Abstract
Di-n-butyl phthalate (DBP) widely used plasticizer in the plastic industry, affects regulation of the endocrine system and causes toxicity in animals. In the present study, the aim was to study the toxic effects/damages of DBP exposure using Hsp70 levels and histopathological changes in Carp liver and gill. Hsp70 expression levels were assessed as specific biomarker of in vivo ecotoxicological stress. Carp (Cyprinus carpio) were exposed to sub-lethal concentration of DBP (di-n-butyl phthalate, 1 mg/L) for 4, 24 and 96 h. Gill and liver tissues were evaluated histopathologically and RNA quantifications for Hsp70 expression levels were carried out using a two-step real-time RT-PCR. In liver, a rapid but non-significant increase in mRNA levels in the first 4 h was observed. mRNA levels significantly increased up to 2–3 fold after 24 and 96 h (p < 0.05). However, irregular mRNA level changes were also recorded: Gill specific and time-dependent regulation of Hsp70 expression were 4–5 fold inhibition after 4 and 24 h (p < 0.05), then increased up to 4 fold after 96 h (p < 0.05). Histopathological findings support altered transcription results as: Epithelial lifting, hyperplasia, fusion of secondary lamellae, telangiectasis, passive hyperemia and hydropic degeneration. Significant alterations of Hsp70 levels were likely due to a tissue specific response against chemical stress, cellular damage and lesions due to DBP. Carp was found to be a suitable experimental model for toxicology, and Hsp70 mRNA levels are reliable, specific biomarkers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates are used in industry widespread as solvents and additives [1]; i.e. in the production process of polyvinyl chloride as plasticizer [2], in cosmetic paints, varnishes and perfumes, repellents and carrier fluids in biocides (Annex XV Rest. Report Ver. 2, 2011). As nonreactive plasticizers, phthalate esters increase the flexibility and workability of high molecular weight polymers [2], therefore phthalates are not chemically bonded to the polymer and can contaminate to the environment.
DBP (di-n-butyl phthalate, CAS: 84-74-2) is also accepted as a potential EDC (Endocrine Distruptive Chemical) with carcinogenic potential [3] and disrupting effects on reproductive physiology [4, 5]. Kruger et al. [6] has demonstrated the dose dependent induction of the AhR transactivity mediated by DBP and stated that DBP may elicit anti-estrogenic effects by altering activity of the ligand dependent AhR pathway.
Global concern in exposure to estrogens, metals, organics, plasticizers, pesticides, industrial wastes, nanomaterials and organic pollutants have led to increased efforts and strengthening of biomonitoring programs, prevention of environmental impacts and taking legislative action [4]; necessitating the development and use of rapid, reliable, sensitive methods/approaches with emphasis on molecular biology and organismal toxicology methodologies [7].
Gill is the first organ to react to environmental factors and the liver is the major organ with detoxification enzyme systems activated/altered when exposed to stressors and toxicants specifically [8]. Environmental stress such as alterations in environmental salinity and exposure to chemical pollutants are known to alter heat shock protein expression, as a general stress response and validating their importance as biomarkers [9]. Therefore useful for monitoring heavy metals [10] and EDCs [11]. Potent environmental contaminants, EDCs, organic pollutants and some other toxicants can modulate the expression of Hsps in fish gills and liver [9, 12] and organ specific induction of Hsp70 was reported [13].
Common carp is one of the economically important freshwater fish and preferred as an experimental model organism for screening environmental pollution and following toxicity [14]. We hypothesize that alterations in fish Hsp70 transcription levels in gills and liver may serve as a rapid, reproducible, sensitive and simple ecotoxicological biomarker. According to reports that demonstrate the relation between toxicity and alteration of Hsp70 transcription levels, we can conclude that transcriptional upregulation of Hsp70 is not able to lead to an increase in Hsp70 protein levels [15]. Gene expression studies are promising to determine protein levels, and mRNA levels can be accepted as prognostic parameters for predicting protein levels [16].
This study aimed to describe the effects of DBP on gills and liver of the common carp (Cyprinus carpio), assessing tissue alterations at the molecular level in relation to a sublethal concentration and exposure time.
Materials and methods
Fish exposure
Carp (Cyprinus carpio) obtained from State Hydraulic Works General Directorate (DSI, Yedikir Dam Lake, Amasya, Turkey) were adapted to 12 h day and 12 h night period in 120 L fish tank for 15 days. Carp were fed with commercial fish food in normal period and were starved prior to chemical treatment. Afterwards, carp were exposed to a graded concentration of DBP (1, 5 and 10 mg/L in acetone; DBP CAS Nr: 84-74-2, 100 %, MERCK,). 10 mg/L and 5 mg/L exposures were determined as lethal dose. Therefore, carp were exposed to sublethal concentration of di-n-butyl phthalate (1 mg/L) which was completely dissolved in water without any deposition for 4 h (n = 5), 24 h (n = 7) and 96 h (n = 7) in 120 L fish tanks. A vehicle control group (n = 16) was included. After sacrifice and macroscopic examination on ice, sexually immature carp (avg. wt. 63.14 g ± 18.9; avg. length 15.87 cm ± 1.59) were necropsied and tissues were evaluated histopathologically. The protocol (G.Ü.ET-07 dated 19 September 2012) for using carp in the experiments was reviewed and approved by the Gazi University Experimental Animals Ethical Council. Guiding principles for experimental procedures found in the Gazi University Experimental Animals Ethical Council and Declaration of Helsinki of the World Medical Association regarding animal experimentation were followed in the study.
Water quality
Fish tanks were filled with municipal tap water and used for the exposure experiments after a 2-days period for chlorine evaporation. A field type oxygen meter (YSI, model 51B) was used for monitoring water quality of the media: pH 7.52, salinity 0.12 ppt, temp 16.2 °C, total hardness = 65 mg/L CaCO3, DO 6.33 mg/L, conductivity 204.7 µs/cm, TDS 159.9 mg/L, ammonia >1 mg/L N, nitrite-N 0.081 mg/L.
Tissue preparations
At the end of treatment periods, fish of all the groups were sacrificed and dissected. Gills and livers of control and treated fish were fixed in 10 % formalin fluid, dehydrated in graded alcohol series and embeded in paraffin. Paraffin blocks of gill tissue were cut at 5 µm thickness using rotary microtome. After deparaffinization, sections were stained with Haematoxylin and eosin (H&E staining). Four to six gill and liver sections from each individual fish were analyzed by light microscopy and were photographed on a Carl Zeiss Primostar microscope fitted with a Canon EOS 1000D camera. Histopathological alterations in the tissues were examined in the randomly selected 5 sections from each fish. The mean prevalence of each histopathological parameter was categorized as no histopathology, mild (<25 % of sections), moderate (25–50 % of sections) and severe (>50 % of sections). This categorization was modified according to Mallatt [17].
Total RNA extraction
Gills and livers of control and treated fish were dissected and submerged immediately into liquid nitrogen within aluminium to prepare flash-frozen tissues and then stored at −80 °C. Total RNA was isolated from 30 mg gill tissue with “Qiagen® RNeasy Total RNA extraction from tissue kit (Limburg, NL)” and DNase I treatment was performed. Quantity of extracted total RNA was evaluated spectrophotometrically at 260 nm; 260/280 ratios were 1.8–2. RNA integrity was determined by 1 % agarose gel electrophoresis. Intact and well separated 28S and 18S rRNA bands were detected.
Reverse transcription and semi-quantitative real-time PCR
To one μg total RNA, 1 μM oligo(dT) primer, dNTP mix (1 mM each), 1 × RT reaction buffer and 10 U reverse transcriptase (Roche® Diagnostics) were added and brought to a total reaction volume of 20 μl with RNase-free distilled water for the RT reaction. Reaction was by incubation for 30 min at 55 °C and enzyme was inactivated 5 min at 85 °C. Real-time PCR reaction mix was prepared using 2x SYBR Green Master mix (Roche® Diagnostics), 0.2 U Uracil-DNA glycosylase, to avoid carry over DNA contamination (Roche® Diagnostics); forward and reverse primers for Hsp70 and β-actin at 0.4–0.6 μM final concentration and mix was completed to a total volume of 20 μl with sterile dH2O. Real-time PCR conditions were optimized. Annealing temperatures of gene specific primers and incubation times were determined after a few reaction experiments. Primers (for Hsp70, forward 5′-GTCCCTGGGTATTGAAACCGCA-3′, reverse 5′-GCTGGTTGTCTGAGTAGGTGGT-3′; for β-actin, forward 5′-ATCCGTAAAGACCTGTATGCCA-3′, reverse 5′-GGGGAGCAATGATCTTGATCTTC-3′) were designed by using Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome). A first step initial denaturation and hot start enzyme activation at 95 °C for 10 min, and 30 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s three step real-time PCR was performed with Nano Lightcycler and Lightcycler Nano SW 1.0 software (both from Roche). A negative control without cDNA template and a no-RT control RNA sample without reverse transcription were included to determine the specificity of target cDNA amplification and to control for genomic DNA contamination. A pool of cDNAs was prepared from control samples and diluted for standards. Standard curves were drawn to a concentration gradient and Cq values, efficiencies and r values were calculated between 1.9–2 and 0.99 respectively, with Nano SW 1.0 software. Melting curve program was used to heat 60–95 °C at a heating rate of 0.1 °C/s and continuous fluorescence measurement and finally a cooling step to 40 °C for 20 s. Melting curve analysis of PCR products was performed at the end of each polymerase reaction to confirm a single PCR product was detected. Quantities of specific mRNA levels were measured from the corresponding standard curves. Expression ratio of Hsp70 was measured relative to the housekeeping gene β-actin as described before by Aoki et al. [18]. Lightcycler® Nano SW 1.0 software was used to compare amplification in samples to the standard curve. Software has calculation function to determine efficiency corrected ΔCq and standard error.
Western blotting
Protein extracts were prepared as described by Puerto et al. [19]. Protein concentrations were determined using a BCA (bicinchoninic acid) protein assay kit (Pierce® Biotechnology). Equal amounts of protein (100 µg) were electrophoresed on 8 % SDS–polyacrylamide gels and transferred to PVDF membrane (Bio-Rad Laboratories). Membrane was blocked for 1 h with 5 % skim milk powder (Sigma, USA) in TBS (Tris-buffered saline, 200 mM NaCl, 50 mM Tris, pH 7.6). Membrane was incubated with anti-Hsp70 antibody (mouse, Cayman Chemical, Michigan, USA), diluted 1:500 at 4 °C by shaking overnight. After serial washing steps with TBS-T (0.1 % Tween-20 in TBS), membrane was incubated for 2 h at room temperature with HRP conjugated secondary antibody (anti-mouse IgG, Sigma, USA) that was diluted 1:4000. Blot was incubated with ECL substrate and visualized by MP image system (Biorad, CA).
Data analysis
The data are expressed as mean ± standard error of the mean (SEM). After assessing the normality distribution and homogeneity of variances of the data by Levene’s test; and differences between groups were analyzed by Student’s t test. The critical significance level for the statistical tests performed was set to be 0.05.
Results
Gill histopathology
The histopathological changes of gills noticed in the control and DBP exposed fish are shown in Table 1. The control fish showed no histopathological lesions in the gill tissue (Fig. 1A). Exposure to DBP (1 mg/L) caused obvious histopathological changes in the gill tissue (Fig. 1B). In the DBP exposed fish, hyperemia, lamellar fusion, epithelial hyperplasia and epithelial lifting and edema were observed (Figs. 1B, 2A). Lamellar telangiectasia was observed in fish exposed to DBP (1 mg/L) for 96 h (Fig. 2B). The observed histological alterations were more evident in fish exposed to DBP for 96 h. To our knowledge, this is the first investigation on alterations of gill tissue in C. carpio exposed to sublethal concentration of DBP.
Gill histological sections showing gill histopathology on DBP exposures. A Epithelial hyperplasia of secondary lamellae (arrowhead), cellular proliferation between secondary lamellae leading to fusion of the secondary lamellae (arrowhead; H&E). B Telangiectasia was evident in 96 h exposed group (star; H&E)
Liver histopathology
The histopathological changes of liver noticed in the control and DBP exposed fish are shown in Table 1. The control fish showed no histopathological lesions in the liver tissue. The liver histology of the control group revealed normal structure, with typical hepatic cells with rounded nuclei with evident nucleoli (Fig. 3). The histopathological lesions in the liver observed in the present study were passive hyperemia and hydropic degeneration. After 24 h of exposure to DBP, carp liver displayed only passive hyperemia (Fig. 4A). 96 h of exposure to DBP, passive hyperemia and hydropic vacuolation were observed (Fig. 4B). The observed histological alterations were more severe in fish exposed to DBP for 96 h.
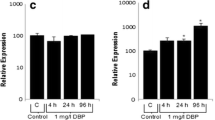
Hsp70 expression in gill
Real-time PCR methodology presently serves as a rapid, simple and sensitive alternative to conventional analytical and biochemical methods, including the time consuming western blotting. In PCR, total tissue RNA can be prepared and results available only within 1–1.5 h. In addition, sensitivity is very high. Levels of Hsp70 expression in experimental group are presented as percentage of Hsp70 expression levels in controls (Fig. 5). In gill, Hsp70 mRNA levels decreased to five-fold relative to controls in the first 4 h after DBP exposure (1 mg/L). After 24 h, inhibition was not altered as it was five-fold reduction in expression levels. But after 96 h following DBP exposure, Hsp70 expression was induced 4 fold in experimental group. Alterations in levels of Hsp70 expression were statistically significant (p < 0.05).
Hsp70 expression in liver
Levels of Hsp70 expression in the experimental group are presented as percentage of Hsp70 expression levels relative to controls (Fig. 5). Hsp70 mRNA levels were not altered in experimental group after 4 h following DBP exposure, however, 2.5-fold induction was observed in experimental group after 24 h (p < 0.05). After 96 h, Hsp70 expression levels of experimental group were altered with a little change significantly as it was 1.8-fold induction relative to controls (p < 0.05). Comparatively, Hsp70 expression was more strongly altered in the gills than in the liver. Moreover, heat-shock response was considerably weaker in the liver than in the gills of carp.
Hsp70 protein levels
Hsp70 protein levels in liver were analyzed using western blotting and presented in Fig. 6. Hsp70 levels in the experimental group were drawn relative to control group. Protein levels were not altered in the experimental group after 4 h. In contrast, 1.3 and 1.6-fold induction were observed in 24 h and 96 h after DBP exposure. Liver Hsp70 induction showed correlation with liver Hsp70 mRNA expression levels. This shows that the regulation of Hsp70 synthesis is controlled in transcription levels.
Discussion
Of many aquatic organisms, fish are highly sensitive to waterborne pollutants, and gills and liver of fish serve as indicators of environmental stress [17, 20, 21]. Histopathology can be used to evaluate the health of fish populations by assessing selected target organs [22] and the results from histopathological studies are useful to evaluate the long and short-term toxic effects at the cellular level [23]. The effect of different organic compounds and heavy metals on biochemical and morphological changes in liver and gill tissues of fish have been reported [24–26].
Since gills present a large surface area in contact with the surrounding water, they are the first target organ of toxic pollutants in fish [17]. Histopathological changes observed in gills of fish recognized as a fast and valid method of evaluating the tissue damage induced by exposure to different kinds of pollutants [27, 28]. DBP-caused alterations in gill tissue like epithelial lifting, hyperplasia of epithelial cells, and partial or complete fusion of secondary lamellae are examples of defense mechanisms since they may cause an increase in the pollutant-blood diffusion distance, thus acting as a barrier to toxicant diffusion [17, 27]. Telangiectasia (lamellar aneurysm) is related to the rupture of the pillar cell system, with dilatation of the blood vessels [29]. Telangiectasia is considered a severe type of lesion, which is often irreversible [27]. This lesion damages the blood flow and leads to an impairment of the gill’s gas exchange [29]. Histological alterations found in this study are consistent with the changes in the gills of different fish species exposed to variety of xenobiotics; pesticides, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and heavy metals [21, 24, 30].
In the current study, the other vital organ “liver” displayed passive hyperemia and hydropic degeneration. The liver is known as the primary organ for metabolism, contaminant detoxification and excretion of harmful substances [31]. Therefore histopathological alterations in the liver are useful biomarkers to see the effects of environmental pollutants. Hyperemia is associated with the pathological conditions of tissue and blood flow [31]. Cellular swelling or hydropic vacuolation was observed in the livers of carp exposed to DBP for 96 h. Hydropic vacuolation of liver is one of the major signs of toxic damage [32]. Histopathological alterations observed in liver have been reported with other pollutants, such as pesticides [33] and heavy metals [24].
In recent years, investigation of alterations in expression levels of stress proteins, such as heat shock protein 70, metallothionein and AhR dependent potential biomarker genes, are increasingly used in the study of the impacts of environmental contamination and organismal stress response showing the necessity for inclusion of molecular biology approach [34–36]. Hsp70 is among the most important potential biomarkers, because of its rapid response to stressors as Hsp70 gene is lacking intron sequence [9]. Moreover, it is known that Hsp70 expression is controlled at transcriptional levels [37]. It can be concluded that alteration of Hsp70 levels can be reliably predicted by RT-PCR in mRNA levels. In this study, Hsp70 mRNA levels were investigated in gill and liver tissues following sublethal DBP exposure. A rapid increase in mRNA levels in the first 4 h after DBP exposure was observed, but it was not statistically significant. Besides, after 24 h, mRNA levels significantly increased up to 2.5 fold and this induction was stabilized at approximately 2.0 fold level (Fig. 5) during 96 h. It is known from the literature that DBP can exert endocrine disrupting activity [4, 5]; DBP exposure induced heat shock protein expression in MCF-7 cells. [38]. In addition, previous studies on exposure of fish to chemical contaminants have shown that increasing Hsp70 levels could be part of a cellular defense mechanism to chemical stress [12]. As a major sign of toxic damage, hydropic vacuolation observed in liver in this study indicates cellular stress and potential damage, we can conclude that a protective mechanism could emerge by inducing Hsp70 expression to accommodate refolding damaged proteins.
Reduced Hsp70 levels were reported in oxidative stress conditions by acute exposure to chemical agents, but regular alterations were not observed [16]. In contrast, some authors reported the early phase expression of fish and rat Hsp70 induction under oxidative stress conditions [39–41]. Moreover, DBP exposure was known to induce oxidative stress [42], and enhanced activities of SOD, GPx and CAT [43]. In this study, increased expression of Hsp70 gene in liver, in 96 h, may be related to oxidative damage which presumably occurred by DBP exposure and this result shows correlation with microscopic evaluation of liver in experimental group. In addition, liver Hsp70 protein levels showed correlation with mRNA levels, meaning upregulation in mRNA levels directly affect protein synthesis. Besides, Xing et al. [14] reported an organ specific expression profile of Hsp70 as there were more rapid and severe induction in liver than in other organs in all experiments performed. Furthermore, some researchers reported that DBP exposure can lead to an inhibitory effect on Hsp70 expression in rat [44]. We observed rapid decrease in Hsp70 expression in gills within 4 and 24 h significantly, but in contrast, a stable fold increase was observed in 96 h (Fig. 5). Irreversible and severe lesions were observed in gills in this study (Figs. 1B, 2A, B). Similarly, Hsp70 has a pleiotropic role in lesions, necrosis or apoptosis [45, 46]. In case of irreversible cell damage, Hsp70 is known to drive the cells to eventual death [46]. Hsp70 can be inhibited even with inhibition of HSF-1 to repress transcriptional activation as HSF-1 is the transcription factor in Hsp70 gene transcription [47]. Inhibition of Hsp70 expression in gills was likely related to combined response against microscopic lesions. Reduced expression of Hsp70 was reported in case of cell damage following exposure of juvenile Ictalurus punctatus to chemical agents [48]. Moreover, a several fold increase of Hsp70 levels in 96 h can be interpreted as a result of recovery effort/effects or a repair process to cope with protein denaturation and following proteotoxicity. We can conclude that dramatic increase in Hsp70 levels was probably due to repairing, thickening and increasing protein synthesis in gill tissue as a response to cellular damage and microscopic lesions.
Alteration of Hsp70 levels were studied in fish liver, gills, kidneys and in other aquatic or terrestrial organisms and used as contamination biomarker [12, 13, 16, 34, 40]. Researchers have identified contaminants which can affect regulation of Hsp70 synthesis, such as PAHs, heavy metals, pesticides, arsenite, chlorpyrifos and nickel [49]. These studies indicate that Hsp70 is a sensitive and integrative marker of chemical toxicity and shows molecular approaches in ecotoxicology to be highly promising.
Conclusions
With the present study we demonstrated that DBP, as a chemical contaminant, is able to change and regulate Hsp70 expression in mRNA levels in a time-dependent manner. The gills are the first organs to react to environmental contaminants and freshwater is a unique supply for the life of fish and freshwater organisms. Besides, since liver is the major organ in metabolism to cope with xenobiotics and toxicants, then alterations in expression levels of this stress protein demonstrate us that DBP is able to affect metabolism and cause stress and toxicity. Hsp70 has highly conserved expression levels normally, but it can be up- or down-regulated with exposure to toxicants, oxidative stressors and most of industrial wastes as shown and determined totally by ecotoxicologists. We evaluated carp as an ecotoxicologic experimental model and demonstrated that Hsp70 levels can be used as a specific biomarker of in vivo ecotoxicological stress originated from chemical contamination and pollution. Besides, a new perspective on methodology of ecotoxicology studies is presented by using real-time RT-PCR as a rapid, sensitive, reproducible and simple molecular approach.
Abbreviations
- HSP70:
-
Heat shock protein 70
- DBP:
-
Di-n-butyl phthalate
- HSF-1:
-
Heat shock factor-1
- AhR:
-
Aryl hydrocarbon receptor
References
Huang P-C, Tien C-J, Sun Y-M et al (2008) Occurrence of phthalates in sediment and biota: relationship to aquatic factors and the biota-sediment accumulation factor. Chemosphere 73:539–544. doi:10.1016/j.chemosphere.2008.06.019
Staples CAS, Dams WIJA, Arkerton THFP et al (1997) Aquatic toxicity of eighteen phthalate esters. Environ Toxicol Rev 16:875–891
Van Wezel AP, van Vlaardingen P, Posthumus R et al (2000) Environmental risk limits for two phthalates, with special emphasis on endocrine disruptive properties. Ecotoxicol Environ Saf 46:305–321. doi:10.1006/eesa.2000.1930
Clewell RA, Campbell JL, Ross SM et al (2010) Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol Vitro 24:327–334. doi:10.1016/j.tiv.2009.08.003
Thompson CJ, Ross SM, Gaido KW (2004) Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology 145:1227–1237. doi:10.1210/en.2003-1475
Krüger T, Long M, Bonefeld-Jørgensen EC (2008) Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246:112–123. doi:10.1016/j.tox.2007.12.028
Kim SJ, Park H, Yu SY et al (2009) Toxicogenomic effect of liver-toxic environmental chemicals in human hepatoma cell line. Mol Cell Toxicol 5:310–316
Arinç E, Sen A, Bozcaarmutlu A (2000) Cytochrome P4501A and associated mixed-function oxidase induction in fish as a biomarker for toxic carcinogenic pollutants in the aquatic environment. Pure Appl Chem 72:985–994. doi:10.1351/pac200072060985
Deane EE, Woo NYS (2011) Advances and perspectives on the regulation and expression of piscine heat shock proteins. Rev Fish Biol Fish 21:153–185. doi:10.1007/s11160-010-9164-8
Fulladosa E, Deane E, Ng AHY et al (2006) Stress proteins induced by exposure to sublethal levels of heavy metals in sea bream (Sparus sarba) blood cells. Toxicol In Vitro 20:96–100. doi:10.1016/j.tiv.2005.06.005
Planelló R, Martínez-Guitarte JL, Morcillo G (2008) The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius. Chemosphere 71:1870–1876. doi:10.1016/j.chemosphere.2008.01.033
Vijayan MM, Pereira C, Kruzynski G, Iwama GK (1998) Sublethal concentrations of contaminant induce the expression of hepatic heat shock protein 70 in two salmonids. Aquat Toxicol 40:101–108
Ali KS, Dorgai L, Ábrahám M, Hermesz E (2003) Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun 307:503–509. doi:10.1016/S0006-291X(03)01206-3
Xing H, Li S, Wang X et al (2013) Effects of atrazine and chlorpyrifos on the mRNA levels of HSP70 and HSC70 in the liver, brain, kidney and gill of common carp (Cyprinus carpio L.). Chemosphere 90:910–916. doi:10.1016/j.chemosphere.2012.06.028
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186. doi:10.1152/japplphysiol.01267.2001
Chen C, Zhou Q, Liu S, Xiu Z (2011) Acute toxicity, biochemical and gene expression responses of the earthworm Eisenia fetida exposed to polycyclic musks. Chemosphere 83:1147–1154. doi:10.1016/j.chemosphere.2011.01.006
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648. doi:10.1139/f85-083
Aoki KA, Harris CA, Katsiadaki I, Sumpter JP (2011) Evidence suggesting that di-n-butyl phthalate has anti-androgenic effects in fish. Environ Toxicol Chem 30:1338–1345. doi:10.1002/etc.502
Puerto M, Gutiérrez-Praena D, Prieto AI, Pichardo S, Jos A, Miguel-Carrasco JL (2011) Subchronic effects of cyanobacterial cells on the transcription of antioxidant enzyme genes in tilapia (Oreochromis niloticus). Ecotoxicology 20:479–490. doi:10.1007/s10646-011-0600-x
Lukin A, Sharova J, Belicheva L, Camus L (2011) Assessment of fish health status in the Pechora river: effects of contamination. Ecotoxicol Environ Saf 74:355–365. doi:10.1016/j.ecoenv.2010.10.022
Abdel-Moneim AM, Al-Kahtani MA, Elmenshawy OM (2012) Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 88:1028–1035. doi:10.1016/j.chemosphere.2012.04.001
Van Dyk JC, Pieterse GM (2008) A histo-morphological study of the testis of the sharptooth catfish (Clarias gariepinus) as reference for future toxicological assessments. J Appl Ichthyol 24:415–422. doi:10.1111/j.1439-0426.2008.01127.x
Van Dyk JC, Marchand MJ, Smit NJ, Pieterse GM (2009) A histology-based fish health assessment of four commercially and ecologically important species from the Okavango Delta panhandle, Botswana. Afr J Aquat Sci 34:273–282
Rajeshkumar S, Munuswamy N (2011) Impact of metals on histopathology and expression of HSP 70 in different tissues of Milk fish (Chanos chanos) of Kaattuppalli Island, South East Coast, India. Chemosphere 83:415–421. doi:10.1016/j.chemosphere.2010.12.086
Agamy E (2013) Impact of laboratory exposure to light Arabian crude oil, dispersed oil and dispersant on the gills of the juvenile brown spotted grouper (Epinephelus chlorostigma): a histopathological study. Marine Environmental Research 86:46–55. doi:10.1016/j.marenvres.2013.02.010
Authman MMN, Abbas WT, Gaafar AY (2012) Metals concentrations in Nile tilapia Oreochromis niloticus from illegal fish farm in Al-Minufiya Province, Egypt, and their effects on some tissues structures. Ecotoxicol Environ Saf 84:163–172. doi:10.1016/j.ecoenv.2012.07.005
Poleksic V, Mitrovic-Tutundzic V (1994) Fish gills as amonitor of sublethal and chronic effects of pollution. In: Muller R, Lloyd R (eds) Sublethal and Chronic effects of pollutants on freshwater fish. Fishing News Books, Oxford, pp 339–352
Schwaiger J, Wanke R, Adam S et al (1997) The use of histopathological indicators to evaluate contaminant-related stress in fish. J Aquat Ecosyst Stress Recover 6:75–86
Heath AG (1995) Water pollution and fish physiology, 2nd edn. CRC Press, Boca Raton, p 384
Costa PM, Diniz MS, Caeiro S et al (2009) Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquat Toxicol 92:202–212. doi:10.1016/j.aquatox.2008.12.009
Van Dyk JC, Pieterse GM, van Vuren JHJ (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol Environ Saf 66:432–440. doi:10.1016/j.ecoenv.2005.10.012
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 17–57
Velmurugan B, Selvanayagam M, Cengiz EI, Unlu E (2009) Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Braz Arch Biol Technol 52:1291–1296
Simpkins AM, Tatum TE, Cardin DL, Wolf WC (2013) Metallothionein and heat-shock protein 70 induction in caged and wild fathead minnows (Pimephales promelas) exposed to the Ouachita River, Louisiana. J Toxicol Environ Health Part A 76:98–106. doi:10.1080/15287394.2013.738174
Sen A, Ulutas OK, Tutuncu B et al (2010) Determination of 7-ethoxyresorufin-o-deethylase (EROD) induction in leaping mullet (Liza saliens) from the highly contaminated Aliaga Bay, Turkey. Environ Monit Assess 165:87–96. doi:10.1007/s10661-009-0928-3
Deane EE, Woo NYS (2006) Impact of heavy metals and organochlorines on hsp70 and hsc70 gene expression in black sea bream fibroblasts. Aquat Toxicol 79:9–15. doi:10.1016/j.aquatox.2006.04.009
Silver JT, Noble EG (2012) Regulation of survival gene hsp70. Cell Stress Chaperones 17:1–9. doi:10.1007/s12192-011-0290-6
Parveen M, Inoue A, Ise R et al (2008) Evaluation of estrogenic activity of phthalate esters by gene expression profiling using a focused microarray (EstrArray). Environ Toxicol Chem 27:1416–1425. doi:10.1897/07-399
Kukreja RC, Kontos MC, Loesser KE et al (1994) Oxidant stress increases heat shock protein 70 mRNA in isolated perfused rat heart. Am J Physiol Heart Circ Physiol 267:H2213–H2219
Jin Y, Zhang X, Shu L et al (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852. doi:10.1016/j.chemosphere.2009.11.044
Barrera G (2012) Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012:1–22. doi:10.5402/2012/137289
Zhou D, Wang H, Zhang J (2011) Di-n-butyl phthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol Ind Health 27:65–71. doi:10.1177/0748233710381895
Xu H, Shao X, Zhang Z et al (2013) Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol Environ Saf 93:39–44. doi:10.1016/j.ecoenv.2013.03.038
Papaconstantinou AD, Fisher BR, Umbreit TH et al (2002) Increases in mouse uterine heat shock protein levels are a sensitive and specific response to uterotrophic agents. Environ Health Perspect 110:1207–1212
Schett G, Steiner CW, Gröger M et al (1999) Activation of Fas inhibits heat-induced activation of Hsf1 and up-regulation of Hsp70. FASEB 13:833–842
Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther 101:227–257. doi:10.1016/j.pharmthera.2003.11.004
Bijur GN, Jope RS (2000) Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem 75:2401–2408. doi:10.1111/j.1471-4159.2007.04792.x
Weber LP, Janz DM (2001) Effect of beta-naphthoflavone and dimethylbenz[a]anthracene on apoptosis and HSP70 expression in juvenile channel catfish (Ictalurus punctatus) ovary. Aquat Toxicol 54:39–50. doi:10.1016/S0166-445X(00)00179-X
Gupta SC, Sharma A, Mishra M et al (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384. doi:10.1016/j.lfs.2009.12.015
Acknowledgments
The present study was partially supported by the: Gazi University, Research Fund, through project contract no: 04/2012-11 and The Turkish Scientific and Technological Research Council of Turkey, contract no: 212T185. Special thanks to Pınar Arslan, graduate student from Ankara University for her help with the experimentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Agus, H.H., Erkmen, B., Sümer, S. et al. Impact of DBP on histology and expression of HSP 70 in gill and liver tissue of Cyprinus carpio . Mol Biol Rep 42, 1409–1417 (2015). https://doi.org/10.1007/s11033-015-3920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-015-3920-8