Abstract
Thallium is a rare-earth element, but widely distributed in water environments, posing a potential risk to our health. This study was designed to investigate the chronic effects of thallium based on physiological responses, gene expression, and changes in the activity of relevant enzymes in adult zebra fish exposed to thallium at low doses. The endpoints assessed include mRNA expression of metallothionein (MT)2 and heat shock protein HSP70; enzymatic activities of superoxide dismutase (SOD) and Na+/K+-ATPase; and the histopathology of gill, gonad, and liver tissues. The results showed significant increases in HSP70 mRNA expression following exposure to 100 ng/L thallium and in MT2 expression following exposure to 500 ng/L thallium. Significantly higher activities were observed for SOD in liver and Na+/K+-ATPase activity in gill in zebra fish exposed to thallium (20 and 100 ng/L, respectively) in comparison to control fish. Gill, liver, and gonad tissues displayed different degrees of damage. The overall results imply that thallium may cause toxicity to zebra fish at environmentally relevant aqueous concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Thallium exists in both mono- and trivalent forms, and is a notable contaminant in the natural environment (Peter and Viraraghavan 2005; Chen et al. 2001). Thallium exists widely in aquatic environments, originating mainly from coal mining, coal processing, coal-fired power plants, other coal-based industrial processes, and the mining and refining of sulfide-rich ores for copper, gold, lead, uranium, and zinc (Cheamet al. 2000). Thus, thallium has been released over a wide geographical scale and is present in water, sediment, and biota. For example, thallium is present in the North River of Guangdong Province in southeast China at concentrations of over 0.49 μg/L (Li et al. 2011), and in the Lanmuchang mine water at concentrations of up to 1100 µg/L (Su et al. 2014). In addition, at the Yunfu sulfuric acid plant, thallium was detected in wastewater at concentrations of 15.4 to 400 µg/L (Chen et al. 2002).
Thallium is reported to be a rare toxic element (Zitko et al. 1975). The well-known mechanism of thallium toxicity is related to its interference with vital potassium-dependent processes, as well as its high affinity for sulfhydryl groups in proteins and other biomolecules (Aoyama et al. 1988). Because both thallium and potassium are univalent ions with similar ionic radii, thallium is able to interfere with potassium-dependent processes (e.g., Na+/K+-ATPase) and can mimic potassium in its movement and intracellular accumulation (Mulkey and Oehme 1993).
Many investigations have been undertaken to study the effects of acute and chronic thallium toxicity on fish (Zitko et al. 1975; LeBlanc and Dean 1984), as well as bio concentration (Zitko et al. 1975; Cataldo and Wildung 1983). Excess thallium intake in humans, which is reported frequently, can give rise to dizziness, tinnitus, fatigue, loss of appetite, inflammation, hair loss, headache, limb pain, abdominal pain, nerve palsy, and even blindness and death (Mulkey and Oehme 1993). For example, the mining of thallium/mercury ore deposits led to the pollution of the surrounding environment and gave rise to the intoxication of hundreds and the death of dozens of people in southwest China (Xiao et al. 2012). Similar to humans, thallium could cause reproductive and developmental toxicity in fish at low concentrations, where the ultra structural responses of various fish organs are useful in assessing the impact of environmental contaminants (Gernhofer et al. 2001). Until recently, few studies have reported the adverse effects of thallium in fish at high concentrations (Zitko et al. 1975; LeBlanc et al. 1984). Despite this, the adverse effects of thallium on histopathology, enzyme activities, and gene expression associated with ion exchange and oxidative stress in fish remain unclear.
An imbalance between the generation and neutralization of reactive oxygen species by antioxidants in an organism may cause oxidative stress (Helmut 1985). Exposure to substances such as transition metal ions, pesticides, and hydrocarbon pollutants can induce the production of reactive oxygen species, and it has been suggested that HSP70 is involved in the response to oxidative protein damage (Wheeler et al. 1995). It is observed that MT protects against oxidative damage induced by thallium (TI) and decreases thallium accumulation in rat liver, while the response of HSP70 to thallium remains unclear.
It is important to develop a detailed understanding of the toxic effects of thallium, from organismal to ecosystem levels, to ascertain putative common mechanisms of toxicity, and to elucidate the biological responses elicited by fish to cope with this metal. Therefore, the objective of this study was to investigate the chronic effects of environmentally relevant concentrations of thallium in adult female zebrafish, including physiological responses, and changes in the gene expression and enzyme activities of proteins likely to be involved in thallium toxicity. The endpoints assessed include MT2 and HSP70 mRNA levels, the enzymatic activities of SOD and Na+/K+-ATPase, and the histopathology of gill, gonad, and liver tissues.
Materials and Methods
Analytical grade thallium acetate was purchased from Sinopharm Chemical Reagent, (Shanghai, China). All other chemicals were obtained from Sangon Biotech (Shanghai, China).
Adult female zebra fish (average wet weight: 0.88 ± 0.03 g; average length: 3.63 ± 0.05 cm) were placed randomly in three tanks containing 100 L of permanently oxygenated and charcoal dechlorinated tap water (pH 7.0 ± 0.1; hardness 42.0–56.0 mg CaCO3/L) at a constant temperature (25 ± 1°C) with a photoperiod of 14:10 h (light:dark). The brood stock was fed three times daily: once with newly hatched brine shrimp and twice with commercial food (TetraMin, Tetra Werke, Melle, Germany) at a rate of 5% of wet fish weight per day.
Zebra fish were exposed to nominal, environmentally relevant thallium concentrations of 20, 50, 100, 500, and 1000 ng/L, with three replicate tanks each and 30 fish per replica. In addition, controls (0 ng/L) were included in the experimental design. For thallium exposure, fish in each group were placed in a glass container containing 10 L of the test solution and maintained at a constant temperature (25 ± 1°C) and a 14:10 h light:dark photoperiod. 40% of the test solution was renewed every 24 h. Fish were observed daily, at which time dead fish were removed. Fish were maintained continuously at the same thallium concentrations for 96 days.
Since the exposure solutions were renewed daily, water samples (500 mL, n = 3 replicate tanks) were randomly collected once at 50 dpf from all treatment groups prior to 40% of the test solution was renewed (24 h).
All Tl concentrations were determined by ICP-MS (Perkin Elmer ElanDRCe, PerkinElmer USA) under standard analytical condition. All acids and reagents used were super-pure grade. Indium (10 μg L−1) in 2% HNO3 (v/v) was used as internal standard for Tl calibration of the ICP-MS. Each sample was analyzed three times, and quality control standards were run after every ten samples to ensure consistent instrument performance during the analysis. The precision was generally better than 5%. The detection limits of Thallium were below 10 ng L−1. Detection limits presented are according to the 3σ definition (three times the measured standard deviation of the on-peak signal for a blank divided by the net sensitivity), with an integration period of 3 s per element per replica. The accuracy of measurements was <5% RSD and recoveries of 85%–103.5%. Quality control of thallium analyses, using the Accutrace reference standard NIST SRM 3158 (National Institute of Standards and Technology, USA), showed good agreement with the certified values.
At the end of the thallium exposure, all fish were anesthetized by keeping them on ice for 1–2 min. Body weight was subsequently measured and tissues were excised. Livers, gills, and gonads were immediately frozen in liquid nitrogen and stored at −80°C for further testing.
Six zebra fish were taken randomly from each treatment group. Total RNA was isolated from frozen samples using RNA Isolater Total RNA Extraction Reagent (Vazyme Biotech, Nanjing, China). RNA samples were treated with DNase I (Ambion, Huntingdon, UK) to remove contaminating genomic DNA and purified by an RNeasy spin column (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA concentrations were determined at 260 nm. The 260/280 ratios were used to verify the quality of RNA in each sample (Zhang et al. 2008). RNA samples were dissolved in diethyl pyro carbonate-treated water and stored at −80°C.
Real-time PCR was performed according to previously described methods (Li et al. 2009). Reverse transcription reaction mixtures contained 10 μL (100 ng/μL) of total RNA and 2 μL of random primers. The primers for MT2 and HSP70 are shown in Table 1. All samples were analyzed in triplicate and the mean value was used for calculating mRNA expression levels. β-actin was used as the housekeeping gene. The relative mRNA expression levels of each gene controlled were analyzed using the 2−△△Ct method (Schmittgen and Livak 2008).
Tissue samples were homogenized using an automatic grinding machine (Jingxin, Shanghai, China). After homogenization, a clear supernatant was obtained after three 10-min centrifugations of 12,000×g at 4°C. The final supernatant served as the enzyme source. Protein content was estimated using a total protein assay kit (Jiancheng Bioengineering Institute, Nanjing, China). The activities of Na+/K+-ATPase and SOD were measured using commercial kits (Jiancheng Bioengineering) according to the manufacturers’ instructions.
Ten adult female fish from each thallium concentration were selected for gill, liver, and gonadal histological examination. Liver and gonads were excised and fixed for 24 h in Bouin solution. Tissues were then dehydrated using an ethyl alcohol series, hyalinized in xylene baths, and embedded in paraffin. After solidification of the paraffin block, thin Sections (5 μm) were cut using a rotary microtome (Cut 4055; Olympus American, Melville, NY, USA). Sections were flattened, stained with hematoxylin and eosin, mounted on slides with neutral resins, and examined via light microscopy. Five sections per fish were chosen randomly to determine cell deformation and lesions and analyzed with an imaging software cell Sens Standard (Olympus, Tokyo, Japan). In the ovaries, maturity was evaluated with respect to oocytes in the following stages: perinuclear oocyte (PO), corticolar alveolar (CO), early vitellogenic oocyte (EV), late vitellogenic oocyte (LV), atretic follicles (AF), and postovulatory follicles (POF). In addition, the presence and/or absence of interstitial proteinaceous fluid (PF) was annotated. The data were evaluated by calculating the mean of every ovary developmental stage, in two different layers per dose groups, and used for graphical representation.
To assess hepatic damage, the number of akaryotes and nucleated cells was counted in both the control group and the thallium-treated group. Five fields of visions for each section were randomly selected and analyzed. To assess gonad damage, the number of perinucleolar oocytes and degenerated oocytes in the histological sections was counted. To assess gill damage, the number of abnormal lamellae was counted in both the control group and the thallium-treated group. Five fields of visions for each section were randomly selected and analyzed. Statistical significance was set at a level of p < 0.05 after student’s t-test (n = 10).
All statistical analyses were performed using SPSS 13.0 (IBM, Chicago, IL, USA) and Origin 8.0 (OriginLab, Northampton, MA, USA). Data was subjected to a one-way analysis of variance with specific mean comparisons by Dunnett’s test. Prior to analysis, experimental data was examined for assumptions of normality using the Kolmogorov–Smirnov one-sample and Shapiro–Wilk test. To avoid bias associated with size-specific indices, an analysis of covariance (with body weight of zebra fish as the covariate) was used to compare the gonad somatic and hepatosomatic indexes between all experimental groups. Data are expressed as means ± SE. A p < 0.05 was considered statistically significant differences relative to the solvent control.
Results and Discussion
Throughout the exposure period, experimental tanks varying in thallium concentrations were maintained ≥90% of their nominal concentrations i.e. mean measured concentrations were 18 ± 1.1, 48 ± 1.2, 97 ± 2.1, 496 ± 2.3, and 990 ± 1.2 ng/L corresponding to the nominal concentrations of 20, 50, 100, 500, and 1000 ng/L, respectively. Measured concentrations are given as mean ± S.D. (n = 3 replicates). Thallium was below the detection limit in the test chambers of control fish.
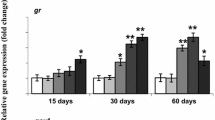
MT2 mRNA levels were unregulated in response to thallium concentrations of 500 and 1000 ng/L (p < 0.05) (Fig. 1a, b). MT2 functions in heavy metal detoxification, MTs are also closely related to antioxidant systems, cellular protection against apoptosis, as well as being stress indicators (Formigari et al. 2007). Wu et al. (2012a) demonstrated that MT2 mRNA levels were increased following exposure to cadmium in zebra fish, while Lee et al. (2010) observed that MT2 was induced after cadmium in mud loach (Misgurnus mizolepis). In addition, MT2 was induced after exposure to copper in the rare minnow (Gobiocypris rarus) (Wanget al. 2014). These results suggest that MTs could play an important physiological role in the liver in response to long-term exposure to environmental levels of heavy metals.
HSP70 expression has received considerable attention as an indicator of oxidative stress in various organisms. The significant induction of HSP70 mRNA observed in adult zebra fish exposed to thallium concentrations of 100, 500 and 1000 ng/L in the present study indicates that thallium may activate the anti-oxidant defense system in zebra fish at environmentally-relevant levels. Numerous other experimental studies have also reported concomitant increases in HSP70 mRNA in the livers of fish exposed to heavy metals (Gonzalez et al. 2006; Kim et al. 2014). For example, the exposure of Carassius auratus to lead, copper, cadmium, and zinc significantly increased the expression of HSP70 mRNA in liver tissue (Shen et al. 2003, 2004). These findings are consistent with Rajeshkumar et al. (2013), who reported a significant up regulation of HSP70 in milk fish (Chanos chanos) exposed to water polluted by copper, lead, zinc, cadmium, manganese and iron.
SOD activity increased and was significantly higher in the gills of fish exposed to 100 and 1000 ng/L thallium in comparison to control fish (Fig. 2a). In liver tissue, exposure to thallium also resulted in a significant increase in SOD activity. An increase was evident at the lowest concentration (20 ng/L), and was not further increased with increasing thallium concentrations 1000 ng/L (Fig. 2b). It is well known that heavy metal exposure results in elevated ROS production in fish (Manzl et al. 2004; Craig et al. 2007). The results of the present study show that thallium stimulated SOD activity in liver and gill tissues at environmentally relevant levels. Furthermore, there appears to be a threshold response rather than a typical concentration response.
There were significant increases in Na+/K+-ATPase activity in gills at all thallium concentrations except 500 ng/L. However, as previously observed, increases did not appear to be concentration-dependent (Fig. 2c). Liver Na+/K+-ATPase activity was not significantly different from the control group at all thallium concentrations (Fig. 2d). Thallium is capable of interfering with Na+/K+-ATPase and potassium in its transfer and intracellular accumulation (Mulkey and Oehme 1993). These results were consistent with Watson and Beamish (1981), who showed that exposure to a low concentration of zinc increased Na+/K+-ATPase activity in the gills of Salmo gardineri. Changes in Na+/K+-ATPase activity were also observed in Scylla serrata exposed to low concentrations of cadmium, and Litopenaeus vannamei exposed to low concentrations of cadmium, zinc, and copper (Dhavale et al. 1988; Wu et al. 2004).
Several stages of follicular development were visible in female gonads from the control fish, illustrating the asynchronous nature of zebra fish ovaries (Fig. 3A). However, no histologic abnormalities were present (Fig. 3A). Degenerate oocytes and a decrease in the number of mature oocytes were noted in ovaries from thallium-treated groups (Fig. 3B–F). The organizational architecture of ovaries in adult zebrafish exposed either to the thallium and the control exposures, showed no significant difference in the proportion of PO, EV, LV and CO. However, AF and POF were present with significant increased at high (>50 ng/L) thallium(Fig. 4). Degenerate and damaged oocytes may be the net result of adverse physiological and biochemical changes inside the cells of the organism. This change indicated toxic effects of thallium on cellular functioning and reflects the imbalance of antioxidant and detoxification related parameters. The lesions confirmed that exposure to heavy metals can induce histological and pathological changes of gonad (Ebrahimi and Taherianfard 2010), but there is no data on ovarian toxicity of thallium in fish.
Female zebra fish gonadal histopathology following exposure to different concentrations of thallium. A Control: (a) indicates a perinucleolar oocyte; (b) indicates the cortical alveolus stage; (c) indicates a vitellogenic oocyte. B–F were exposed to 20, 50, 100, 500, and 1000 ng/L thallium, respectively. Black arrows indicate atrophic and distorted cell membranes. White arrows indicate pyknotic primary oocytes. Bars = 100 μm
Relative percentages of stages of oocytes in ovary. PO perinuclear oocyte, CO corticolar alveolar, EV early vitellogenic oocyte, LV late vitellogenic oocyte, AF atretic follicles, and POF postovulatory follicles. *, ** and *** denote significant difference compared to solvent control at p < 0.05, p < 0.01 and p < 0.001, respectively
Liver cells appeared to have a stronger tolerance to thallium than oocytes. Compared to the control fish, no obvious histological changes were seen in the livers of the zebrafish following exposure to <100 ng/L thallium (Fig. 5). In the liver tissue of control fish, hepatocytes were located among the sinusoids, forming cordlike structures known as hepatic cell cords. Hepatocytes had a roundish, polygonal cell body containing a clear, spherical nucleus that usually had one nucleolus (Fig. 5A). There was a significant increase in Kupffer cells, circulatory disturbances, widespread nuclear pyknosis, focal necrosis, and fatty degeneration in liver tissues of zebra fish exposed to >1000 ng/L thallium (data not shown). Disruption of the spatial architecture and cellular vacuolization were observed in the 500 and 1000 ng/L thallium treatment group (Fig. 5C, D). Low thallium concentrations (<100 ng/L) caused oocyte abnormalities, but no effect on hepatocytes, indicating that the gonads were more sensitive than livers to this metal.
Liver histopathology following exposure of zebra fish to different concentrations of thallium. A Control. (a) indicates liver cells and (b) indicates a hepatic sinusoid. Panels B, C, and D represent fish exposed to 100, 500, and 1000 ng/L of thallium, respectively. The black arrow in B indicates an apparently normal liver cell. The black arrow in C indicates some gradual vacuolization of a liver cell. The black arrows in D point to vacuolar liver cells. Bars = 100 μm
Structurally, gills showed primary lamellae arranged in double rows, projecting towards the lateral side with a series of alternately arranged secondary lamellae (respiratory lamellae). Each lamella was composed of a single layer of cells (Fig. 6A). There was a significant increase in hyperplasia at the ends, and deformation of secondary lamellae in gill tissues of zebra fish exposed to >20 ng/L thallium (data not shown). Hyperplasia at the ends, and deformation of secondary lamellae were observed in the 20 and 50 ng/L treatment group. (Fig. 6B, C). Similarly, hypertrophy and fusion at the ends of secondary lamellae, irregular inter-lamellar spaces, and circulatory disturbances were observed in the gills of fish exposed to 100 ng/L thallium (Fig. 6D). Hypertrophies of primary lamellae with circulatory disturbances were observed at a concentration of 500 ng/L (Fig. 6E). The fusion of secondary lamellae, lamellar swelling, and reduced inter-lamellar space was also observed in the gills of fish exposed to 1000 ng/L thallium (Fig. 6F).
Gill histopathology following the exposure of zebra fish to different concentrations of thallium. A–F are from fish exposed to 0, 20, 50, 100, 500, and 1000 ng/L thallium, respectively. A The gill of control fish shows a normal arrangement of PL primary lamellae and SL secondary lamellae; B The black arrow indicates extreme expansion of the SL; C (a) indicates transformation of the SL and (b) indicates extreme expansion of the SL; D (a) indicates transformation of the SL and (b) indicates extreme expansion of the SL and accumulation of hemocytes; E. The black arrow indicates extreme expansion of the PL and accumulation of hemocytes; F The black arrow indicates a disrupted SL and fusion of the SL. Bars = 100 μm
Gills are considered a prime target of aquatic contaminants. They provide a significant route for the uptake, bio concentration, and excretion of toxicants due to their broad surface area, reduced distance between internal and external media, and direct contact with the environment. Zitko et al. (1975) suggested that thallium accumulation was greatest in gills, followed by liver, gonads, and muscle. Thus, an analysis of gill pathology is essential when studying the effect of thallium in vivo. Wu et al. (2012b) reported vacuoles in branchial lamellae, and the necrosis and hypertrophy of epithelial cells in fish exposed to ferrous ions. Ameur et al. (2015) reported the thickening of primary lamellae, cellular hyperplasia, aneurism, curving, shortening, and fusion of secondary lamellae, as well as, the increased activity of SOD Mugil cephalus from the polluted Bizerte lagoon in Tunisia. These results are consistent with the results of the present study, where changes in HSP70 expression and SOD activity, and thus the anti-oxidant defense system, were observed in response to thallium exposure, which also results in histological lesions.
Exposure of fish to thallium induces a number of biochemical alterations and histological lesions reflecting that thallium at environmentally relevant concentrations may result in oxidative stress and toxicity in zebra fish.
References
Ameur WB, El Megdiche Y, de Lapuente J, Barhoumi B, Trabelsi S, Ennaceur S, Camps L, Serret J, Ramos-López D, Gonzalez-Linares J (2015) Oxidative stress, genotoxicity and histopathology biomarker responses in Mugil cephalus and Dicentrarchus labrax gill exposed to persistent pollutants. A field study in the Bizerte Lagoon. Tunis Chemosphere 135:67–74
Aoyama H, Yoshida M, Yamamura Y (1988) Induction of lipid peroxidation in tissues of thallous malonate-treated hamster. Toxicology 53:11–18
Cataldo DA, Wildung RE (1983) The role of soil and plant metabolic processes in controlling trace element behavior and bioavailability to animals. Sci Total Environ 28:159–168
Cheam VJ, Garbai G, Lechner J, Rajkumar J (2000) Local impacts of coal mines and power plants across Canada. I. thallium in waters and sediments. Water Qual Res J Can 35:581–607
Chen YH, Xie WB, Wu YJ, Wang ZH (2001) Utilization of mineral resources containing thallium and thallium pollution in China. J Shenzhen Univ Sci Eng 18:57–63 ( in Chinese)
Chen YH, Xie WB, Wu YJ, Cao XA, Wang ZH, Yang CX, Chen SL, Wu HM, Zhang HY (2002) Thallium Migration and Diffusion in the Ecological Environment. J Guangzhou Univ 1:62–66 (in Chinese)
Craig PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 293:1882–1892
Dhavale DM, Masurekar V, Giridhar B (1988) Cadmium induced inhibition of Na+/K + ATPase activity in tissues of crabScylla serrata (Forskal). Bull Environ ContamToxicol 40:759–763
Ebrahimi M, Taherianfard M (2010) Concentration of four heavy metals (cadmium, lead, mercury, and arsenic) in organs of two cyprinid fish (Cyprinuscarpioand Capoeta sp.) from the Kor River (Iran). Environ Monit Assess 168:575–585
Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C 146:443–459
Gernhofer M, Pawert M, Schramm M, Müller E, Triebskorn R (2001) Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. J Aquat Ecosyst Stress Recovery 8:241–260
Gonzalez P, Baudrimont M, Boudou A, Bourdineaud JP (2006) Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio). Biometals 19:225–235
Helmut S (1985) Oxidative stress: Introductory remarks.Oxidative Stress1-8
Kim BM, Rhee JS, Jeong CB, Seo JS, Park GS, Lee YM, Lee JS (2014) Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (HSP) modulation in the intertidal copepod Tigriopusjaponicus. Comp Biochem Physiol C 166:65–74
LeBlanc GA, Dean JW (1984) Antimony and thallium toxicity to embryos and larvae of fathead minnows (Pimephalespromelas). Bull Environ Contam Toxicol 32:565–569
Lee SY, Stoliar O, Nam YK (2010) Transcriptional alteration of two metallothionein isoforms in mud loach (Misgurnusmizolepis) fry during acute heavy metal exposure. Fish Aquat Sci 13:112–117
Li W, Zha J, Li Z, Yang L, Wang Z (2009) Effects of exposure to acetochlor on the expression of thyroid hormone related genes in larval and adult rare minnow (Gobiocypris rarus). Aquat Toxicol 94:87–93
Li X, Qi J, Chen Y (2011) Prelmiinary health risk assessment of heavy metals in the main drinking water sources of Guangzhou. Acta Sci Circumst 31:547–553
Manzl C, Enrich J, Ebner H, Dallinger R, Krumschnabel G (2004) Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology 196:57–64
Mulkey JP, Oehme FW (1993) A review of thallium toxicity. Vet Hum Toxicol 35:445–453
Peter AJ, Viraraghavan T (2005) Thallium: a review of public health and environmental concerns. Environ Int 31:493–501
Rajeshkumar S, Mini J, Munuswamy N (2013) Effects of heavy metals on antioxidants and expression of HSP70 in different tissues of Milk fish (Chanoschanos) of Kaattuppalli Island, Chennai, India. Ecotoxicol Environ Saf 98:8–18
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Shen H, Wang X, Zhang J (2003) Application of the stress protein (HSP70) as the biomarker in studying zinc and copper, and the joint toxicity to fish live. Acta Sci Circumst 24:895–899
Shen H, Wang XR, Zhang JF, Zhao YJ (2004) Effects of pb~(2+) and cd~(2+) on the induction of HSP70 in the fish liver. Environ Pollut Control 26:244–246 (in Chinese)
Su LX, Chen YH, Liu J, Wang J, Qin SQ, Qi JY (2014) Distribution of containing thallium deposit in china and effects of resource development on environment. J Anhui Agric Sci 42:7588–7591 (in Chinese)
Wang C, Zhang F, Cao W, Wang J (2014) The identification of metallothionein in rare minnow (Gobiocypris rarus) and its expression following heavy metal exposure. Environ Toxicol Pharmacol 37:1283–1291
Watson T, Beamish F (1981) The effects of zinc on branchial adenosine triphosphatase enzymes in vitro from rainbow trout, Salmo gairdneri. Comp Biochem Physiol C 68:167–173
Wheeler JC, Bieschke ET, Tower J (1995) Muscle-specific expression of Drosophila hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci USA 92:10408–10412
Wu ZW, Qing PL, Xia ZH, Yun RJ (2004) Effects of metal ions on Na~+-K~+-ATPase activity in gill of Litopenaeus vannamei. Mar Environ Sci 23:27–29 (in Chinese)
Wu SM, Tsai PR, Yan CJ (2012a) Maternal cadmium exposure induces mt2 and smtB mRNA expression in zebrafish (Danio rerio) females and their offspring. Comp Biochem Physiol C 156:1–6
Wu Z, You F, Liu H, Liu M, Li J, Zhang P (2012b) Effects of waterborne Fe (II) on juvenile turbot Scophthalmus maximus: analysis of respiratory rate, hematology and gill histology. Chin J Oceanol Limnol 30:193–199
Xiao T, Yang F, Li S, Zheng B, Ning Z (2012) Thallium pollution in China: a geo-environmental perspective. Sci Tot Environ 421:51–58
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (pseudosciaenacrocea). Aquaculture 280:136–139
Zitko V, Carson WV, Carson WG (1975) Thallium: occurrence in the environment and toxicity to fish. Bull Environ Contam Toxicol 13:23–30
Acknowledgements
The authors would like to acknowledge the financial support of the National Natural Science Foundation of China (NSFC21607032, 40821003, 21037001 and 41101462) and State Key Laboratory of Organic Geochemistry, GIGCAS (Grant No. SKLOG-2016).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hou, LP., Yang, Y., Shu, H. et al. Changes in Histopathology, Enzyme Activities, and the Expression of Relevant Genes in Zebrafish (Danio rerio) Following Long-Term Exposure to Environmental Levels of Thallium. Bull Environ Contam Toxicol 99, 574–581 (2017). https://doi.org/10.1007/s00128-017-2176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2176-5