerotic processes as a point of crosstalk between immunity and lipoprotein metabolism [21]. This is summarized in Table 1. Through mouse studies, FOXP3+ Treg cells have been shown to inhibit atherosclerosis by modulating lipoprotein metabolism [22]. Conversely, depleting functional Treg cells aggravates atherosclerosis, as seen through increased atherosclerotic lesion size and reduced immune cell infiltration into the lesion [22]. This depletion impairs lipoprotein catabolism with reduction of very-low-density lipoprotein (VLDL) and chylomicron remnant clearance [22]. The atherosclerotic environment is also able to impact phenotype and plasticity of Treg cells [21]. The major role of regulatory T cells in atherosclerosis is displayed in Table 1.
Alterations in intracellular cholesterol homeostasis affects Treg cell development as cholesterol is a modulator of both innate and adaptive immunity [24]. Cholesterol enrichment in T cell lipid rafts leads to the clustering of T-cell receptor (TCR) signaling complex, which leads to T-cell activation and proliferation, Th17 and Th1 differentiation, and downregulation of Treg [21]. As described above, FOXP3 is a Treg cell lineage-specification factor that plays a crucial role in Treg cells by upholding immunologic tolerance [23]. FOXP3 is found in different isoforms in humans based on exclusion of exon 2 and/or exon 7 [24]. Co-expressed on Treg cells, FOXP3fl and FOXP3Δ2 comprise 95% of total FOXP3 protein [24]. However, mice models lack different isoforms of FOXP3 which demands additional models for Treg research [24]. FOXP3Δ2 has been shown to be the predominant isoform in the increase of FOXP3 expression during Treg cell activation in humans [23]. Plaque instability is associated with lower transcript usage of FOXP3Δ2, suggesting that FOXP3Δ2 controls transcriptional program in Treg cells for protection in human atherosclerotic plaques [23]. FOXP3Δ2 induces the expression of glycoprotein A repetitions predominant (GARP), which tethers TGF-β to the surface of activated Treg cells [23]. The multiple pathways connected to TGF-β signaling are known to be important for cell proliferation, cell migration, matrix synthesis, wound contraction, calcification, and immune response [25]. A constitutive level of TGF-β signaling is present and considered atheroprotective since it functions for normal tissue repair [25]. Besides TGF‐β, IL-10 is another cytokine associated with Treg cells. IL-10 has been shown to have anti-inflammatory properties which decelerates the progression of atherosclerosis by inhibiting inflammation and cell apoptosis [26]. Comparing control to patient groups, higher levels of Treg cells and IL-10 were present in the control group as reported in a seminal study [27]. IL-10 was the only cytokine found to have such a correlation with Treg cell level [27]. Similarly, in a separate study with human patients, serum IL-10 along with Treg cells, and other IL-10 producing CD4+ T lymphocytes, are initially lower in patients with progression of coronary atherosclerosis than controls [26]. In particular, low baseline IL-10 levels and levels of CD4 + lymphocytes that produce IL-10 have been found to be associated with coronary atherosclerosis progression in stable coronary artery disease (CAD) patients [26]. Furthermore, patients with stable CAD and a history of at least two myocardial infarctions presented lower Treg cells and IL-10 levels in the blood than those without myocardial infarctions [26]. The interplay between Treg and TH17 cells are shown in Fig. 3.
Imbalance between Treg and Th17 cells: Treg/Th17 balance is regulated by FOXP3/STAT5 and RORγt/STAT3 transcription factors, respectively. Treg/Th 17 ratio can be influenced by ox-LDL, HMGB1, and IL-35. Ox-LDL and HMGB1 decreases the Treg/Th17 ratio by inducing Treg apoptosis and Th17 proliferation. IL-35, on the other hand, promotes Treg levels while preventing Th17 levels. Treg/Th17 ratio has been a therapeutic target with pioglitazone and Angong niuhuang pill both showing anti-atherosclerotic properties and modulation of Treg/Th17 balance. Conversely, high dietary salt intake and P. Gingivalis infection have been associated with atherosclerosis development through Treg/Th17 ratio
Regulatory T cells in Atherosclerosis: Proportion with Th17 cells
The impact of Treg cells not only depends on their characteristics and actions, but also its relative proportion with other cells. For example, the imbalance between Treg and Th17 cells is intimately associated with atherosclerosis and acute coronary syndrome [28]. The balance between Th17 and Treg cells depends on transcription factors especially RORγt (factors retinoic acid receptor-related orphan receptor γt) and STAT3 (signal transducer and activator of transcription 3), and FOXP3 (forkhead box P3) and STAT5 (signal transducer and activator of transcription 5) respectively [29]. Moreover, Treg/Th17 negatively correlates with serum levels of ox-LDL, high-sensitivity C-reactive protein, lipoprotein A, and creatine kinase-MB [28]. The predictive specificity and sensitivity possible with Treg/Th17 ratio makes it a potential indicator for early diagnosis [28].
Oxidized low-density lipoprotein influences Treg/Th17 ratio as ox-LDL induces Treg apoptosis through the Fas/FasL pathway as well as NF-κB-associated Th17 proliferation [30]. In addition, the balance between Treg cells and Th17 cells is modulated by high mobility group box-1 protein (HMGB1), an endogenous damage-associated molecular pattern (inflammatory DAMP) molecule released from necrotic or injured cells that stimulate expression and/or secretion of cytokines, adhesion molecules, chemokines, lipid mediators and plasminogen activator in smooth muscle cells or endothelial cells [31]. HMGB1 levels tend to be significantly higher in serum of atherosclerosis patients than that of control patients [31]. Recombinant HMGB1 induces Treg cell apoptosis and promotes Th17 cells, which ultimately lead to an overall decrease in the Treg/Th17 cell ratio [31]. Furthermore, the anti-inflammatory cytokine IL-35 has been reported to attenuate atherosclerosis [32]. In mice, IL-35 promotes Treg levels, prevents Th17 levels, and does not affect Th1 or Th2 levels [32]. The deficiency of the IL-12p35 subunit of IL-35 in particular has been shown to aggravate Treg/Th17 cell balance [32].
Regulating Treg/Th17 cell ratio has been explored as a method for promoting anti-atherosclerotic responses. The anti-diabetic thiazolidinediones drug, Pioglitazone (PIO), induces AMPK phosphorylation, decreases IL-17+ cells, and increases FOXP3+ cells in mice [33]. The treatment shows ability to stabilize atherosclerotic plaques through AMPK-dependent regulation of Treg/Th17 cell ratio [33]. In addition, Angong Niuhuang Pill (ANP), a well-known patented Chinese medicine used to treat stroke, encephalitis, and meningitis, has been shown to have anti-atherosclerosis effects [34]. In a mouse model, ANP significantly downregulates mRNA expression levels of RORγt and significantly upregulates mRNA expression levels of FOXP3. From a lifestyle perspective, high-salt diet promotes Th17 cells [29]. Additionally, peripheral tolerance and maintenance of tolerance to self-antigens by Treg cells are inhibited following high-salt diet in human subjects [29]. The resulting imbalance of these cells from the combined effects results in inflammation and organ damage [29]. Other diseases and infections associated with atherosclerosis development have impact on Treg cell and Th17 cell frequency. For instance, Porphyromonas gingivalis, a Gram-negative anaerobic bacterium, is a known risk factor for atherosclerosis [35]. Upon P. gingivalis-challenge, splenic lymphocytes of mice had significantly increased proportion of CD4+ IL-17+ cells along with CD4+ FOXP3+ [35]. Interestingly, Treg/Th17 balance varies with different stages of atherosclerosis [36]. Frequencies of CD4+ FOXP3+ and CD4+ IL10+ T cells were decreased and frequencies of CD4+ IL17+ T cells were increased in patients with severe coronary atherosclerosis [36]. Overall, Treg/Th17 ratio was lower in patients with either severe atherosclerosis or disease progression in non-invaded coronary arteries following coronary stenting than patients with intact coronary arteries or no disease progression following coronary stenting [36]. This suggests relevance of Treg/Th17 ratio in the progression of atherosclerosis [36].
Regulatory T cells in atherosclerosis: proportion with other cells
While the balance of Tregs and Th17 cells is the most studied, research has also been conducted on the ratio of Tregs with other cells [37]. The balance between Treg and Th2 cells has been studied using an allergic asthma model in mice [37]. Accelerated atherosclerosis induced by allergic asthma is accompanied by increased Th2 cells and decreased Treg cells in the spleen of the mouse model [37]. Additionally, curcumin ameliorates the aggravation of atherosclerotic lesions and modulates the balance of Treg and Th2 cells [37]. The balance of frequencies between Treg cells and CD4+CD28null T cells has been considered in atherosclerosis. CD4+CD28null T cells are unique helper T lymphocyte subset that induce damage to tissues or amplify inflammation by releasing inflammatory cytokines and cytotoxic molecules [38]. In addition, significantly higher frequency of CD4+CD28null T lymphocytes has been observed in Non-ST Elevation Myocardial Infarction (NSTEMI) patients with evidence of ruptured fibrous cap compared to those with evidence of intact fibrous cap and stable angina [39]. These CD4+CD28null T cells represent a subset of long-living cytotoxic cells with increased resistance to apoptosis, which are shown to have pro-atherogenic and plaque-destabilizing properties [39]. Overall, the CD4+CD28null T cell/Treg ratio suggests that plaque rupture involves an immune imbalance toward aggressive effector T cells [39].
Regulatory T cells in atherosclerosis: T follicular helper cells
As a key regulator in the development and function of Treg cells, diminished FOXP3 expression is shown to impair the suppressive phenotype of Treg cells [40]. In a study on mice, loss of FOXP3 expression leads to Treg cells losing their immunosuppressive function [41]. A fraction of these cells then convert into T follicular helper (Tfh) cells [41]. This conversion correlates with decreased IL-2Rα and pSTAT5 expression and increased IL-6Rα expression [41]. Tfh cells are found to be pro-atherogenic and the mice that lack Tfh cells retain normal Treg cell percentages compared to controls show reductions of atherosclerosis development [41]. This finding likely translates in human cells as a significant inverse correlation between plasma levels of IL-21, a major cytokine in Tfh cells, and FOXP3 has been observed [41]. In addition, this finding suggests a possibility that preventing the conversion from Tregs to Tfh cells can have a positive impact for atherosclerosis. Interestingly, elevated expression of apolipoprotein AI (ApoAI), a structural protein found in high-density lipoprotein (HDL), is associated with reduced Tfh cells and its injection into mice has been shown to prevent the conversion of Tregs to Tfh cells [41]. This appears to occur through increasing IL-2Rα and decreasing IL-6Rα expression, which is accompanied by a reduction of plasma IL-6 levels [41]. Analysis of IL-21 and FOXP3 levels in plasma of human subjects with coronary artery disease showed an inverse correlation, consistent with findings using mice [41].
A subtype of Treg cells, follicular regulatory T (Tfr) cells express the receptor CXCR5 along with FOXP3 and CD25 [13]. Tfr cells negatively regulate follicular T (Tfh) cell populations; however, their protective function can extend past this regulation [13]. Part of the protective properties of Tfr cells have been traced to the stimulation of lymphangiogenesis and regulatory B (Breg) cell proliferation [13]. Control on Breg cell population occurs through direct cell interaction; and together, the Breg and Tfr cell populations form an amplification loop of regulation [13]. Additionally, the associated decreased Tfh cell population leads to nonantigen-specific B cells expansion under pathological conditions [13].
Treg cells in clinical implication of atherosclerosis
The role of Treg cells and the downstream signaling have been intimately associated with the clinical presentation and therapeutic implications of atherosclerosis. Interestingly, a causal relationship between deficiency of CD4+ CD25+ Treg cells and development of atherosclerotic lesions has been shown in mice. Administration of mice with splenocytes lacking in Treg cells and CD28 exhibited lesions more than double size displaying advanced plaque phenotypes compared to those injected with wild-type splenocytes, despite no significant difference in serum cholesterol level [42]. Moreover, the co-transfer with CD4+ CD25+ Treg cells attenuated the induction of atherosclerosis [42]. Transfer with CD4+ cells without CD25 resulted in development of inflammatory lipid lesions comparable to those injected with wild-type splenocytes [42]. This was further supported through study of atherosclerosis development in mice treatment with a CD25-depleting antibody that depletes Treg cells [42]. Compared to mice treated with control IgG, there was a 50% increase in lesion size with less collagen and more T cell and macrophage accumulation in those lesions, which indicate more plaque inflammation and reduced healing in mice treated with the CD25-depleting antibody [42].
In addition, the Treg cells plays potential role in graft survival. A seminal study, using genetically engineered donor pig hearts and non-human primates to consider therapeutic potential of Treg cells, revealed that the explanted cardiac xenografts of long-term survivors dislayed increased Treg cells among the graft-infiltrating lymphocytes compared to those of recipients with early graft failures [43]. This was consistent with the increased Treg cell numbers within the peripheral blood of cardiac xenograft recipients with long-term survival [43].
The ability to transfer Treg cells into patients has been considered to be highly beneficial for exploring the therapeutic potential of these cells. Adoptive transfer of ex vivo expanded CD4+ CD25+ CD127- Treg cells have already been demonstrated in graft versus host disease (GvHD) treatment [44]. In the first-in-man trial, Treg cells were sorted from buffy coats of family donors, expanded ex vivo, and transferred into recipients with GvHD [44]. In the patient with chronic GvHD, the transfer was associated in increase in Treg cell level and decrease in cytokine level in peripheral blood [44]. Even with acute GvHD, there was temporary stabilization of symptoms [44]. Brunstein et al. considered the safety and efficacy of ex vivo-expanded natural Treg cells using umbilical cord blood (UCB)-derived CD4+ CD25+ Treg cells in the context of UCB transplantation [45]. Treg cells were isolated from a UCB unit that was 4–6/6 HLA matched to the each patient [45]. There was no dose-limiting toxicity observed from Treg infusions nor was risk of opportunistic infection or relapse adversely affected [45]. The observed decrease in risk of acute GvHD in Treg recipients is promising; however, a randomized trial is necessary for definitive results [45]. The safety and feasibility of use of Treg cells as therapy makes understanding their implication in atherosclerosis relevant for therapeutic considerations.
The benefit of a potential new therapeutic option is highlighted by the limitations of other tested approaches. The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) tested effects of canakinumab, a monoclonal antibody targeting interleukin-1β, in the context of atherosclerotic disease via reducing inflammation without affecting lipid levels [46]. Patients receiving 150 mg of dose of canakinumab displayed 15% lower risk in terms of the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death [46]. This was observed with a reduction in high-sensitivity C-reactive protein and interleukin-6 levels; however, reduction in LDL and HDL cholesterol level was not evident [46]. This suggests that the inflammatory hypothesis of atherothrombosis holds as a strategy for intervening for cardiovascular events. However, there were significantly more deaths due to infection or sepsis in the canakinumab groups versus the placebo and no significant difference in all-cause mortality [46]. The Cardiovascular Inflammation Reduction Trial (CIRT) tested the inflammatory hypothesis of atherothrombosis by studying effect of low-dose methotrexate on rates of myocardial infarction, stroke, and cardiovascular death [47]. The low-dose methotrexate treatment failed to reduce the levels of interleukin-1β, interleukin-6, or C-reactive protein and atherosclerosis [47].
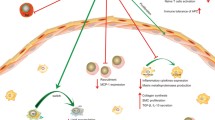
Table 2 displays the overview of the anti-atherogenic implications associated with the common complications including plaque formation, restenosis, neointimal hyperplasia, graft failure and ischemic injury associated with vascular interventions and this section focusses on the current understanding regarding the Treg cell signaling associated with such complications. The schematic representation of these ailments is depicted in Fig. 4.
Clinical implications of atherosclerosis progression and interventions: There are variations in atherosclerosis progression, especially based on potential intervention. Disease develops starting with plaque formation leading to ischemia in which there is diminished blood, and thus oxygen, flow. Interventions to prevent harmful consequences of atherosclerotic plaque include vessel graft and stent placement. However, these interventions are followed by neointimal hyperplasia and thus restenosis
Plaque formation
Lipoprotein drives the plaque formation at specific sites during atherosclerosis [48]. This plaque formation involves lipoprotein retention, intimal inflammation, foam cell formation, necrosis, smooth muscle cell proliferation, fibrous cap rupture, arterial remodeling, and calcification [49]. As mentioned previously, LDL accumulation at arterial sites result in the formation of arterial plaques as the LDLs oxidize and aggregate through a process that involves immune cells [49]. Early plaque progression due to intimal thickening involves macrophage infiltration [50]. Macrophage infiltration releases matrix metalloproteinase and cellular apoptosis leads to the formation of acellular necrotic core [50]. T lymphocytes have been observed to simultaneously be present in all stages of plaque formation and progression [48].
The slow build-up of atherosclerotic plaques is asymptomatic; however, it can be identified through changes in composition. Analysis has shown that the composition and phenotype of T lymphocytes differ between plaques and blood [51]. This includes CD4+ T cells, which show more activation in plaques than in peripheral blood [52]. There tends to be an absence of CD4+ T cells in early plaques [53]. The CD4+ T cell level increases with the development into late fibrous plaques [53] suggesting that CD4+ T cells in general are not substantially involved in the initial stages of plaque formation, but rather with the subsequent development [53]. Furthermore, CD4+ T cells are more abundant in unstable plaques compared to stable plaques [53]. In particular, T helper type 1 (Th1) cells are triggered by the formation of atherosclerotic plaques [54]. Treg cells show a similar trend to CD4+ T cells overall in that they are higher in lipid-rich advanced plaques compared to early lesions [53]. Treg cells can control the response of helper T cell subsets such as Th1 cells [54]. Treg cells act through IL-10 synthesis to arrest Th1 cell proliferation and decrease in IFN-γ production [55]. Overall, Treg cells have been shown to limit inflammation and inhibit lesion formation playing an essential role in modulating plaque development [56].
In-stent restenosis
Coronary artery stents are self-expanding metal-mesh tubes with struts inserted via a minimally invasive procedure. They hold open narrowed arteries to allow for improved blood flow. Stents can fail to maintain their function over time. Upon the insertion of a stent, new tissue with healthy cells from endothelium grows so that eventually the normal lining covering the stent allows blood to flow smoothly over the stented area [57]. However, scar tissue can also grow and obstruct blood flow. The blockage of a treated artery is restenosis, with in-stent restenosis specifically referring to the restenosis arising after the use of stents [57]. Inflammation plays an important role for in-stent restenosis [58]. In fact, c-reactive protein level, which is used for assessment of inflammatory status, is predictive of in-stent restenosis risk [59]. The stress during the stent implantation procedure triggers vascular inflammation [60]. Restenosis has been considered to be a form of hypertrophic wound healing resulting from interaction between monocyte-derived macrophages, T cells, and the normal cellular elements of the arterial wall [60]. Both Th1 and Th2 lymphocytes have been implicated in restenosis [58]. However, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio were reported to be significantly higher for patients who developed restenosis [61]. Lymphocyte-to-monocyte ratio, on the other hand, exhibits no association [61]. Correlation between restenosis and the IL-10 gene has been observed [62]. The particular relationship with restenosis six months following femoropopliteal percutaneous transluminal angioplasty with covered stent implantation has been studied [63]. There was significant increase in anti-inflammatory cytokines of TGF-β and IL-10 and decrease in pro-inflammatory cytokines [63]. TGF-β signaling has been found to be important for restenosis as they are upregulated at sites of arterial injury and mediate vascular fibrosis [64]. The upregulation of these inflammatory cytokines in restenosis suggests the possibility that Treg cells play a crucial role for restenosis. Treg cells have been shown to play a key role in regulating inflammation involved in carotid artery stenosis [65, 66]. Since the mechanical injury involved with stent placement leads to local inflammation, it can stimulate vascular smooth muscle cell proliferation and extracellular matrix deposition, leading to restenosis [67]. Together these results suggest the possibility that Treg cells may play a role development of in-stent restenosis; however, further research regarding the specific role of Treg cell in-stent restenosis is still warranted.
Neointimal hyperplasia
Surgical interventions for atherosclerosis including surgical bypass grafting or balloon angioplasty fail from restenosis due to neointimal hyperplasia [68]. Neointimal hyperplasia is the healing response to injury of the blood vessel wall [69]. Vascular wall thickening and loss of luminal patency caused by vascular smooth muscle cell proliferation and migration characterize neointimal hyperplasia [68]. These smooth muscle cells lose their contractile phenotype in favor of a predominantly proliferative or secretory phenotype which secrete growth factors, growth factor receptors, proteinases, and inflammatory mediators [68]. Neointimal growth and proliferation have been shown to involve leukocyte recruitment and pro-inflammatory cytokines [70]. CCR5 deficiency has been shown to significantly reduces neointimal area through association with a decrease in macrophages, CD3+ T lymphocytes, and CCR2 + cells [70]. The CCR5 deficiency involves an upregulation of IL-10 in the neointimal smooth muscle cells and lesions [70]. In CCR5-negative mice, reduced neointima formation appears to result from a shift toward Th2-type immune response with increased IL-10 expression [70]. However, another study found that mice lacking IL-10 developed identical neointimal hyperplasia similar to IL-10 positive controls [71]. This suggests that IL-10 fails to modulate low shear stress-induced neointimal hyperplasia [71]. Another study showed that IL-10 knock-out mice had significantly delayed re-endothelialization and enhanced neo-intimal growth, suggesting the differential regulation through IL-10 possibly inhibits neointimal hyperplasia[72]. Additionally, TGF‐β signaling promotes the expansion of progressive fibrotic neointimal hyperplasia [73]. With steady neointimal hyperplasia growth, there is enhanced TGF‐β activation, SMAD2/3 phosphorylation, and connective tissue growth factor (CTGF) production [73]. Together, these suggest that regulatory T cells, which often act through TGF‐β and IL-10, may play a role in inhibiting neointimal hyperplasia. However, regulation and mechanism of Treg cells in neointimal hyperplasia pathology still requires further investigation.
Vessel graft failure
Vascular grafts are often used in aortocoronary bypass graft therapy [74]. Commonly, saphenous vein grafts are used as aortocoronary bypass vessels, which is a lifesaving treatment modality for CADs [74]. However, occlusion of the graft often occurs through thrombosis, intimal hyperplasia, and atherosclerosis [74]. Vein graft atherosclerosis involves biomechanical stress and cell death [74]. The biomechanical stress stimulates the expression of chemokines as well as adhesion molecules by endothelial and smooth muscle cells [74]. Following cell death monocytes are recruited which differentiate into macrophages within the tissue and contributes to inflammatory responses [74]. Increased expressions of pro-inflammatory cytokines and leukocyte adhesion molecules have been associated with graft failure disease [75]. The involvement of inflammation in failure of grafts suggests that the induction of immune response, as Treg cells do, may have a beneficial outcome.
Ischemic arterial injury
Mechanical vascular injury elicits inflammatory response from vessel walls [76]. Specifically, leukocyte engagement is involved for biological response to vascular injury [76]. Vascular injury triggers expression of autocrine or paracrine mediators such as IL-1β and TNF-α [76]. Animal models in which stents produced deep vessel wall trauma show an induction of early inflammatory response with surface-adherent neutrophils, monocytes, platelets, and fibrin deposits at the site of injury [76]. The activated platelets facilitate the attachment of leukocytes onto and rolling along the injured surface [76]. Following ischemic injury, which is caused by diminished or even absence of blood flow, the immune cells that infiltrated the tissue produce inflammatory cytokines [77]. A moderate inflammatory response is beneficial for tissue repair and protect organ function upon ischemia [77]. However, excessive and chronic inflammatory response, including T cells, can be detrimental [77]. Treg cells promote repair following ischemic injury as the cell subset suppresses multiple immune cell types and thus blocks excess immune responses and inflammatory signaling pathways [77]. Treg cells have been shown to decrease IL-1β and TNF-α in acute ischemic myocardial injury and inhibit neutrophil, macrophage, and lymphocyte infiltration as well as CD8+ T cell proliferation and function [77]. In chronic ischemic stress, dysfunctional Tregs plays a role in the pathogenesis of hypertrophy, fibrosis, and tissue neovascularization, which ultimately results in adverse cardiac remodeling [77].
Conclusion
The significant role of inflammation and immune cells in atherosclerosis has prompted the exploration of an immune mechanism to protect against disease development and progression. Treg cells are of particular interest due to their anti-inflammatory responses. Recent research regarding Treg cells has supported this hypothesis. Treg cells have been discovered in atherosclerotic lesions and their absence is associated with aggravated disease and larger lesions. This suggests a potential role in decelerating progression of atherosclerosis. Treg cells are known to produce anti-inflammatory cytokines including TGF‐β and IL-10. Besides cytokine signaling, the ratios between Treg and other T cells such as Th17 and Th2 cells are also shown to be associated with disease progression. However, there has been evidence that Treg cells can differentiate into pro-atherogenic T follicular helper (Tfh) cells, which in turn are negatively regulated by a subset of Treg cells, follicular regulatory T (Tfr) cells. Together, this suggests that maintaining and promoting Treg cell populations in atherosclerotic lesions may have therapeutic potential for atherosclerosis. Further studies in the future are warranted to effectively turn this association into a therapeutic strategy and to unveil the mechanism of Treg cell action that opens novel translational avenues. Overall, Treg cells possesses optimal therapeutic value; however, more investigations are required regarding Treg cell involvement in different stages of the disease progression and associated conditions including neointimal hyperplasia or vessel graft failure. TGF‐β and IL-10, produced by Treg cells, seem to play an important role in restenosis and neointimal hyperplasia. Further studies regarding the role of Treg cells in appropriate animal models are needed to confirm these connections. Further translational work would identify strategies to utilize Treg cells to improve effectiveness of existing interventions in addition to decelerating disease progression.
Abbreviations
- Treg cell:
-
Regulatory T cell
- Tfh cell:
-
T follicular helper cell
- ox-LDL:
-
Oxidized low-density lipoprotein
- TGF-β:
-
Transforming growth factor-β;
- IL-10:
-
Interleukin-10
References
Kobiyama K, Ley K (2018) Atherosclerosis: a chronic inflammatory disease with an autoimmune component. Circ Res 123(10):1118–1120. https://doi.org/10.1161/CIRCRESAHA.118.313816
Atherosclerosis. (n.d.). www.heart.org. Accessed October 14, 2020, from https://www.heart.org/en/health-topics/cholesterol/about-cholesterol/atherosclerosis
Schaftenaar F, Frodermann V, Kuiper J, Lutgens E (2016) Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol 27(3):209–215. https://doi.org/10.1097/MOL.0000000000000302
Zhao TX, Mallat Z (2019) Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol 73(13):1691–1706. https://doi.org/10.1016/j.jacc.2018.12.083
Roh JS, Sohn DH (2018) Damage-Associated molecular patterns in inflammatory diseases. Immune Netw. https://doi.org/10.4110/in.2018.18.e27
Sage AP, Tsiantoulas D, Binder CJ, Mallat Z (2019) The role of B cells in atherosclerosis. Nat Rev Cardiol 16(3):180–196. https://doi.org/10.1038/s41569-018-0106-9
Maguire EM, Pearce SWA, Xiao Q (2019) Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc Pharmacol 112:54–71. https://doi.org/10.1016/j.vph.2018.08.002
Lundberg AM, Hansson GK (2010) Innate immune signals in atherosclerosis. Clin Immunol 134(1):5–24. https://doi.org/10.1016/j.clim.2009.07.016
Wolf D, Ley K (2019) Immunity and inflammation in atherosclerosis. Circ Res 124(2):315–327. https://doi.org/10.1161/CIRCRESAHA.118.313591
Martinet W, Schrijvers DM, De Meyer GRY (2011) Necrotic cell death in atherosclerosis. Basic Res Cardiol 106(5):749–760. https://doi.org/10.1007/s00395-011-0192-x
Srikakulapu P, McNamara CA (2017) B cells and atherosclerosis. Am J Physiol Heart Circ Physiol 312(5):H1060–H1067. https://doi.org/10.1152/ajpheart.00859.2016
Saigusa R, Winkels H, Ley K (2020) T cell subsets and functions in atherosclerosis. Nat Rev Cardiol 17(7):387–401. https://doi.org/10.1038/s41569-020-0352-5
Baptista D, Mach F, Brandt KJ (2018) Follicular regulatory T cell in atherosclerosis. J Leukoc Biol 104(5):925–930. https://doi.org/10.1002/JLB.MR1117-469R
Ketelhuth Daniel FJ, Hansson GK (2016) Adaptive Response of T and B Cells in Atherosclerosis. Circ Res 118(4):668–678. https://doi.org/10.1161/CIRCRESAHA.115.306427
van Duijn J, Kuiper J, Slütter B (2018) The many faces of CD8+ T cells in atherosclerosis. Curr Opin Lipidol 29(5):411–416. https://doi.org/10.1097/MOL.0000000000000541
Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y (2016) Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol 13(3):167–179. https://doi.org/10.1038/nrcardio.2015.169
Won HY, Hwang ES (2016) Transcriptional modulation of regulatory T cell development by novel regulators NR4As. Arch Pharmacal Res 39(11):1530–1536. https://doi.org/10.1007/s12272-016-0803-z
Wakamatsu E, Omori H, Ohtsuka S, Ogawa S, Green JM, Abe R (2018) Regulatory T cell subsets are differentially dependent on CD28 for their proliferation. Mol Immunol 101:92–101. https://doi.org/10.1016/j.molimm.2018.05.021
Owen DL, Sjaastad LE, Farrar MA (2019) Brief review: regulatory T cell development in the thymus. J Immunol 203(8):2031–2041. https://doi.org/10.4049/jimmunol.1900662
Arce-Sillas A, Álvarez-Luquín DD, Tamaya-Domínguez B, Gomez-Fuentes S, Trejo-García A, Melo-Salas M, Cárdenas G, Rodríguez-Ramírez J, Adalid-Peralta L (2016) Regulatory T cells: molecular actions on effector cells in immune regulation. J Immunol Res. https://doi.org/10.1155/2016/1720827
Yazdani MR, Khosropanah S, Doroudchi M (2019) Interleukin-17 production by CD4+CD45RO+Foxp3+ T cells in peripheral blood of patients with atherosclerosis. Arch Med Sci Atheroscler Dis 4:e215–e224. https://doi.org/10.5114/amsad.2019.87525
Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S (2013) Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 123(3):1323–1334. https://doi.org/10.1172/JCI63891
Joly AL, Seitz C, Liu S, Kuznetsov NV, Gertow K, Westerberg LS, Paulsson-Berne G, Hansson GK, Andersson J (2018) Alternative splicing of FOXP3 controls regulatory T cell effector functions and is associated with human atherosclerotic plaque stability. Circ Res 122(10):1385–1394. https://doi.org/10.1161/CIRCRESAHA.117.312340
Joly AL, Andersson J (2019) Alternative splicing, FOXP3 and cardiovascular disease. Aging (Albany NY) 11(7):1905–1906. https://doi.org/10.18632/aging.101897
Toma I, McCaffrey TA (2012) Transforming growth factor-β and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 347(1):155–175. https://doi.org/10.1007/s00441-011-1189-3
Filatova AY, Pylaeva EA, Potekhina AV, Ruleva NY, Klesareva EA, Radyukhina NV, Masenko VP, Shchinova AM, Noeva EA, Provatorov SI, Afanas’eva OI. (2019) Low Blood content of IL-10-producing CD4+ T cells as a risk factor for progression of coronary atherosclerosis. Bull Exp Biol Med 166(3):330–333. https://doi.org/10.1007/s10517-019-04344-z
George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, Shamiss A (2012) Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis 222(2):519–523. https://doi.org/10.1016/j.atherosclerosis.2012.03.016
Li Q, Wang Y, Wang Y, Chen K, Zhou Q, Wei W, Wang Y (2014) Treg/Th17 ratio acts as a novel indicator for acute coronary syndrome. Cell Biochem Biophys 70(2):1489–1498. https://doi.org/10.1007/s12013-014-9993-5
Luo T, Ji WJ, Yuan F, Guo ZZ, Li YX, Dong Y, Ma YQ, Zhou X, Li YM (2016) Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans. Sci Rep 6(1):1–2. https://doi.org/10.1038/srep26767
Li Q, Wang Y, Li H, Shen G, Hu S (2014) Ox-LDL influences peripheral Th17/Treg balance by modulating Treg apoptosis and Th17 proliferation in atherosclerotic cerebral infarction. Cell Physiol Biochem 33(6):1849–1862. https://doi.org/10.1159/000362963
Ding J-W, Zhou T, Zheng X-X, Wang X-A, Tong X-H, Luo C-Y et al (2018) The effects of high mobility group box-1 protein on peripheral Treg/Th17 balance in patients with atherosclerosis. Acta Cardiol Sin 34(5):399–408. https://doi.org/10.6515/ACS.201809_34(5).20180419A
Huang Y, Hu H, Liu L, Ye J, Wang Z, Que B et al (2019) Interleukin-12p35 deficiency reverses the Th1/Th2 imbalance, aggravates the Th17/Treg imbalance, and ameliorates atherosclerosis in ApoE-/- mice. Mediat Inflamm 2019:3152040. https://doi.org/10.1155/2019/3152040
Tian Y, Chen T, Wu Y, Yang L, Wang L, Fan X, Zhang W, Feng J, Yu H, Yang Y, Zhou J (2017) Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms. Cardiovasc Diabetol 16(1):1–9. https://doi.org/10.1186/s12933-017-0623-6
Fan Q, Liu Y, Rao J, Zhang Z, Xiao W, Zhu T, Chai X, Ye K, Ning N, Yin Z, Chai Y (2020) Anti-atherosclerosis effect of Angong Niuhuang Pill via regulating Th17/Treg immune balance and inhibiting chronic inflammatory on ApoE-/- mice model of early and mid-term atherosclerosis. Front Pharmacol. https://doi.org/10.3389/fphar.2019.01584
Cai Y, Kobayashi R, Hashizume-Takizawa T, Kurita-Ochiai T (2014) Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch Oral Biol 59(11):1183–1191. https://doi.org/10.1016/j.archoralbio.2014.07.012
Potekhina AV, Pylaeva E, Provatorov S, Ruleva N, Masenko V, Noeva E, Krasnikova T, Arefieva T (2015) Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis 238(1):17–21. https://doi.org/10.1016/j.atherosclerosis.2014.10.088
Gao S, Zhang W, Zhao Q, Zhou J, Wu Y, Liu Y et al (2019) Curcumin ameliorates atherosclerosis in apolipoprotein E deficient asthmatic mice by regulating the balance of Th2/Treg cells. Phytomedicine 52:129–135. https://doi.org/10.1016/j.phymed.2018.09.194
Dumitriu IE (2015) The life (and death) of CD4+CD28null T cells in inflammatory diseases. Immunology 146(2):185–193. https://doi.org/10.1111/imm.12506
Ruggio A, Pedicino D, Flego D, Vergallo R, Severino A, Lucci C et al (2019) Correlation between CD4+CD28null T lymphocytes, regulatory T cells and plaque rupture: an optical coherence tomography study in acute coronary syndromes. Int J Cardiol 276:289–292. https://doi.org/10.1016/j.ijcard.2018.08.101
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+ CD25 + regulatory T cells. Nat Immunol 4(4):330–336. https://doi.org/10.1038/ni904
Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM et al (2018) Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 9(1):1095. https://doi.org/10.1038/s41467-018-03493-5
Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J et al (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12(2):178–180. https://doi.org/10.1038/nm1343
Singh A, Goerlich CE, Braileanu G, Hershfeld A, Zhang T, Tatarov I et al (2020) Presence of graft-infiltrating regulatory t cells are associated with long term cardiac xenograft survival in non-human primate. Transplantation 104(S3):S641. https://doi.org/10.1097/01.tp.0000702068.56688.88
Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N et al (2009) First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol 133(1):22–26. https://doi.org/10.1016/j.clim.2009.06.001
Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J et al (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117(3):1061–1070. https://doi.org/10.1182/blood-2010-07-293795
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131. https://doi.org/10.1056/NEJMoa1707914
Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M et al (2019) Low-dose methotrexate for the prevention of atherosclerotic events. New Engl J Med 380(8):752–762. https://doi.org/10.1056/NEJMoa1809798
Rognoni A, Cavallino C, Veia A, Bacchini S, Rosso R, Facchini M, Secco G, Lupi A, Nardi F, Rametta FS, Bongo A (2015) Pathophysiology of atherosclerotic Plaque development. Cardiovasc Hematol Agents Med Chem 13(1):10–13
Fog BJ, Fumiyuki O, Renu V, Erling F (2014) Mechanisms of plaque formation and rupture. Circ Res 114(12):1852–1866. https://doi.org/10.1161/CIRCRESAHA.114.302721
Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R (2013) Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 22(6):399–411. https://doi.org/10.1016/j.hlc.2013.03.001
Grivel J-C, Ivanova O, Pinegina N, Blank PS, Shpektor A, Margolis LB, Vasilieva E (2011) Activation of T lymphocytes in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 31(12):2929–2937. https://doi.org/10.1161/ATVBAHA.111.237081
Paul VSV, Paul CMP, Kuruvilla S (2016) Quantification of Various Inflammatory Cells in Advanced Atherosclerotic Plaques. J Clin Diagn Res 10(5):EC35–EC38. https://doi.org/10.7860/JCDR/2016/19354.7879
Lee S, Bartlett B, Dwivedi G (2020) Adaptive immune responses in human atherosclerosis. Int J Mol Sci 21(23):9322. https://doi.org/10.3390/ijms21239322
Gisterå A, Hansson GK (2017) The immunology of atherosclerosis. Nat Rev Nephrol 13(6):368–380. https://doi.org/10.1038/nrneph.2017.51
Albany CJ, Trevelin SC, Giganti G, Lombardi G, Scottà C (2019) Getting to the heart of the matter: the role of regulatory t-cells (Tregs) in cardiovascular disease (CVD) and atherosclerosis. Front Immunol. https://doi.org/10.3389/fimmu.2019.02795
Ketelhuth DFJ, Gisterå A, Johansson DK, Hansson GK (2013) T cell-based therapies for atherosclerosis. Curr Pharm Des 19(33):5850–5858. https://doi.org/10.2174/1381612811319330003
George D, Frank K (2002) Restenosis: repeat narrowing of a coronary artery. Circulation 105(22):2586–2587. https://doi.org/10.1161/01.CIR.0000019122.00032.DF
Demyanets S, Tentzeris I, Jarai R, Katsaros KM, Farhan S, Wonnerth A, Weiss TW, Wojta J, Speidl WS, Huber K (2014) An increase of interleukin-33 serum levels after coronary stent implantation is associated with coronary in-stent restenosis. Cytokine 67(2):65–70. https://doi.org/10.1016/j.cyto.2014.02.014
Niccoli G, Montone RA, Ferrante G, Crea F (2010) The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol 56(22):1783–1793. https://doi.org/10.1016/j.jacc.2010.06.045
Schillinger M, Minar E (2005) Restenosis after percutaneous angioplasty: the role of vascular inflammation. Vasc Risk Manag 1(1):73–78
Lee S, Hoberstorfer T, Wadowski PP, Kopp CW, Panzer S, Gremmel T (2020) Platelet-to-lymphocyte and Neutrophil-to-lymphocyte ratios predict target vessel restenosis after infrainguinal angioplasty with stent implantation. J Clin Med 9(6):1729. https://doi.org/10.3390/jcm9061729
Monraats PS, Kurreeman FA, Pons D, Sewgobind VD, de Vries FR, Zwinderman AH, de Maat MP, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J (2007) Interleukin 10: a new risk marker for the development of restenosis after percutaneous coronary intervention. Genes Immun 8(1):44–50. https://doi.org/10.1038/sj.gene.6364343
Guimaraes TS, da Rocha LA, Becari C, Piccinato CE, Joviliano RD, Ribeiro MS, Joviliano EE (2018) The Role of interleukins and inflammatory markers in the early restenosis of covered stents in the femoropopliteal arterial segment. Ann Vasc Surg 50:88–95. https://doi.org/10.1016/j.avsg.2017.11.064
McCaffrey TA (2009) TGF-beta signaling in atherosclerosis and restenosis. Front Biosci (Schol Ed) 1:236–245. https://doi.org/10.2741/s23
Del Porto F, Cifani N, Proietta M, Dezi T, Tritapepe L, Raffa S, Micaloni A, Taurino M (2019) Lag3+ regulatory T lymphocytes in critical carotid artery stenosis. Clin Exp Med 19(4):463–468. https://doi.org/10.1007/s10238-019-00570-x
Del Porto F, Cifani N, Proietta M, Perrotta S, Dito R, di Gioia C, Carletti R, Rizzo L, Orgera G, Rossi M, Ferri L (2017) Regulatory T CD4+ CD25+ lymphocytes increase in symptomatic carotid artery stenosis. Ann Med 49(4):283–290. https://doi.org/10.1080/07853890.2016.1241427
Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI (2011) Vascular inflammation and repair: implications for reendothelialization, restenosis, and Stent thrombosis. JACC Cardiovasc Interv 4(10):1057–1066. https://doi.org/10.1016/j.jcin.2011.05.025
Zain MA, Jamil RT, Siddiqui WJ (2020) Neointimal hyperplasia. StatPearls. StatPearls Publishing, Treasure Island (FL)
Braga, S. F., Neves, J. R., Ferreira, J., Carrilho, C., Simões, J. C., & Mesquita, A. (2019). Neointimal hyperplasia. Revista Portuguesa De Cirurgia Cardio-Toracica E Vascular: Orgao Oficial Da Sociedade Portuguesa De Cirurgia Cardio-Toracica E Vascular, 26(3), 213–217
Zernecke A, Liehn EA, Gao J-L, Kuziel WA, Murphy PM, Weber C (2006) Deficiency in CCR5 but not CCR1 protects against neointima formation in atherosclerosis-prone mice: involvement of IL-10. Blood 107(11):4240–4243. https://doi.org/10.1182/blood-2005-09-3922
Rectenwald JE, Minter RM, Moldawer LL, Abouhamze Z, La Face D, Hutchins E, Seeger JM, Ozaki CK (2002) Interleukin-10 fails to modulate low shear stress-induced neointimal hyperplasia. J Surg Res 102(2):110–118. https://doi.org/10.1006/jsre.2001.6283
Verma SK, Garikipati VNS, Krishnamurthy P, Khan M, Thorne T, Qin G, Losordo DW, Kishore R (2016) IL-10 accelerates re-endothelialization and inhibits post-injury intimal hyperplasia following carotid artery denudation. PLoS ONE. https://doi.org/10.1371/journal.pone.0147615
Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA (2009) Established neointimal hyperplasia in vein grafts expands via TGF-β-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol 297(4):H1200–H1207. https://doi.org/10.1152/ajpheart.00268.2009
Xu Q (2004) Mouse models of arteriosclerosis. Am J Pathol 165(1):1–10
Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J (2006) Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg 82(4):1458–1464. https://doi.org/10.1016/j.athoracsur.2006.05.038
Simon DI (2012) Inflammation and Vascular Injury. Circ J 76(8):1811–1818
Zhuang R, Feinberg MW (2020) Regulatory T cells in ischemic cardiovascular injury and repair. J Mol Cell Cardiol 147:1–11. https://doi.org/10.1016/j.yjmcc.2020.08.004
Funding
The research work of FGT was supported by the startup research funds from Western University of Health Sciences and DKA received funding from NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest. The authors declared no conflict of interest.
Consent to participate
This article does not contain any studies with human participants or animal models performed by any of the authors.
Consent to publish
As the corresponding author, I verify that all authors significantly contributed to various aspect of the study, have read the manuscript and consented to submit for publication in Molecular Biology Reports.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuan, R., Agrawal, D.K. & Thankam, F.G. Treg cells in atherosclerosis. Mol Biol Rep 48, 4897–4910 (2021). https://doi.org/10.1007/s11033-021-06483-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06483-x