Abstract
The adaptor protein Grb10 is a close homolog of Grb7 and Grb14. These proteins are characterized by an N-terminal proline-rich region, a Ras–GTPase binding domain, a PH domain, an SH2 domain and a BPS domain in between the PH and SH2 domains. Human Grb10 gene encodes three splice variants. These variants show differences in functionality. Grb10 associates with multiple proteins including tyrosine kinases in a tyrosine phosphorylation dependent or independent manner. Association with multiple proteins allows Grb10 to regulate different signaling pathways resulting in different biological consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In all the aspects of cellular processes protein–protein interactions play a role. Interactions are mediated through many different ways. Functional domains are one of the basic components for protein–protein interactions. Among the many different types of protein–protein interactions, association of the receptor and signaling proteins is one of the important event in signal transduction from extracellular stimuli. In many cases tyrosine phosphorylation of the receptor is involved in this process that in turn recruits SH2 domain containing proteins. The human genome encodes approximately 112 SH2 domain containing proteins which are divided into 38 families [1]. The Grb7 family proteins include three members referred to as Grb7, Grb10 and Grb14 [2]. Besides the presence of the SH2 domain, these proteins possess a multi-domain structure including RA, PH and BPS domains. The presence of multiple functional domains facilitates recruitment of multiple signaling proteins and thus Grb7 family proteins act as adaptors to link signaling proteins to the receptor. This review will address our current understanding of Grb10 biology and its roles in cell signaling.
Grb10 structure and splice variants
Human Grb10 was first cloned in 1995 after cloning of mouse Grb10 in the same year [3, 4]. While mouse Grb10 encodes a 621 amino acids proteins, the first cloned human Grb10 was a 548 amino acids protein and was named as Grb-IR, as it was found to be associated with insulin receptor, InsR [3]. The human Grb10 gene is located on the chromosome 7p11.2-p12 and is mainly expressed three splice variants, Grb10α, Grb10β and Grb10γ (Fig. 1). Grb10α is expressed as a 548 amino acids protein which is also known as Grb-IR or Grb10β. Human Grb10β was also named as Grb-IRβ or Grb10-IR-SV1 and it is expressed as a 536 amino acids protein. The longest human Grb10 splice variant is Grb10γ that is expressed as a 594 amino acids protein, which has also been described as Grb10ζ. Grb10 possesses four functional domains and an N-terminal proline-rich region. All three splice variants contain an intact RA domain, a BPS domain and an SH2 domain. Grb10α lacks a part of the PH domain, while Grb10β lacks a part of the N-terminal proline-rich region. The SH2 domain facilitates interaction with phosphotyrosine residues of receptors or signaling proteins [5, 6]. The PH domain mainly recruits Grb10 to the cell membrane through interaction with phospholipids. The RA domain is known to bind to the Ras superfamily proteins and the BPS (between PH and SH2 domains) domain interacts with the insulin receptors and acts as a negative regulator of insulin signaling [7].
Grb10 splice variants. Grb10 gene expresses three splice variants. The hGrb10α expresses a 548 amino acids protein that lacks a part of PH domain. The hGrb10β expresses a 536 amino acids proteins that lacks N-terminal proline rich regions. The hGrb10γ expresses the longest protein with all functional domains presence in mouse Grb10
Grb10 expression
Grb10 expression was initially identified in mouse fibroblasts [4]. Although a low level Grb10 mRNA expression was detected in mouse liver, an abundant mRNA expression was detected in skeletal muscle, heart, liver, brain, cartilage and in adipose tissue [6, 8]. In humans, higher Grb10 expression was detected in pancreas and skeletal muscle, intermediate expression was detected in brain and cardiac muscle, and lower expression was reported in liver, lung, kidney, placenta, spleen, ovary, prostate, colon, testis and in small intestine [3, 9–11]. The mRNA expression of Grb10 has been found to be up-regulated in primary cervical squamous cell cancers and depletion of Grb10 mRNA by siRNA resulted in marked cell growth inhibition of the cervical squamous cell suggesting that Grb10 acts as a survival factor in this disease [12].
The role of Grb10 in insulin receptors signaling
Grb10 was found to be associated with InsR in response to the insulin stimulation and this association resulted in negative regulation of insulin signaling [3, 5]. Interaction was dependent on InsR-pY1322 and Grb10-SH2 domain [5]. Similar to InsR, insulin-like growth factor 1 receptor (IRS-1R) associated with Grb10 and the association was mediated through the carboxy-terminus of activated receptor [5, 13, 14]. However, unlike other InsR interacting proteins, Grb10 did not associate with the insulin receptor substrate-1 (IRS-1) [5] suggesting that Grb10 plays unique role in the insulin signaling independent of IRS-1. Although Grb10 has been shown to negatively regulate the insulin signaling, contradictory results have also been reported. Microinjection of the SH2 domain of Grb10 in fibroblasts blocked insulin and IGF-1 induced mitogenesis but had no effect on the EGF-induced mitogenesis [9] suggesting that Grb10 cooperates with insulin in downstream signaling. Furthermore, in response to PDGF, IGF-1 and insulin, Grb10 potentiates cell proliferation [15]. This biological effect is mediated through association of Grb10 with Gab1. Overexpression of mouse Grb10α in p6 or other mouse embryo fibroblast cell lines partially blocked insulin induced transformation but not cell proliferation [16]. Thus, Grb10 acts differentially in InsR signaling.
Activation of InsR induces tyrosine phosphorylation of Grb10 on Y67 residue. Although Grb10 directly associates with InsR, Grb10 is not a direct substrate of the receptor. Insulin induced Grb10 tyrosine phosphorylation is mediated through the Src family of non-receptor tyrosine kinases [17]. Grb10 displays specificity in association to the insulin receptor, as compared to the IGF-1R [6]. Besides interaction through the SH2 domain, it appears that Grb10 BPS domain interacts with the catalytically active InsR and IGF-1R [7]. While InsR displays equal affinity to the SH2 and BPS domains, IGF-1R displays higher affinity to the BPS domain and EGFR does not bind to the BPS domain but to the SH2 domain [7]. Recombinant BPS domain inhibits IGF-1-induced substrate phosphorylation [18].
Grb10 interacts with the regulatory domain of PI3-K, p85 in response to insulin stimulation suggesting that Grb10 can recruit PI3-K to the InsR [19]. Grb10 creates bridges between the IGF-1R receptor and the GYF domain containing proteins GIGYF1 and GIGYF2 [20]. These associations are mediated through the proline-rich region of Grb10 and the GYF domain of GIGYF1 and GIGYF2 suggesting that Grb10 is capable of interacting with multiple proteins at the same time using different functional regions. Additionally Grb10 can create dimers through its SH2 domain which further creates additional sites for complex formation [21]. Mouse Grb10 interacts constitutively with the E3 ubiquitin ligase, NEDD4 and this interaction is mediated through the BPS and/or SH2 domain of Grb10 [22]. Association with NEDD4 did not induce Grb10 ubiquitination. However, it affected the stability of the IGF-1R in response to the ligand stimulation, suggesting that Grb10 links ubiquitin ligase to the receptor and induces receptor ubiquitination, internalization and degradation [23]. Thus, by limiting stability Grb10 negatively regulates of IGF-1 signaling (Fig. 2).
Grb10 in insulin receptor signaling. Grb10 associates with activated insulin receptor through the SH2 domain when it is attached to the cell membrane through PH domain. Then it recruits ubiquitin ligase NEDD4 that transfers ubiquitin moieties to the receptor leading to receptor degradation. On the other hand Grb10 can recruit signaling proteins to the receptor and then it can activate downstream signaling
Grb10 negatively regulates receptor signaling not only by destabilizing the receptor but also by competing with substrate proteins for association. For example, human Grb10γ blocks insulin induced IRS1 and IRS2 phosphorylation without affecting the kinase activity of InsR [24]. Grb10 disrupts association of IRS with InsR which further blocks insulin induced Akt phosphorylation. Therefore Grb10γ acts as a negative regulator of the insulin signaling. Expression of Grb10 in the Chinese hamster ovary cells expressing InsR reduced insulin-induced MAPK phosphorylation [25]. Grb10 reduced insulin induced MAPK activation by blocking InsR-mediated Shc phosphorylation and an intact SH2 domain of Grb10 was required for this inhibition suggesting that negative regulation occurred by direct inhibition of the receptor. Knockdown of Grb10 enhanced IGF-1-induced phosphorylation of IRS, Akt and Erk1/2 [26]. Grb10γ underwent serine phosphorylation in response to the insulin stimulation and this phosphorylation is mediated through activation of Erk1/2 by InsR. Three serine residues 150, 418 and 476 are directly phosphorylated by Erk1/2 and Ser 150 and Ser 476 sites are required for Grb10-mediated inhibition of the insulin signaling [27]. Another mechanism is probably involved in the serine phosphorylation of Grb10. Grb10 homolog, Grb14 has been shown to be phosphorylated by PKCζ in response to insulin stimulation [28]. PKCζ is a member of PKC serine/threonine family protein kinases which are involved in various cellular processes and can be regulated by receptor tyrosine kinases [29–36]. Thus it is likely that PKC family proteins might be capable of phosphorylating Grb10 on serine residues. Overexpression of Grb10 inhibited insulin induced receptor auto-phosphorylation and glucose uptake, while only BPS-SH2 domain fragment had no effect on these processes. However, both full length and BPS-SH2 fragment inhibited insulin-induced IRS phosphorylation as well as activation of downstream signaling and could efficiently associate with InsR [37].
Grb10 expression led to reduction in InsR level where depletion increased InsR level but in both cases mRNA levels of InsR remained unchanged [38]. Furthermore, the proteasome inhibitor MG132, but not the lysosome inhibitor chloroquine, reversed Ins-induced InsR reduction in Grb10 expressing cells and Grb10 expression increased InsR ubiquitination suggesting that Grb10 not only reduces InsR kinase activity but also reduces InsR turnover. Grb10 binds to the E3 ubiquitin ligase Nedd4 and promotes IGF-I-stimulated ubiquitination, internalization, and degradation of the IGF-IR through multi-ubiquitination, and clathrin-dependent and-independent internalization [39]. In addition, Grb10 associates with mTORC1 and mTORC1 phosphorylates and stabilizes Grb10 that led to feedback inhibition of the PI3-K signaling [40, 41]. Grb10 is also a negative regulator of the insulin signaling in pancreatic beta cells [42].
Regulation of type III receptor tyrosine kinases by Grb10
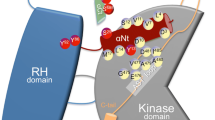
The type III receptor tyrosine kinases include PDGFRα, PDGFRβ, CSF-1R, KIT and FLT3. These receptor tyrosine kinases are associated with cancer and many of family members found to be mutated in several cancers [43, 44]. Downstream signaling of this family of receptor tyrosine kinases have been shown to be regulated by interaction with various proteins [45–50]. The N-terminal truncated isoform Grb10β was found to be associated with multiple proteins including PDGFR, EGFR and c-Abl [10]. Grb10 was associated with PDGFRβ in response to the PDGF and this association was mediated through pY771 of PDGFRβ and the SH2 domain of Grb10 [51]. Overexpression of full-length Grb10 potentiated mitogenic signaling from PDGFR as well as PDGF-induced cell proliferation but the SH2 domain alone suppressed these events suggesting that Grb10 positively regulates PDGF signaling. Grb10 was associated with KIT in response to the stem cell factor (SCF)-induction through the SH2 domain and then it interacted with AKT resulting in aberrant activation of mitogenic signaling [52]. Probably in this way KIT can bypass PI3-K activity in AKT activation. KIT mutant that lacks PI3-K binding site can still activate AKT in Grb10 expressing cells suggesting that Grb10 can transduce signal from the receptor to AKT directly. Grb10 directly associates with ligand-stimulated wild-type FLT3 as well as with oncogenic FLT3 and recruits p85 leading to activation of AKT and STAT5 in hematopoietic cells [53]. Thus it is suggested that although Grb10 is mainly negative regulator of insulin signaling, it cooperates with type III receptor tyrosine kinase signaling by recruiting multiple signaling proteins (Fig. 3).
Grb10 in VEGFR regulation
The vascular endothelial growth factor receptor (VEGFR) is involved in both vasculogenesis and angiogenesis. In response to the VEGF-stimulation Grb10 was tyrosine phosphorylated and this phosphorylation was mediated partly through the activation of Src. In endothelial (HUVEC) cells VEGF-induction increased transcriptional activation of Grb10. Elevated Grb10 expression further potentiated expression and tyrosine phosphorylation of the VEGFR3 (KDR) followed by accelerated mitogenic signaling [54]. This effect was mediated by direct association of Grb10 to the activated receptor through the Grb10 SH2 domain. Grb10 stabilized VEGFR2 by inhibiting NEDD4-mediated VEGFR2 degradation [55]. The inhibition was mediated through direct interaction of Grb10 with NEDD4 and over-expression of Grb10 in VEGFR2 expressing cells accelerated VEGF signaling. Thus, like with type III receptor tyrosine kinases, Grb10 potentiates VEGFR signaling.
The role of Grb10 in EGFR signaling
The epidermal growth factor receptor (EGFR) is a family of four receptor tyrosine kinases including EGFR, ERBB2, ERBB3 and ERBB4 [56, 57]. This family proteins are over-expressed in many cancers. Grb10 was first identified as an EGFR interacting protein in a screen of bacterial expression libraries in 1995 [4]. Grb10 was found to be poorly associated with EGFR and EGF induction did not induce a tyrosine phosphorylation of Grb10. However, EGF induced serine phosphorylation of Grb10 suggesting that Grb10 is not a direct substrate of EGFR and its role in EGFR signaling has to be defined.
Regulation of other tyrosine kinases
The receptor tyrosine kinase RET plays a role in the development of the enteric nervous, endocrine, and renal systems. Grb10 was found to be associated with RET in a yeast two-hybrid screening of a mouse embryonic library using the cytoplasmic domain of RET [58]. Using an EGFR extracellular domain with RET cytoplasmic chimera, it has been demonstrated that Grb10 associates with RET in an activation dependent manner [58]. The activated hepatocyte growth factor receptor (MET) has also been found to be associated Grb10 [51]. However the role of Grb10 in RET and MET downstream signaling still remains unknown.
Grb10 associates with receptor tyrosine kinase ELK in vascular endothelial cells through ELK-pY929 and Grb10-SH2 domain [59]. Besides interaction with activated receptor tyrosine kinases, Grb10 interacts with a number of non-receptor tyrosine kinases. Although the interaction between receptor and Grb10 is mainly dependent on Grb10 SH2 domain, in many cases Grb10 associates with non-receptor tyrosine kinases in an SH2-domain independent manner. For example, interaction with c-Abl is mediated through the SH3 domain of c-Abl [10].
AKT directly binds to Grb10 and phosphorylates Grb10 on serine 428 residue that further facilitates binding to 14–3–3 [60]. Furthermore, Grb10 creates complex with Raf1 and 14–3–3 and inhibits Bad-mediated apoptosis [61]. Despite of its interaction with phospho-tyrosine residue, the Grb10 SH2 domain interacts with Raf1 and MEK1 in a phosphotyrosine independent manner [62]. MEK1 associates with Grb10 through Thr-386 and this association is needed for MEK1 induced survival of the HTC-IR and COS-7 cells [62]. The oncogenic fusion protein BCR-ABL also associates with Grb10 through phosphotyrosine residues in BCR site and the kinase activity of BCR-ABL is indispensable for this interaction [63]. This interaction is important for BCR-ABL induced cell transformation. Another non-receptor tyrosine kinase, Tec, binds with Grb10 and phosphorylates Grb10 on tyrosine residues, and this association results in a negative regulation of Tec induced c-Fos-induction in Ba/F3 cells [64]. In addition, Grb10 has been shown to be associated with mitochondria and IGF-1-induction induces translocation of Grb10 to the membrane. Mitochondrial localization is probably mediated through Raf-1, as it was reported to be associated with activated Raf-1 in mitochondria following ultraviolet radiation [65].
Growth hormone (GH) receptor and Grb10
Loss of Grb10 function in mice results in fetal and placental overgrowth suggesting that Grb10 play a role in negative regulation of growth factor signaling. Although Grb10 transgenic mice displayed growth retardation [66], disruption of Grb10 in mice peripheral tissues had no significant effect on fasting glucose or insulin levels but peripheral-tissue-specific knockout led to significant over growth [67]. Dual Grb10 and Grb14 knockout mice results in deregulation of whole-body glucose homeostatis [68]. Adult mice deficient in Grb10 have elevated body mass and muscle mass throughout the adulthood [69]. Thus it is likely that Grb10 maintains normal growth of mice. Grb10 associated with growth hormone receptor (GHR) in response to growth hormone (GH) in the Huh-7 hematoma cells [70]. Grb10 associated with Jak2 as well, and overexpression of Grb10 blocked GH-induced induction of serum response element (SRE) but not STAT5 tyrosine phosphorylation [70] suggesting that Grb10 negatively regulates selective downstream pathways of GHR.
Conclusions
Grb10 has been implicated mainly in insulin signaling. Despite of its regulation of insulin signaling Grb10 plays important roles in other receptors regulation (Table 1). The presence of multiple domains, proline-rich region and ability of self-dimerization facilitate multiple protein complex formation by Grb10. In this way Grb10 can recruit multiple signaling proteins to the receptor. In the case of insulin receptor regulation Grb10 associates with phosphotyrosine residues of the receptor and then recruits either ubiquitin ligase or signaling proteins or blocks access of substrate proteins. In this manner Grb10 can negatively regulate insulin signaling or can transmit signal from InsR depending on interacting partners. In a similar fashion Grb10 regulates other receptors. Grb10 associates with FLT3 and KIT, and recruits p85 and AKT resulting in accelerated activation of downstream signaling. In some cases Grb10 associates with ubiquitin ligase and blocks receptor ubiquitination. Since the Grb10 gene is expressed as three different splice variants and the variants are structurally different, it is possible that depending on tissue specific abundance of the different splice variants, Grb10 regulates receptor downstream signaling differentially.
References
Liu BA, Shah E, Jablonowski K, Stergachis A, Engelmann B, Nash PD (2011) The SH2 domain-containing proteins in 21 species establish the provenance and scope of phosphotyrosine signaling in eukaryotes. Sci Signal 4(202):ra83. doi:10.1126/scisignal.2002105
Daly RJ (1998) The Grb7 family of signalling proteins. Cell Signal 10(9):613–618
Liu F, Roth RA (1995) Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc Natl Acad Sci USA 92(22):10287–10291
Ooi J, Yajnik V, Immanuel D, Gordon M, Moskow JJ, Buchberg AM, Margolis B (1995) The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene 10(8):1621–1630
Hansen H, Svensson U, Zhu J, Laviola L, Giorgino F, Wolf G, Smith RJ, Riedel H (1996) Interaction between the Grb10 SH2 domain and the insulin receptor carboxyl terminus. J Biol Chem 271(15):8882–8886
Laviola L, Giorgino F, Chow JC, Baquero JA, Hansen H, Ooi J, Zhu J, Riedel H, Smith RJ (1997) The adapter protein Grb10 associates preferentially with the insulin receptor as compared with the IGF-I receptor in mouse fibroblasts. J Clin Investig 99(5):830–837. doi:10.1172/JCI119246
He W, Rose DW, Olefsky JM, Gustafson TA (1998) Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains. J Biol Chem 273(12):6860–6867
Charalambous M, Smith FM, Bennett WR, Crew TE, Mackenzie F, Ward A (2003) Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc Natl Acad Sci USA 100(14):8292–8297. doi:10.1073/pnas.1532175100
O’Neill TJ, Rose DW, Pillay TS, Hotta K, Olefsky JM, Gustafson TA (1996) Interaction of a GRB-IR splice variant (a human GRB10 homolog) with the insulin and insulin-like growth factor I receptors. Evidence for a role in mitogenic signaling. J Biol Chem 271(37):22506–22513
Frantz JD, Giorgetti-Peraldi S, Ottinger EA, Shoelson SE (1997) Human GRB-IRbeta/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains. J Biol Chem 272(5):2659–2667
Dong LQ, Du H, Porter SG, Kolakowski LF Jr, Lee AV, Mandarino LJ, Fan J, Yee D, Liu F (1997) Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma. J Biol Chem 272(46):29104–29112
Okino K, Konishi H, Doi D, Yoneyama K, Ota Y, Jin E, Kawanami O, Takeshita T (2005) Up-regulation of growth factor receptor-bound protein 10 in cervical squamous cell carcinoma. Oncol Rep 13(6):1069–1074
Dey BR, Frick K, Lopaczynski W, Nissley SP, Furlanetto RW (1996) Evidence for the direct interaction of the insulin-like growth factor I receptor with IRS-1, Shc, and Grb10. Mol Endocrinol 10(6):631–641
Morrione A, Valentinis B, Li S, Ooi JY, Margolis B, Baserga R (1996) Grb10: a new substrate of the insulin-like growth factor I receptor. Cancer Res 56(14):3165–3167
Deng Y, Zhang M, Riedel H (2008) Mitogenic roles of Gab1 and Grb10 as direct cellular partners in the regulation of MAP kinase signaling. J Cell Biochem 105(5):1172–1182. doi:10.1002/jcb.21829
Morrione A, Valentinis B, Resnicoff M, Xu S, Baserga R (1997) The role of mGrb10alpha in insulin-like growth factor I-mediated growth. J Biol Chem 272(42):26382–26387
Langlais P, Dong LQ, Hu D, Liu F (2000) Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family. Oncogene 19(25):2895–2903. doi:10.1038/sj.onc.1203616
Stein EG, Gustafson TA, Hubbard SR (2001) The BPS domain of Grb10 inhibits the catalytic activity of the insulin and IGF1 receptors. FEBS Lett 493(2–3):106–111
Deng Y, Bhattacharya S, Swamy OR, Tandon R, Wang Y, Janda R, Riedel H (2003) Growth factor receptor-binding protein 10 (Grb10) as a partner of phosphatidylinositol 3-kinase in metabolic insulin action. J Biol Chem 278(41):39311–39322. doi:10.1074/jbc.M304599200
Giovannone B, Lee E, Laviola L, Giorgino F, Cleveland KA, Smith RJ (2003) Two novel proteins that are linked to insulin-like growth factor (IGF-I) receptors by the Grb10 adapter and modulate IGF-I signaling. J Biol Chem 278(34):31564–31573. doi:10.1074/jbc.M211572200
Stein EG, Ghirlando R, Hubbard SR (2003) Structural basis for dimerization of the Grb10 Src homology 2 domain. Implications for ligand specificity. J Biol Chem 278(15):13257–13264. doi:10.1074/jbc.M212026200
Morrione A, Plant P, Valentinis B, Staub O, Kumar S, Rotin D, Baserga R (1999) mGrb10 interacts with Nedd4. J Biol Chem 274(34):24094–24099
Vecchione A, Marchese A, Henry P, Rotin D, Morrione A (2003) The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol 23(9):3363–3372
Wick KR, Werner ED, Langlais P, Ramos FJ, Dong LQ, Shoelson SE, Liu F (2003) Grb10 inhibits insulin-stimulated insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase/Akt signaling pathway by disrupting the association of IRS-1/IRS-2 with the insulin receptor. J Biol Chem 278(10):8460–8467. doi:10.1074/jbc.M208518200
Langlais P, Dong LQ, Ramos FJ, Hu D, Li Y, Quon MJ, Liu F (2004) Negative regulation of insulin-stimulated mitogen-activated protein kinase signaling by Grb10. Mol Endocrinol 18(2):350–358. doi:10.1210/me.2003-0117
Dufresne AM, Smith RJ (2005) The adapter protein GRB10 is an endogenous negative regulator of insulin-like growth factor signaling. Endocrinology 146(10):4399–4409. doi:10.1210/en.2005-0150
Langlais P, Wang C, Dong LQ, Carroll CA, Weintraub ST, Liu F (2005) Phosphorylation of Grb10 by mitogen-activated protein kinase: identification of Ser150 and Ser476 of human Grb10zeta as major phosphorylation sites. Biochemistry 44(24):8890–8897. doi:10.1021/bi050413i
Cariou B, Perdereau D, Cailliau K, Browaeys-Poly E, Bereziat V, Vasseur-Cognet M, Girard J, Burnol AF (2002) The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase Czeta. Mol Cell Biol 22(20):6959–6970
Kazi JU, Soh JW (2007) Isoform-specific translocation of PKC isoforms in NIH3T3 cells by TPA. Biochem Biophys Res Commun 364(2):231–237. doi:10.1016/j.bbrc.2007.09.123
Kazi JU, Soh JW (2008) Induction of the nuclear proto-oncogene c-fos by the phorbol ester TPA and v-H-Ras. Mol Cells 26(5):462–467
Kabir NN, Kazi JU (2013) Selective mutation in ATP-binding site reduces affinity of drug to the kinase: a possible mechanism of chemo-resistance. Med Oncol 30(1):448. doi:10.1007/s12032-012-0448-9
Kabir NN, Ronnstrand L, Kazi JU (2013) Protein kinase C expression is deregulated in chronic lymphocytic leukemia. Leuk Lymphoma 54(10):2288–2290. doi:10.3109/10428194.2013.769220
Kazi JU, Kabir NN, Ronnstrand L (2013) Protein kinase C (PKC) as a drug target in chronic lymphocytic leukemia. Med Oncol 30(4):757. doi:10.1007/s12032-013-0757-7
Kazi JU, Soh JW (2008) Role of regulatory domain mutants of PKC isoforms in c-fos induction. Bull Korean Chem Soc 29(1):252–254
Kazi JU, Kim CR, Soh JW (2009) Subcellular localization of diacylglycerol-responsive protein kinase C isoforms in HeLa cells. Bull Korean Chem Soc 30(9):1981–1984
Kazi JU (2011) The mechanism of protein kinase C regulation. Frontiers Biol 6(4):328–336. doi:10.1007/s11515-011-1070-5
Mori K, Giovannone B, Smith RJ (2005) Distinct Grb10 domain requirements for effects on glucose uptake and insulin signaling. Mol Cell Endocrinol 230(1–2):39–50. doi:10.1016/j.mce.2004.11.004
Ramos FJ, Langlais PR, Hu D, Dong LQ, Liu F (2006) Grb10 mediates insulin-stimulated degradation of the insulin receptor: a mechanism of negative regulation. Am J Physiol Endocrinol Metab 290(6):E1262–E1266. doi:10.1152/ajpendo.00609.2005
Monami G, Emiliozzi V, Morrione A (2008) Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J Cell Physiol 216(2):426–437. doi:10.1002/jcp.21405
Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J (2011) Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332(6035):1322–1326. doi:10.1126/science.1199484
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332(6035):1317–1322. doi:10.1126/science.1199498
Li L, Li X, Zhu Y, Zhang M, Yin D, Lu J, Liu F, Wang C, Jia W (2013) Grb10 Inhibits glucose-stimulated insulin release from pancreatic beta-cells associated with suppression of insulin/IGF-1 signaling pathway. Clin Exp Pharmacol Physiol. doi:10.1111/1440-1681.12160
Kabir NN, Ronnstrand L, Kazi JU (2013) FLT3 mutations in patients with childhood acute lymphoblastic leukemia (ALL). Med Oncol 30(1):462. doi:10.1007/s12032-013-0462-6
Lennartsson J, Ronnstrand L (2012) Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev 92(4):1619–1649. doi:10.1152/physrev.0 0046.2011
Kazi JU, Ronnstrand L (2012) Src-Like adaptor protein (SLAP) binds to the receptor tyrosine kinase Flt3 and modulates receptor stability and downstream signaling. PLoS ONE 7(12):e53509. doi:10.1371/journal.pone.0053509
Kazi JU, Sun J, Phung B, Zadjali F, Flores-Morales A, Ronnstrand L (2012) Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3. J Biol Chem 287(43):36509–36517. doi:10.1074/jbc.M112.376111
Lin DC, Yin T, Koren-Michowitz M, Ding LW, Gueller S, Gery S, Tabayashi T, Bergholz U, Kazi JU, Ronnstrand L, Stocking C, Koeffler HP (2012) Adaptor protein Lnk binds to and inhibits normal and leukemic FLT3. Blood 120(16):3310–3317. doi:10.1182/blood-2011-10-388611
Kazi JU, Agarwal S, Sun J, Bracco E, Ronnstrand L (2013) Src-Like Adaptor Protein (SLAP) differentially regulates normal and oncogenic c-Kit signaling. J Cell Sci. doi:10.1242/jcs.140590
Kazi JU, Ronnstrand L (2013) Suppressor of cytokine signaling 2 (SOCS2) associates with FLT3 and negatively regulates downstream signaling. Mol Oncol 7(3):693–703. doi:10.1016/j.molonc.2013.02.020
Kazi JU, Vaapil M, Agarwal S, Bracco E, Pahlman S, Ronnstrand L (2013) The tyrosine kinase CSK associates with FLT3 and c-Kit receptors and regulates downstream signaling. Cell Signal 25(9):1852–1860. doi:10.1016/j.cellsig.2013.05.016
Wang J, Dai H, Yousaf N, Moussaif M, Deng Y, Boufelliga A, Swamy OR, Leone ME, Riedel H (1999) Grb10, a positive, stimulatory signaling adapter in platelet-derived growth factor BB-, insulin-like growth factor I-, and insulin-mediated mitogenesis. Mol Cell Biol 19(9):6217–6228
Jahn T, Seipel P, Urschel S, Peschel C, Duyster J (2002) Role for the adaptor protein Grb10 in the activation of Akt. Mol Cell Biol 22(4):979–991
Kazi JU, Ronnstrand L (2013) FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia. Mol Oncol 7(3):402–418. doi:10.1016/j.molonc.2012.11.003
Giorgetti-Peraldi S, Murdaca J, Mas JC, Van Obberghen E (2001) The adapter protein, Grb10, is a positive regulator of vascular endothelial growth factor signaling. Oncogene 20(30):3959–3968. doi:10.1038/sj.onc.1204520
Murdaca J, Treins C, Monthouel-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E, Giorgetti-Peraldi S (2004) Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem 279(25):26754–26761. doi:10.1074/jbc.M311802200
Kabir NN, Kazi JU (2011) Comparative analysis of human and bovine protein kinases reveals unique relationship and functional diversity. Genet Mol Biol 34(4):587–591. doi:10.1590/S1415-47572011005000035
Kazi JU, Kabir NN, Soh JW (2008) Bioinformatic prediction and analysis of eukaryotic protein kinases in the rat genome. Gene 410(1):147–153. doi:10.1016/j.gene.2007.12.003
Pandey A, Duan H, Di Fiore PP, Dixit VM (1995) The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J Biol Chem 270(37):21461–21463
Stein E, Cerretti DP, Daniel TO (1996) Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem 271(38):23588–23593
Urschel S, Bassermann F, Bai RY, Munch S, Peschel C, Duyster J (2005) Phosphorylation of grb10 regulates its interaction with 14-3-3. J Biol Chem 280(17):16987–16993. doi:10.1074/jbc.M501477200
Kebache S, Ash J, Annis MG, Hagan J, Huber M, Hassard J, Stewart CL, Whiteway M, Nantel A (2007) Grb10 and active Raf-1 kinase promote Bad-dependent cell survival. J Biol Chem 282(30):21873–21883. doi:10.1074/jbc.M611066200
Nantel A, Mohammad-Ali K, Sherk J, Posner BI, Thomas DY (1998) Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases. J Biol Chem 273(17):10475–10484
Bai RY, Jahn T, Schrem S, Munzert G, Weidner KM, Wang JY, Duyster J (1998) The SH2-containing adapter protein GRB10 interacts with BCR-ABL. Oncogene 17(8):941–948. doi:10.1038/sj.onc.1202024
Mano H, Ohya K, Miyazato A, Yamashita Y, Ogawa W, Inazawa J, Ikeda U, Shimada K, Hatake K, Kasuga M, Ozawa K, Kajigaya S (1998) Grb10/GrbIR as an in vivo substrate of Tec tyrosine kinase. Genes Cells 3(7):431–441
Nantel A, Huber M, Thomas DY (1999) Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool. J Biol Chem 274(50):35719–35724
Shiura H, Miyoshi N, Konishi A, Wakisaka-Saito N, Suzuki R, Muguruma K, Kohda T, Wakana S, Yokoyama M, Ishino F, Kaneko-Ishino T (2005) Meg1/Grb10 overexpression causes postnatal growth retardation and insulin resistance via negative modulation of the IGF1R and IR cascades. Biochem Biophys Res Commun 329(3):909–916. doi:10.1016/j.bbrc.2005.02.047
Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, DeFronzo RA, Liu F (2007) Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol 27(18):6497–6505. doi:10.1128/MCB.00679-07
Holt LJ, Lyons RJ, Ryan AS, Beale SM, Ward A, Cooney GJ, Daly RJ (2009) Dual ablation of Grb10 and Grb14 in mice reveals their combined role in regulation of insulin signaling and glucose homeostasis. Mol Endocrinol 23(9):1406–1414. doi:10.1210/me.2008-0386
Holt LJ, Turner N, Mokbel N, Trefely S, Kanzleiter T, Kaplan W, Ormandy CJ, Daly RJ, Cooney GJ (2012) Grb10 regulates the development of fiber number in skeletal muscle. FASEB journal 26(9):3658–3669. doi:10.1096/fj.11-199349
Moutoussamy S, Renaudie F, Lago F, Kelly PA, Finidori J (1998) Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins. J Biol Chem 273(26):15906–15912
Wick MJ, Dong LQ, Hu D, Langlais P, Liu F (2001) Insulin receptor-mediated p62dok tyrosine phosphorylation at residues 362 and 398 plays distinct roles for binding GTPase-activating protein and Nck and is essential for inhibiting insulin-stimulated activation of Ras and Akt. J Biol Chem 276(46):42843–42850. doi:10.1074/jbc.M102116200
Tezuka N, Brown AM, Yanagawa S (2007) GRB10 binds to LRP6, the Wnt co-receptor and inhibits canonical Wnt signaling pathway. Biochem Biophys Res Commun 356(3):648–654. doi:10.1016/j.bbrc.2007.03.019
Hu ZQ, Zhang JY, Ji CN, Xie Y, Chen JZ, Mao YM (2010) Grb10 interacts with Bim L and inhibits apoptosis. Mol Biol Rep 37(7):3547–3552. doi:10.1007/s11033-010-0002-9
Acknowledgments
We thank Professor Lars Rönnstrand for comments on manuscript. This research was funded by the Stiftelsen Olle Engkvist Byggmästare, Kungliga Fysiografiska Sällskapet i Lund, Ollie Elof Ericssons Stiftelse and Stiftelsen Lars Hiertas Minne.
Conflict of interests
Authors declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kabir, N.N., Kazi, J.U. Grb10 is a dual regulator of receptor tyrosine kinase signaling. Mol Biol Rep 41, 1985–1992 (2014). https://doi.org/10.1007/s11033-014-3046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3046-4