Abstract
The imbalance of CD4+CD25+ regulatory T (Treg) cells and Th17 cells has shown to be involved in pathogenesis of atherosclerosis and acute coronary syndrome [ACS, including unstable angina (UA) and acute myocardial infarction (AMI)]. The purpose of this study is to explore the significance of Treg/Th17 ratio in early diagnosis for ACS. We detected expression of Treg and Th17 in patients with AMI, UA, stable angina, and subjects with normal coronary arteries at the time of admission. Our results showed that ACS patients have a significant increase of Th17 number, but a marked decline of Treg/Th17 ratio, Treg number, and Treg function. Significant positive correlations in Th17 frequency and negative correlation in Treg frequency, Treg/Th17 ratio were found to levels of oxidized low-density lipoprotein (Ox-LDL), high sensitive C-reactive protein (hsCRP), Lipoprotein (a) [Lp(a)], and Creatine kinase-MB(mass) (CK-MBmass) in serum. Receiver-operating characteristic curves shown that the predictive specificity and sensitivity of Treg/Th17 ratio for ACS and AMI was the highest among all the five markers: Ox-LDL, hsCRP, Lp(a), CK-MBmass, and Treg/Th17 ratio. In conclusion, Treg/Th17 ratio appeared to be a novel indicator for early diagnosis of ACS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherogenesis involves various immune cells, particularly CD4+ T-helper cells [1, 2]. It is now recognizable that most atherosclerotic plaques that cause acute coronary syndrome [ACS, including unstable angina (UA) and acute myocardial infarction (AMI)] exhibit angiographic obstruction, and approximately 60 % of ACS is caused by rupture of plaques. ACS occurs as a consequence of coronary plaque rupture, and T-helper cells also play an important role in these coronary events [3].
Distinguishing patients that present to the hospital with chest pain have an acute coronary syndrome (ACS) or a non-coronary problem is often difficult, time-consuming, and expensive [4]. Several cardiac biomarkers, such as creatine kinase-MB, troponins-T(TnT), C-reactive protein, have emerged as strong predictors of risk among patients presenting with suspicion of an ACS and are now routinely available to clinicians [5].
Recently, CD4+CD25+ regulatory T (Treg) cells and Th17 cells have been described as two new subsets in CD4+ T-helper cells. Treg cells, a fraction of inflammatory regulated negative cells, have important effects on the maintenance of immune tolerance and immune homeostasis by contact-dependent suppression or releasing anti-inflammatory cytokines, such as interleukin (IL)-10 [6], while Th17 cells exert an important effect on the pathogenesis of many autoimmune diseases and inflammatory conditions by producing IL-17 [7].
Treg and Th17 cells are important in the development of tissue inflammation and autoimmune diseases [8]. In our pervious studies and others, both Treg cells and Th17 cells have shown to be involved in pathogenesis of atherosclerosis (AS) and ACS, and Treg/Th17 imbalance plays a potential role in the development of AS, especially ACS [9–11]. The aim of this study was to further explore the significance of Treg/Th17 ratio in early diagnosis for ACS. Here, we report that ACS patients have a significant increase of Th17 number, but a marked decline of Treg/Th17 ratio, Treg number, and Treg function. Treg/Th17 ratio and Treg frequency correlated negatively with levels of serum Ox-LDL, hsCRP, Lp(a), and CK-MBmass, while Th17 frequency correlated positively with these biomarkers levels. Receiver-operating characteristic (ROC) curves shown that the predictive specificity and sensitivity of Treg/Th17 ratio for ACS and AMI was higher as comparison to Ox-LDL, hsCRP, Lp(a), and CK-MBmass. Treg/Th17 ratio could be a useful indicator for early diagnosis of ACS.
Materials and Methods
Study Protocol
The study conformed to protocols approved by the institutional review boards of Anhui Medical University and conformed with the declaration of Helsinki. All patients gave written informed consent before enrollment into this study. Patients at Anhui provincial hospital between January 2010 and December 2012 who underwent diagnostic catheterization (163 males and 89 females) were examined. Patients were classified into 4 groups: Group 1, AMI patients (37 males and 25 females, mean age 57.8 ± 16.7 years). Myocardial infarction was confirmed by definite (>2 mm) ST-segment elevations in at least two consecutive leads and significant rise of creatine kinase-MB and troponin I levels. Group 2, UA patients (39 male and 22 female, mean age 63.9 ± 12.4). UA was defined as chest pain at rest accompanied by definite ischemic electrocardiographic changes (ST-segment changes and/or T-wave inversions). Group 3, SA patients (45 male and 24 female, mean age 60.6 ± 11.8). SA was diagnosed by typical exertional chest discomfort associated with downsloping or horizontal ST-segment depression >1 mm in an exercise test. Group 4, subjects with normal coronary arteries (NCA). Control subjects were selected on a basis of a recent angiography showing NCA (42 male and 18 female, mean age 56.7 ± 15.9). Patients with ACS and stable angina had similar extent of coronary atherosclerosis. There were no evident differences between the four groups with regard to age.
Patients with the following conditions were excluded from the study: (1) thromboembolism, disseminated intravascular coagulation, advanced liver disease, renal failure, malignant disease; (2) inflammatory disease or inflammatory conditions probably associated with an acute-phase response; (3) chronic-immune-mediated disorders; (4) valvular heart disease, atrial fibrillation or pacemaker user; (5) use of anti-inflammatory drugs and/or immunosuppressive agents; (6) Individuals with alcohol or drug abuse history.
Blood Samples
5–10 ml of peripheral blood was obtained from all the patients in a fasting state at the time of admission to the hospital. In ACS cases, the mean time interval from the symptom onset to the blood sampling was 4 h. Blood samples were anti-coagulated with heparin and examined within 4 h. Peripheral blood mononuclear cells (PBMCs) were prepared immediately by Ficoll density gradient for analysis of flow cytometry (FCM). Serum was obtained after centrifugation for the measurement of oxidative low-density lipoprotein (Ox-LDL), heat-sensitive C-reaction protein (hsCRP), mass concentration of creatine kinase-MB (CK-MBmass), and other biochemistry indicators.
Cell Separation and Flow Cytometry
Cell Preparation
For the analysis of Th17, PBMCs were suspended at a density of 2 × 106 cells/ml in complete culture medium (RPMI 1640 supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin, 2 mM glutamine and with 10 % heat-inactivated fetal calf serum, Gibco BRL). The cell suspension was transferred to each well of 24-well plates. Cultures were stimulated with phorbol myristate acetate (PMA, 25 ng/ml) plus ionomycin (1 μg/ml) for 4 h, in the presence of monensin (1.7 μg/ml, all from Alexis Biochemicals, San Diego, CA). The incubator was set at 37 °C under a 5 % CO2 environment. After culture, the contents of the well were transferred to 5 ml sterile tubes. The cells were then centrifuged at 1,200 rpm for 5 min. For the analysis of Treg, 100 μl of PBMCs (106) was added to tubes for further staining.
Treg and Th17 Detection
As for Treg analysis, cell surface staining was performed by the use of isothiocyanate(FITC)-conjugated anti-CD4(13B8.2 clone; Beckman Coulter-Immunotech, Marseille, France), phycoerythrin(PE)-conjugated anti-CD25(B1.49.9 clone, Beckman Coulter-Immunotech), PE-cy7-conjugated anti-CD127 (ebioRDR5 clone, eBioscience, San Diego, CA), and appropriate isotype controls for 20 min at room temperature in the dark followed by washing in PBS (for purified cells). Cells were then fixed and permeabilized with Fix/Perm reagent, incubated with PE-cy5-conjugated anti-Foxp3(PCH101 clone, eBioscience, San Diego, CA) and its isotype control antibody, washed with PBS and analyzed by FCM. For Th17 analysis, the cells were incubated with FITC-conjugated anti-CD4 at 4 °C for 15 min, then stained with PE-conjugated anti-IL-17A (ebio64DEC17 clone, eBioscience, San Diego, CA) after fixation and permeabilization according to the manufacturer’s instructions. Stained cells were assessed by FCM using COULTER EPICS ALTRA HyPerSort™ flow cytometer with EXPO 32 MULTICOMP Software (Beckman Coulter, Miami, FL, USA). The frequency of Treg (CD4+CD25high, CD4+CD25+CD127low, and CD4+CD25+Foxp3+) and Th17(CD4+IL17+)T cells was expressed as a percentage of CD4+ T cells by sequential gating on lymphocytes and CD4+ T cells.
Treg Function
For functional assays of Tregs, CD4+CD25+CD127low and CD4+CD25− cells were sorted using the gates (see Fig. 1) on a Beckman Counter flow cytometric cell-sorter (ALTRA HyPerSort™ System), after cells were dyed with FITC-conjugated anti-CD4, PE-conjugated anti-CD25, and PE-cy7-conjugated anti-CD127. Consistent purity of >90 % was obtained for both CD4+CD25+CD127low and CD4+CD25− cells fractions.
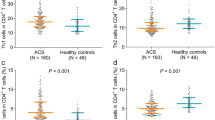
Tregs, Th17, and Treg/Th17 ratio in ACS patients. Quantification of flow cytometry results showed that Tregs frequencies, Th17 frequencies, and Treg/Th17 ratio were significantly altered in AMI and UA patients than in the SA and NCA groups. The values above were also markedly altered in SA patients than in the NCA groups. ▲ P < 0.01 compared with NCA and SA, *P < 0.01 compared with NCA, # P < 0.05 compared with NCA
Freshly purified CD4+CD25+CD127low T cells from NCA controls and from patients with AMI, UA, SA (n = 10 in each group) were assayed for suppressive activity in allogeneic mixed lymphocyte response (MLR). Irradiated (3,000 rad) PBMCs from healthy controls were used as allogeneic stimulator cells. CD4+CD25− cells were used as responder cells. CD4+CD25− cells (104 cells per well) were co-cultured with CD4+CD25+CD127low cells (104 cells per well, 1:1 ratio) in the presence of irradiated PBMCs (2 × 104 cells per well) in a 96-well flat-bottom plate. Wells without CD4+CD25+CD127low cells (responders and stimulators only) served as positive controls. Wells containing CD4+CD25+CD127low cells and irradiated PBMCs (none responders) served as baseline controls. All incubations were run in triplicate with a final volume of 150 μl at 37 °C and 5 % CO2. After 48 h of incubation, 10 μl per well Cell Proliferation Reagent WST-1 was added. After incubation for 4 more hours, the absorbance of the samples against a background control as blank was measured at 450 nm using 650 nm as a reference wavelength on a Biocell HT1 ELISA microplate reader (Salzburg, Austria). Suppression was expressed as percentage of the positive control.
Measurement of Ox-LDL, Blood Biochemistry, and Biomarkers
The levels of Ox-LDL in serum were examined by enzyme-linked immunosorbent assay (ELISA) and measured at 450 nm on Biocell HT1 ELISA microplate reader. The concentrations were then calculated by the absorbance of the samples against a background control. (Ox-LDL ELISA kits, from Uscnlife, USA). The minimal detectable concentrations were 4.5 μg/L for Ox-LDL. Intra-assay and inter-assay coefficients of variation for ELISA were <5 %, respectively. All samples were measured in duplicate.
Blood sugar and lipids were determined by the enzymatic method. Biomarkers, high sensitive C-reactive protein (hsCRP), and Lipoprotein (a) [Lp(a)] were measured by immunoturbidimetric method. All the assays were conducted on an Olympus AU2700 biochemical autoanalyzer (Olympus, Janpan). CK-MBmass was detected by chemiluminescent immunoenzymatic “sandwich” assay, which was performed an access chemiluminescent analyzer (Beckman-Coulter, USA). The upper reference limit (URL) for CK-MBmassass defined by the manufacturer is 6.3 ng/ml, and the detection limit is 0.1 ng/ml.
Statistical Analysis
Values were expressed as mean ± standard deviation (SD) in the text and figures. Data were analyzed by using statistical software (SPSS 11.0, LEAD Technologies, Inc, Chicago, IL, USA). Statistical significance for the difference in different groups was assessed by one-way analysis of variance (ANOVA). If significance was found, Bonferroni test was performed for post-hoc analysis to detect the difference among groups when equal variance was assumed, while Dunnett’s C test was performed when equal variance was not assumed. Spearman’s correlation was used as a test of correlation between two continuous variables. Receiver-operating characteristic (ROC) analysis was carried out on the levels of Treg/Th17 ratio, Ox-LDL, hsCRP, Lp(a), and CK-MBmass for ACS with non-ACS (including NCA and SA group) as a negative control group, and for AMI with non-AMI (including NCA, SA and UA group) as a negative control group. This analysis plots the true positive fraction (sensitivity) against the false positive fraction (1-specificity) by changing the cut-off value for the test. Areas under the ROC curves indicate the relative accuracy of diagnostic tests. All the P values <0.05 were considered to be statistically significant.
Results
Clinical Characteristics of Patients
There were no significant differences in age, gender, coronary artery disease (CAD) extent, hypertension, smoking rate, obesity, diabetes mellitus, high-density lipoprotein-cholesterol (HDL-C), and very low-density lipoprotein-cholesterol (VLDL-C) concentrations among patients with AMI, UA, and SA. The Levels of total cholesterol (TC) and total triglyeride (TG) in AMI and UA groups were significantly higher than those in SA group and NCA controls (P < 0.05, P < 0.01, respectively). Besides, the Levels of low density lipoprotein cholesterol (LDL-C) in AMI and UA groups were also significantly higher than those in NCA controls. (P < 0.05, P < 0.01, respectively, Table 1).
Decrease of Treg and Increase of Th17 Cells in Patients with ACS
As shown in Fig. 1, the frequencies of Treg (CD4+CD25+CD127lo/CD4+ T cells,) were significantly lower in the patients with AMI (2.86 ± 0.73 %) and UA (3.65 ± 0.70 %/) as compared with the NCA (5.41 ± 0.87 %) group and SA (4.74 ± 0.98 %/) patients (P < 0.01), while Treg frequencies of CD4+CD25+CD127lo in the SA groups were also markedly lower than those of the NCA group (P < 0.05). On the contrary, the frequencies of Th17 (CD4+IL17+/CD4+T cells) were markedly higher in patients with AMI (3.81 ± 0.96 %) and UA (2.94 ± 0.71 %) than those with NCA (1.06 ± 0.30 %) and SA (1.73 ± 0.35 %, P < 0.01), while there was also obvious difference between the SA and NCA group (P < 0.01).
Reduction of the Treg/Th17 Ratio in Patients with ACS
The ratio of Treg/Th17 was markedly lower in the patients with AMI (0.87 ± 0.45) and UA (1.34 ± 0.68) as compared with the NCA (5.29 ± 2.61) and SA (2.78 ± 1.46) patients (P < 0.01), besides, significant decrease of the ratio of Treg/Th17 in the SA groups was noted in comparison to that in the NCA group (P < 0.01, Fig. 1).
Functional Decrease of Tregs in Patients with ACS
The function of Treg cells was assessed by inhibition of the proliferation of CD4+CD25− cells in the 4 groups. CD4+CD25+CD127low cells showed different suppressive rates: 83.2 ± 5.1 %, 74.8 ± 4.2 %, 48.9 ± 3.6 %, and 36.3 ± 2.5 %. Suppressive rates of Tregs in UA and AMI group were significantly lower than those in NCA and SA group (P < 0.01). In addition, suppressive rates of Treg cells in SA patients were also significantly lower than those in NCA group (P < 0.05) (Fig. 2).
Comparison of suppressive rate of Tregs among NCA, SA, UA, and AMI groups (n = 10 in each group). Suppressive functions of Tregs in UA and AMI group were markedly lower than those in NCA and SA group. Tregs suppression in SA patients was also significantly lower than that in NCA group. *P < 0.01 compared with SA and NCA; # P < 0.05 compared with NCA
Concentrations of Serum Ox-LDL and Biochemical Markers in Patients with ACS
The concentrations of Ox-LDL, hsCRP, Lp(a), and CK-MBmass in AMI and UA group significantly increased as compared with SA and NCA groups (P < 0.01 for all). In addition, an increase in the levels of Ox-LDL and hsCRP was significant for SA group in comparison to NCA group (P < 0.05) (Table 2).
Treg/Th17 Cells and Treg/Th17 Ratio Correlated to the Levels of Ox-LDL and Biochemical Markers
Notably, in all the 4 groups, the frequency of CD4+CD25+CD127low Treg cells, Th17 cells, and Treg/Th17 ratio showed significant correlation with Ox-LDL and biochemical markers (Fig. 3). The frequency of Treg cells negatively correlated with Ox-LDL, hsCRP, Lp(a), and CK-MBmass concentrations in serum (P < 0.01 and r = −0.614, −0.536, −0.712, −0.571, respectively), while the frequency of Th17 cells positively correlated with the concentrations of 4 markers in serum (P < 0.01 and r = 0.605, 0.614, 0.738, 0.697, respectively). The ratio of Treg/Th17 negatively correlated with Ox-LDL, hsCRP, Lp(a), and CK-MBmass concentrations in serum (P < 0.01 and r = −0.544, −0.503, −0.668, −0.595, respectively).
Spearman correlation of Treg,Th17 cells, and Treg/Th17 ratio to the levels of Ox-LDL and biochemical markers. a The correlation of Treg cells to Ox-LDL, hsCRP, and CK-MBmass concentrations. b The correlation of Th17 cells to Ox-LDL, hsCRP, and CK-MBmass concentrations. c The correlation of Treg/Th17 ratio to Ox-LDL, hsCRP, and CK-MBmass concentrations
Prediction Values of Treg/Th17 Ratio for ACS and AMI
To compare the predictive specificity and sensitivity for ACS, receiver-operating characteristic (ROC) curves were compared among Treg/Th17 ratio, Ox-LDL, hsCRP, Lp(a), and CK-MBmass (Fig. 4). Area under the curve (AUC) values for Ox-LDL, hsCRP, Lp(a), CK-MBmass, and Treg/Th17 ratio were 0.781, 0.834, 0.807, 0.865, and 0.891, respectively, indicating that Treg/Th17 ratio showed the highest sensitivity and specificity for ACS among these five biomarkers. A 1.7 cut-off value of Treg/Th17 ratio predicted ACS with a sensitivity and specificity of 85.4 % and 89.2 % (Table 3).
To compare the predictive specificity and sensitivity for AMI, ROC curves were compared among these (Fig. 5). Area under the curve (AUC) values of Ox-LDL, hsCRP, Lp(a), CK-MBmass, and Treg/Th17 ratio was 0.788, 0.817, 0.806, 0.854, and 0.873, respectively, also indicating that Treg/Th17 ratio showed the highest sensitivity and specificity for AMI among these five biomarkers. A 1.2 cut-off value of Treg/Th17 ratio predicted AMI with a sensitivity and specificity of 85.1 % and 84.6 %, respectively, (Table 4).
Discussion
Treg and Th17 cells are closely related to inflammation which may affect plaque destabilization and the onset of ACS. Ox-LDL and inflammatory markers are associated with increased plaque inflammation and features of plaque vulnerability [12]. The numbers and functions of Treg/Th17 cells and the ratio of Treg/Th17 were assessed in patients with AMI, UA, SA, and NCA subjects, and the correlations of Tregs and Th17 levels to serum Ox-LDL, hsCRP, Lp(a), and CK-MBmass were analyzed. In ACS patients, a significant increase of Th17 frequencies, a significant decline of Treg/Th17 ratio, Treg frequenies, and function were found in comparison to the SA patients and NCA subjects. Significant positive correlations in Th17 and negative correlation in Tregs were found to level of serum Ox-LDL and biomarkers. ROC curves shown that the predictive specificity and sensitivity of Treg/Th17 ratio for ACS and AMI was the highest among these five markers: Ox-LDL, hsCRP, Lp(a), CK-MBmass, and Treg/Th17 ratio. These results indicate that levels of Th17 and Treg cells in peripheral blood are associated with the onset of ACS, and Treg/Th17 ratio may be useful as a marker for early detection for ACS.
Recent studies have reported that adoptive transfer of Treg cells is able to cause a great reduction in plaque size and inhibit inflammation in animal model [13, 14]. In our study, the number and function of Tregs were found to be reduced significantly in ACS patients, which were in line with the report by Mor et al. [15] suggesting that Treg cells have a protective role in the progression and stability of the plaque. In this study, Tregs from ACS patients exhibited a hampered inhibitory ability on proliferation of responder CD4+CD25− T cell compared to Tregs from SA patients and NCA individuals. Moreover, the frequency of CD4+CD25+CD127low Treg cells and the functional suppressive properties from SA patients were also significantly lower than NCA subjects (P < 0.05).
Th17 cells are the new member of the Th cell family and are characterized by their ability to produce specific cytokines such as IL-17. IL-17 exhibits proinflammatory properties and acts as a key cytokines for the recruitment, activation, and migration of neutrophils [16].
However, the role of Th17 cell or IL-17A remains controversial in ACS patients. In a study by Zhao et al. [17], ACS patients exhibited remarkable rise in the number of peripheral Th17/Th1 (IL-17A+ IFN-γ+) cells but not Th17 (IL-17A+ IFN-γ+) cells. In our study, we found that patients with ACS exhibited a marked increase in Th17 number as compared with patients with SA and NCA subjects, while patients with SA also exhibited a significant increase in Th17 level as compared with patients with NCA subjects. Th17 participation in inflammatory process of plaque destabilization and ACS development could relate to onset of ACS [18].
Therefore, we have further demonstrated that Treg/Th17 imbalance was existed in ACS patients, which was consisted with our pervious studies and others [9–11]. In our study, Treg/Th17 ratio, representing the balance between Treg and Th17, was markedly lower in the patients with ACS as compared with the NCA group and SA patients, and also significantly lower in the SA groups in comparison to that in the NCA group. The results of our study suggest that plaque instability and ACS occurence are related to declined Treg/Th17 ratio. Treg/Th17 ratio also shown significant correlation with Ox-LDL, hsCRP, Lp(a), and CK-MBmass, which suggested that alteration of Th17/Treg is linked to increase of inflammatory mediators and risk factor which lead to myocardial damage and plaque destabilization.
Biochemical markers play a pivotal role in the diagnosis and management of patients with ACS. Ox-LDL is a marker that links the immune system with the atherogenic process, which is considered an instrumental factor that promotes atherosclerosis initiation, progression, and possibly, plaque destabilization [19]. Ox-LDL changes the secretory activities of endothelium and causes it to become dysfunctional. Serum Ox-LDL level not only can predict the presence of atherosclerotic coronary artery disease but can also be a marker of plaque instability and ACS occurence [20]. The increased Ox-LDL content in the circulation is closely related to the vulnerability of plaques. Moreover, increased Ox-LDL levels in the circulation are known to induce the expression of inflammatory factors which play a role in augmenting the inflammatory reaction in plaques. It was also suggested that Ox-LDL level in the circulation is an indicator of the severity and course of ACS [21, 22].
Recent studies suggest that inflammation markers take an important part in the risk of developing coronary artery disease, and may correlate with its severity. Hs-CRP is a well-known inflammatory biomarker which reflects proinflammatory responses. CRP has a direct proinflammatory effect on human endothelial cells, impairing endothelial vasoreactivity, promoting smooth muscle cell proliferation, and migration [23, 24]. It was shown that hs-CRP induced the expression of adhesive factors in endothelium and thus initiated or accelerated the atherosclerosis pathogenesis [25]. Patients with AMI showed a rise in CRP within 6 h of symptom onset, and serum CRP levels can predict future risks in patients with UA and AMI [26, 27].
Lp(a) is a lipoprotein composed of apoprotein(a), which is connected to apo-B by disulfide bonding. Lp(a) has been implicated in the regulation of the expression of plasminogen activator inhibitor-1 in endothelial cells, and has been shown to inhibit endothelial cell surface fibrinolysis, attenuate plasminogen binding to platelets and bind to plaque matrix components. It is also associated to inflammatory reactions, including the recruitment of macrophage [28]. Therefore, Lp(a) is an atherogenic, and thrombogenic lipoprotein, which has been implicated in the pathogenesis of ACS. Lp(a) is considered to be an independent risk factor for ACS, and play a role in plaque instability [29].
Creatinine kinase isoenzyme MB (CK-MB) is the heart specific isoenzyme and has been the gold standard method for the diagnosis of AMI in many laboratories. It exists in large quantity in heart muscle, but is not totally cardiac specific and exists also in skeletal muscles and other tissues. A study by Karras et al. [30] has shown that measurement of CK-MB using mass is better than that measuring CK-MB activity. CK-MBmass measurement has also been reported to be more sensitive in detecting small injuries to the myocardium that occur in patients with non-ST elevation ACS [31]. ROC curves shown that the predictive specificity and sensitivity of CK-MBmass for ACS and AMI was similar to Treg/Th17 ratio, and higher than Ox-LDL, hsCRP, and Lp(a).
Plaque vulnerability is the pathophysiological substrate of ACS. The morbidity and mortality from AMI are significantly reduced and prognosis can be improved if unstable plaque is identified and given proper therapy in advance. Though Intravenous ultrasound (IVUS) imaging is an excellent tool for detecting ruptured and unstable plaque, it is limited because of its invasiveness. It is of potential significance to explore the blood markers indicating plaque characteristics. However, besides detection of CK-MB, hsCRP, Ox-LDL, and Lp(a), few non-invasive examinations can predict ACS occurence and few studies have focused on this subject.
In our study, we compared cut-off values of Treg/Th17 ratio for ACS and AMI with hsCRP, Ox-LDL, Lp(a) and CK-MBmass in the same blood samples at the time of admission, and revealed that Treg/Th17 ratio shows the highest sensitivity and specificity for ACS and AMI diagnosis in this study population. Treg/Th17 ratio <1.7 can be an indicator for ACS, with a sensitivity of 85.4 %, a specificity of 89.2 %, while Treg/Th17 ratio <1.2 can be an indicator for AMI occurrence, with a sensitivity of 85.1 %, a specificity of 84.6 %.
In the present study, we found for the first time that Treg/Th17 ratio could acts as a novel marker to evaluate plaque stability. Moreover, this new marker seems to be a more potent for early diagnosis of ACS and AMI than other biomarkers. However, we need a well-designed evaluation of the marker before their use in the clinical practice.
In summary, alteration of Treg/Th17 has presented in ACS which may contribute to plaque destablilzation and onset of disease. Treg/Th17 ratio appears to be a novel indicator for early diagnosis of ACS and AMI. The analysis of Treg/Th17 ratio may be useful for the development of new strategies for coronary disease prevention and treatment.
References
Shoenfeld, Y., Sherer, Y., & Harats, D. (2001). Artherosclerosis as an infectious, inflammatory and autoimmune disease. Trends in Immunology, 22, 293–295.
Rodríguez, G., Mago, N., & Rosa, F. (2009). Role of inflammation in atherogenesis. Investigacion Clinica, 50, 109–129.
Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine, 352, 1685–1695.
Swap, C. J., & Nagurney, J. T. (2005). Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. Journal of the American Medical Association, 294, 2623–2629.
Sabatine, M. S., Morrow, D. A., de Lemos, J. A., et al. (2002). Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: Simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation, 105, 1760–1763.
Sakaguchi, S., Sakaguchi, N., Shimizu, J., et al. (2001). Immunologic tolerance maintained by CD25+CD4+regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunological Reviews, 182, 18–32.
Miossec, P., Korn, T., & Kuchroo, V. K. (2009). Interleukin-17 and type 17 helper T cells. New England Journal of Medicine, 361, 888–898.
Wang, W., Shao, S., Jiao, Z., et al. (2012). The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatology International, 32, 887–893.
Cheng, X., Yu, X., Ding, Y. J., et al. (2008). The Th17/Treg imbalance in patients with acute coronary syndrome. Clinical Immunology, 127, 89–97.
Li, Qing, Wang, Yi, Chen, Ke, et al. (2010). The role of oxidized low-density lipoprotein in breaking peripheral Th17/Treg balance in patients with acute coronary syndrome. Biochemical and Biophysical Research Communications, 394, 836–842.
Xie, J. J., Wang, J., Tang, T. T., et al. (2010). The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine, 49, 185–193.
Nishi, K., Itabe, H., Uno, M., et al. (2002). Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 1649–1654.
Ait-Oufella, H., Salomon, B. L., Potteaux, S., et al. (2006). Natural regulatory T cells control the development of atherosclerosis in mice. Nature Medicine, 12, 178–180.
Mor, A., Planer, D., Luboshits, G., et al. (2007). Role of naturally occurring CD4 + CD25 + regulatory T cells in experimental atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 893–900.
Mor, A., Luboshits, G., Planer, D., et al. (2006). Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. European Heart Journal, 27, 2530–2537.
Romagnani, S., Maggi, E., Liotta, F., et al. (2009). Properties and origin of human Th17 cells. Molecular Immunology, 47, 3–7.
Zhao, Z., Wu, Y., Cheng, M., et al. (2011). Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis, 217, 518–524.
Hashmi, S., & Zeng, Q. T. (2006). Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coronary Artery Disease, 17, 699–706.
Mitra, S., Goyal, T., & Mehta, J. L. (2011). Oxidized LDL, LOX-1 and atherosclerosis. Cardiovascular Drugs and Therapy, 25, 419–429.
Ehara, S., Ueda, M., Naruko, T., et al. (2001). Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation, 103, 1955–1960.
Yamashita, H., Ehara, S., Yoshiyama, M., et al. (2007). Elevated plasma levels of oxidized low-density lipoprotein relate to the presence of angiographically detected complex and thrombotic coronary artery lesion morphology in patients with unstable angina. Circulation Journal, 71, 681–687.
Zhang, Y. C., Wei, J. J., Wang, F., et al. (2012). Elevated levels of oxidized low-density lipoprotein correlate positively with C-reactive protein in patients with acute coronary syndrome. Cell Biochemistry and Biophysics, 62, 365–372.
Verma, S., Wang, C. H., Li, S. H., et al. (2002). A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation, 106, 913–919.
Venugopal, S. K., Devaraj, S., Yuhanna, I., et al. (2002). Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation, 106, 1439–1441.
Pasceri, V., Willerson, J. T., & Yeh, E. T. (2000). Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation, 102, 2165–2168.
Biasucci, L. M., Liuzzo, G., Colizzi, C., et al. (2001). Clinical use of C-reactive protein for the prognostic stratification of patients with ischemic heart disease. Italian Heart Journal, 2, 164–171.
Sanchis, J., Bodi, V., Llacer, A., et al. (2004). Usefulness of C-reactive protein and left ventricular function for risk assessment in survivors of acute myocardial infarction. American Journal of Cardiology, 15, 766–769.
Dangas, G., Mehran, R., Harpel, P. C., et al. (1998). Lipoprotein(a) and inflammation in human coronary atheroma: Association with the severity of clinical presentation. Journal of the American College of Cardiology, 32, 2035–2042.
Ryu, S. K., Hong, B. K., Kwon, H. M., et al. (2003). Age-related contribution of Lp(a) with coronary artery calcification in patients with acute coronary syndrome: A potential role of metabolic disorder in calcified plaque. Yonsei Medical Journal, 44, 445–453.
Karras, D. J., & Kane, D. L. (2001). Serum markers in the emergency department diagnosis of acute myocardial infarction. Emergency Medicine Clinics of North America, 19, 321–337.
Penttilä, I. M., Laatikainen, A., Penttilä, K., et al. (2007). Imprecision of cardiac marker analyses among laboratories on the basis of external quality assurance results: Finnish experience. Scandinavian Journal of Clinical and Laboratory Investigation, 67, 507–518.
Acknowledgments
This study was supported by Natural Science Foundation of Anhui province of China (No. 1408085MH145, 11040606Q08), Natural Science Key Foundation of Anhui Universities of China (No. KJ2011A166), and Anhui Medical University Research Foundation (No. 2012XKJ002).
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qing Li, Yi Wang and Yiping Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Q., Wang, Y., Wang, Y. et al. Treg/Th17 Ratio Acts as a Novel Indicator for Acute Coronary Syndrome. Cell Biochem Biophys 70, 1489–1498 (2014). https://doi.org/10.1007/s12013-014-9993-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9993-5