Abstract
Final fruit size of apple (Malus domestica) cultivars is related to both mesocarp cell division and cell expansion during fruit growth, but it is unclear whether the cell division and/or cell enlargement determine most of the differences in fruit size between Malus species. In this study, by using an interspecific hybrid population between Malus asiatica “Zisai Pearl” and Malus domestica cultivar “Red Fuji,” we found that the mesocarp cell number was the main causal factor of diversity in fruit size between Malus species. Rapid increase in mesocarp cell number occurred prior to 28 days after anthesis (DAA), while cell size increased gradually after 28 DAA until fruit ripening. Six candidate genes related to auxin signaling or cell cycle were predicted by combining the RNA-seq data and previous QTL data for fruit weight. Two InDels and 10 SNPs in the promoter of a small auxin upregulated RNA gene MdSAUR36 in Zisai Pearl led to a lower promoter activity than that of Red Fuji. One non-synonymous SNP G/T at 379 bp downstream of the ATG codon of MdSAUR36, which was heterozygous in Zisai Pearl, exerted significant genotype effects on fruit weight, length, and width. Transgenic apple calli by over-expressing or RNAi MdSAUR36 confirmed that MdSAUR36 participated in the negative regulation of mesocarp cell division and thus apple fruit size. These results could provide new insights in the molecular mechanism of small fruit size in Malus accession and be potentially used in molecular assisted breeding via interspecific hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple (Malus domestica Borkh.) is an important deciduous fruit crop worldwide which makes a great economic contribution every year (Cornille et al 2019). Apple fruit size (weight) is a crucial agronomic trait that is associated closely with yield and fruit appearance quality. Selection for cultivars with acceptable fruit size is one of the most important goals for apple breeding programs; nevertheless, the molecular regulation of fruit size is nowadays unclear (Bu et al. 2020; Zhou et al. 2021).
Abundant Malus genetic resources for environmental adaptability and biotic/abiotic stress tolerance have been repeatedly reported but these species often bear small fruit. For instance, Malus baccata L. accessions exhibit excellent winter hardiness and quantitative resistance to pathogens such as apple scab (Venturia inaequalis (Cooke) Wint.) but the fruit of most accessions is less than 1 g (Chen et al. 2019; Cho et al. 2021). Malus robusta (Carr.) Rehd. is a Chinese native apple species and it exhibits resistance to apple fire blight (Erwinia amylovora) and powdery mildew (Podosphaera leucotricha), but the fruit usually weights about 12 g (Peil et al. 2007; Gardiner et al. 2012; Gao et al. 2021). Therefore, understanding the inheritance and genetic control of fruit size in Malus genus may contribute not only to breeding for optimum fruit size but also to genetic improvement of disease resistance and stress tolerance in the modern apple industry.
In apples, fruit development from pollination to full ripping presents a simple sigmoidal growth curve (Denne 1963). Final fruit size is determined by both cell division and cell expansion during fruit growth and development (Denne 1963; Olmstead et al. 2007; Malladi & Hirst 2010). Cell number has a much close relationship with fruit size (Bain & Robertson 1951; Westwood et al. 1967; Malladi & Hirst 2010; Karim et al. 2022). The cell division occurs during the fruit set and continues until 35–50 days after anthesis (DAA), and the final fruit cell number is determined in this phase (Denne 1963; Malladi & Johnson 2011; Karim et al. 2022). After the cell division phase, cell expansion becomes the main event for fruit enlargement, which reaches the peak at 40 to 60 DAA, although it continues until fruit ripening (Janssen et al. 2008; Karim et al. 2022). However, it is unclear whether the genetic variation in final fruit size within Malus species is associated dominantly with cell number, cell size, or both.
A considerable number of genes related to cell number and cell size have been identified in plants. An APETALA2–repeat containing transcription factor AINTEGUMENTA (ANT) regulates cell division in ovule development and floral organ growth in Arabidopsis thaliana; ANT mutants reduce the size of floral organs and leaves by decreasing cell number (Elliott et al. 1996; Klucher et al. 1996; Krizek 1999; Mizukami & Fischer 2000). Auxin-inducible gene ARGOS can prolong the expression of ANT and regulate cell proliferation and organ growth (Hu et al. 2003). Similarly in apples, MdANT1 and MdANT2 are closely associated with the regulation of fruit cell production (Dash & Malladi 2012). High levels of MdANT expression were found during the period of cell production and declined rapidly after this period (Dash & Malladi 2012). The expression of MdANTs exhibited higher levels and a longer duration in the larger-fruit cultivar “Smoothee” than in the middle-fruit cultivar “Gala” (Dash & Malladi 2012). The expression level of a microRNA, miRNA172, affects apple fruit size (Yao et al. 2015). A 154-bp miniature inverted-repeat transposable elements (MITEs) insertion at the 3′ end of pri-miRNA172 was found to be co-located with a major apple fruit size QTL, and this MITEs insertion led to reduced expression of miRNA172 (Yao et al. 2015). The fruit size of transgenic apple over-expressing MdMIR172 was significantly smaller than the untransformed control (Yao et al. 2015). In addition, FASCIATED encodes a YABBY-like transcription factor which controls tomato fruit size by regulating the number of carpels during flower and fruit development (Cong et al. 2008).
Protein post-translational modification is involved in the regulation of cell number/expansion and fruit size. An E3 ubiquitin ligase gene BIG BROTHER is a repressor of plant organ growth; its activity limits floral organ size by restricting the period of proliferative growth in Arabidopsis (Disch et al. 2006). In tomato (Solanum lycopersicum), a member of CELL NUMBER REGULATOR (CNR) family gene Slfw2.2 negatively controls cell division during fruit development (Frary et al. 2000; Cong et al. 2002). MdCNR8 in an apple is an ortholog of tomato Slfw2.2; MdCNR8 protein can be SUMOylated by the SUMO E3 ligase MdSIZ1 and further regulate cell proliferation to control plant organ size (Wang et al. 2022).
To explore the genetic variations contributing to the phenotype segregation of apple fruit size, anatomic and transcriptomic analyses were performed in this study by using an interspecific hybrid population between Malus asiatica Nakai and Malus domestica Borkh. cultivars. Then, the functions of the genetic variations were validated by transgenic apple callus. The results could provide new insights into the molecular mechanism of fruit size in Malus species.
Materials and methods
Plant materials
Fruit and leaf samples of 1679 F1 hybrid plants derived from “Zisai Pearl” (M. asiatica Nakai) × “Red Fuji” (M. domestica Borkh.) and the parental cultivars were used in this study, whereas M. baccata L. “Sue” was also used as the small fruit external control. To estimate the genotype effects of genetic variations, a training population was used including 1134 F1 hybrid plants from three bi-parental crosses and 788 Malus accessions (Shen et al. 2022). All the hybrid plants and the parental cultivars were grafted on “SH6” interstem (40 cm length) and “Baleng Crab” seedling rootstock. All plant materials were planted at the experimental station of China Agricultural University under conventional field management and pest control. Fruit weight, fruit length, and width of all individuals have been phenotyped for at least 3 years. Six hybrid plants with fruit weight less than 40 g and six hybrid plants with fruit weight more than 120 g in three consecutive years were chosen for anatomy and RNA-seq analyses from the F1 hybrid population as representative small-fruit group and large-fruit group, respectively.
Phenotyping
Apples from hybrid plants of the representative small- and large-fruit groups were collected at 0, 7, 14, 21, 28, 58, 88, 118, and 148 DAA for phenotyping. Three apples of each plant were collected as three biological replicates. Fruit diameter and fruit length were measured by using a vernier caliper, fruit weight was measured by using a counterbalance, and fruit size was measured referring to the method of Yan et al (2008) based on Archimedes’ principle.
Microstructure of apple fruit at different growth stages
Three fruitlets were collected with almost the same size from one hybrid, two slices with a size of 0.5 × 0.5 × 0.2 cm were cut from mesocarp of each fruit, then the slices were fixed in FAA (10% formalin, 50% ethanol, 5% glacial acetic acid, adjusted to pH 7.0) for 24 h at room temperature. Next, the samples were managed by a series of dehydration, clearing, paraffin embedding, sectioning, and staining steps according to the protocol of Yang et al. (2016).
The paraffin section samples were photographed using a confocal microscope (Olympus, Tokyo, Japan) and the images were analyzed using Images J software. Three rectangular areas from an image were picked out for calculating average cell area. The total fruit cell number was estimated by fruit volume/cell volume.
RNA-seq
Fruitlets of three small- and three large-fruit hybrid plants were collected at 7, 14, and 28 DAA for RNA -seq. Total RNA was extracted using a CTAB method and was quantified using NanoDrop 2000 (Thermo Fisher Scientific). Sequencing libraries were constructed using NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB) and NGS sequencing was performed on an Illumina platform (X-ten). Clean data were obtained after quality control, these clean reads were mapped to the apple genome reference (M. domestica GDDH13 v1.1 genome) using Hisat2 tools.
Gene expression levels were estimated by fragments per kilobase of transcript per million fragments mapped (FPKM). Genes with false discovery rate (FDR) < 0.05 and log2 (fold change) ≥ 1.5 were defined as differentially expressed genes (DEGs). All DEGs were selected for weighted correlation network analysis using the WGCNA software package. Gene functions were annotated based on the databases of Nr (NCBI non-redundant protein sequences, (fttp://ncbi.nih.gov/blast/db/). Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/) and Gene Ontology (GO) (http://www.geneontology.org/) were also analyzed online.
Validation of genetic variations in MdSAUR36
DNA and RNA were both extracted using the CTAB method, and the quality of each DNA or RNA sample was confirmed by agarose gel electrophoresis. All RNA samples were reverse transcribed to first-strand cDNA using 2x Phanta Flash Master Mix (Vazyme Biotech, Nanjing, China). GDDH13 v1.1 apple reference genome (https://www.rosaceae.org/) was used for the prediction of the coding sequence (CDS) and promoter sequence of MdSAUR36. The putative promoter motifs were predicted using the plantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al. 2002). Conserved domain of CDS was analyzed by the CDD: NCBI’s conserved domain database (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Primers were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table S1). The PCR products were purified and linked to CV16-Zero Background pTOPO-TA/Blunt Simple Cloning Kit (Aidlab Biotechnologies, Beijing, China). The constructed cloning vectors were transformed into Escherichia coli DH5α were cultured overnight on LB solid medium. Ten single colonies of each cloning vector were chosen for Sanger’s sequencing (Sangon Biotech, Shanghai, China).
Real-time quantitative PCR analysis
RT-qPCR was performed in a QuantStudio™ 6 Flex Real-Time PCR (Thermo Fisher Scientific, Waltham, USA) with the presence of NovoStrat SYBR qPCR SuperMix Plus (Novoprotein, Suzhou city, Jiangsu province, China). All reactions were repeated three times. Apple elongation factor 1-alpha gene was used as the housekeeping internal reference. The primers were designed using Primer-BLAST (Table S1).
Construction of plant expression vector and transformation of apple callus
Full-length CDS of MdSAUR36 was enzyme digested from cloning vector by HindIII/SalI or HindIII/BamHI and then were linked to the multiple cloning site (MCS) of PRI-101-GFP or PRI-101-RNAi using One Step Seamless Cloning kit (Aidlab Biotechnologies, Beijing, China). Transgenic apple calli (derived from mesocarp of cultivar “Orin”) were obtained by Agrobacterium-mediated transformation method. Plant expression vectors and empty vectors were transformed into Agrobacterium tumefaciens strain GV3101 by heat shock. The apple calli were infected and co-cultured with the transformed Agrobacterium tumefaciens at 25 °C in the dark for 2 days, then cultured on the screening medium with kanamycin and cephalosporin resistance for a least 20 days until positive calli appear to grow. The positive calli were sub-cultured on the screening medium 3–4 times. The successful transgenesis was confirmed by RT-qPCR.
Promoter activity analysis
Promoter sequences were linked to the expression vector Pcambia1391 prior to the β-glucuronidase (GUS) report gene. Then, the PRI-101-GFP empty vector, Pcambia1391 empty vector, and target promoter-linked vector were transformed into Agrobacterium tumefaciens strain GV3101 by heat shock. The Agrobacterium solution transformed by Pcambia1391 empty vector and target promoter-linked vector were mixed with the Agrobacterium solution transformed by PRI-101-GFP with a volume ratio of 1:1. Next, these different types of Agrobacterium solutions were injected into tobacco (Nicotiana benthamiana) leaves; three tobacco individuals for each vector were injected as three biological replicates. All the infected tobacco individuals were grown in the dark for 1 day and then grown in light to dark of 16 h:8 h rhythm. Transgenic tobacco samples were immersed into GUS reaction buffer (X-Gluc) in the dark at 37 °C for 1 day, bleached with 75% ethanol for 3 days, and then observed. The expression of GUS gene in transiently transformed tobacco was analyzed by RT-PCR.
Results
Phenotype of fruit size in large- and small-fruit F1 hybrid plants
The phenotypes of fruit weight, length, and width in the F1 population of Zisai Pearl × Red Fuji were segregated extensively (Figure S1). Shapiro–Wilk test indicated that the fruit weight, fruit length, and fruit width were all skew-distributed and had positive kurtosis (Table S2). The coefficient of variation was estimated as 0.41, 0.17, and 0.17 for fruit weight, fruit length, and fruit width, respectively. The broad sense heritability was 0.73, 0.60, and 0.54 for fruit weight, fruit length, and fruit width, respectively (Table S2).
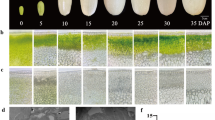
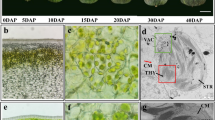
During fruit development period, the small- and large-fruit groups and “Sue” exhibited a single sigmoid pattern of fruit growth curve, the intensive increase in fruit weight, size, length, and width occurred from 28 to 118 DAA (Fig. 1). Since 28 or 58 DAA, significantly higher phenotype values in fruit weight, size, length, and width were found in the large-fruit group than the small-fruit group, whereas Sue exhibited significantly lower phenotype values (Fig. 1a–e). Sue had the smallest fruit weight, but its cell size was significantly larger than the small- and large-fruit groups from 7 to 28 DAA, whereas the cell size of the small-fruit group was larger than that of the large-fruit group without statistical significance (Fig. 2a, c). From 58 to 148 DAA, no significant differences in mesocarp cell size among either large-fruit group, small-fruit group, or Sue were observed (Fig. 2a, c). However, the mesocarp cell number increased more rapidly and was significantly higher in large-fruit hybrid plants than in small-fruit plants from 7 to 28 DAA, while Sue showed almost no obvious increase in cell number and the lowest mesocarp cell number throughout fruit development (Fig. 2b). No considerable increase in mesocarp cell number was found after 28 DAA in all fruit samples (Fig. 2b). These data indicated that the phenotype variations in final fruit weight and size between large- and small-fruit groups were mainly attributed to the mesocarp cell number before 28 DAA.
Comparison in apple fruit growth in large- and small-fruit groups of F1 hybrid plants derived from “Zisai Pearl” × “Red Fuji.” Each of the small-fruit (S) and large-fruit (L) groups included six hybrid plants; three apples were measured for each time point of sampling. A Malus baccata L. accession “Sue” with very small fruit was used as external control. Lowercase letters indicate statistical significance (p < 0.05) via analysis of variance and multiple comparison
Differences in gene expression between mesocarp of large- and small-fruit hybrid plants
As mentioned above, a significant difference in fruit size appeared in the early stage of fruit development before 28 DAA, so RNA-seq was performed using fruitlets of three large-fruit and three small-fruit hybrid plants sampled at 7, 14, and 28 DAA. After library construction, RNA sequencing, and quality control, 472.36 Gb clean reads were obtained in total (Table S3). The reads of each sample were mapped to the apple reference genome (GDDH13 v1.1) ranging from 86.71 to 89.00% (Table S4). Totally, 50,159 genes were annotated while 481, 815, and 611 DEGs between large- and small-fruit were identified at 7, 14, and 28 DAA, respectively. Finally, 1256 DEGs were defined in all three fruit developmental stages excluding repeats (Fig. 3a).
Weighted correlation network analysis of the differentially expressed genes (DEGs) between apple fruitlets of large- and small-fruit hybrid plants at 7, 14, and 28 days after anthesis (DAA). a Venn diagram of DEGs between apple fruitlets of large- and small-fruit hybrid plants at 7, 14, and 28 DAA. b Hierarchical clustering tree (dendrogram) of DEGs showing nine modules based on co-expression network analysis. Pearson’s correlation coefficient and the e-value of each module compared with fruit size were indicated in the center of each module grid. c Eigengene expression of six modules which were most correlated with fruit size. Columns represent the eigengene expression of DEGs in each sample. S, small-fruit group; L, large-fruit group
WGCNA revealed that 555 of the 1256 DEGs were classified into nine modules according to their expression patterns (Fig. 3b). Among the nine modules, six modules (black, blue, cyan, green, magenta, and pink) had a relatively high correlation with fruit size (Fig. 3c). The magenta module contained 166 genes which showed the most significant positive correlation with fruit size (r = 0.98) (Fig. 3b). The genes in magenta module exhibited a significantly high positive eigengene expression in the large-fruit group in 28 DAA and had a negative eigengene expression under other conditions (Fig. 3c). The genes in magenta module were mainly related to defense response, sucrose catabolic process, protein refolding, and DNA recombination (Figure S2). The blue module included 80 genes showing also a relatively high positive correlation with fruit size (r = 0.54) (Fig. 3b). The genes in the blue module were enriched mainly in the GO terms of transcription, DNA-templated, positive regulation of stomatal complex development, chromosome condensation, and lipid phosphorylation (Figure S2). There were 45 genes clustered in the green module which displayed the highest negative correlation with fruit size (r = − 0.77) (Fig. 3b). The GO terms of the genes in the green module were the tryptophan biosynthetic process, cytokinin metabolic process, and carbon fixation (Figure S2). Black and pink modules had similar expression pattern; the genes in these two modules showed significantly high-positive eigengene expression in the small-fruit group at 28 DAA and had low eigengene expression under other conditions (Fig. 3b, c). The genes in black and pink modules were mainly enriched in the GO terms of hormone biosynthetic process, indoleacetic acid biosynthetic process, monocarboxylic acid biosynthetic process, auxin biosynthetic process, and cell wall macromolecule catabolic process (Figure S2). The cyan module contained 33 genes showing a negative correlation with fruit size (r = − 0.48) (Fig. 3b). The genes in the cyan module were mainly enriched in the GO terms of phloem development and positive regulation of abscisic acid-activated signaling pathway (Figure S2).
Screening for fruit size related candidate genes
We previously identified 48 significant QTLs for fruit weight on eight chromosomes by using Zisai Pearl × Red Fuji F1 population (Shen et al. 2022). Here, we found that 229 of the 1256 DEGs were co-located in 15 of the 48 QTL regions. Considering the gene function annotation and the result of WGCNA, six candidate genes were predicted in six QTLs, respectively (Fig. 4; Table 1).
Images showing the location of the six candidate genes within the regions of QTLs associated with apple fruit weight identified using the F1 population of “Zisai Pearl” × “Red Fuji.” The Y axis denotes the G′ value, and the X axis denotes the physical location of the apple GDDH13 v1.1 genome. The blue line represents that the variations were homozygous in the maternal parent and heterozygous in the pollen parent. The red line indicates that the variations were heterozygous in the maternal parent and homozygous in the pollen parent. The black line means the variations were heterozygous in both parents (Shen et al. 2022)
Of the six candidate genes, MdSAUR36 (MD02G1205700) was annotated as a small auxin-upregulated RNA gene (SAUR) homologous to SAUR36 in Arabidopsis (Table 1). MdSAUR36 was clustered into the cyan module with 0.74 kME and was located 36.80 kb apart from the peak of QTL Z02.2 with a considerably high G′ value of 12.97 (Fig. 4a; Table 1). The expression (in FPKM) of MdSAUR36 in the small-size group of hybrid plants was significantly higher than that in the large-fruit group hybrid plants at 28 DAA (Fig. 5).
Two candidate genes, MdCYCB1-2 (MD12G1093900) and MdSMR3 (MD12G1140600), were located within the regions of QTL Z12.2 and Z12.3, respectively, and they were annotated to be related to cell cycle and cell division (Fig. 4b; Table 1). The expression of MdCYCB1-2 in the small-fruit group was significantly lower than that in the large-fruit group at 14 DAA (Fig. 5). MdSMR3 belongs to the pink module with the kME of 0.83 and the expression in the small-fruit group was significantly higher than that in the large-fruit group at 7 and 14 DAA (Fig. 5; Table 1). Within the significant region of QTL Z13.2, MdILL2 (MD13G1086300) was annotated as the IAA-amino acid hydrolase ILR1-like 2, which can hydrolyze certain amino acid conjugates of IAA (Fig. 4c; Table 1). MdILL2 was clustered into the green module with a kME of 0.85 and the expression was significantly higher in the small-fruit group than that in the large-fruit group at 14 DAA (Fig. 5; Table 1). Within QTL Z13.7, an undefined gene MD13G1158300 was clustered into the magenta module with a significantly high kME of 0.93 (Fig. 4c; Table 1) The expression of MD13G1158300 in the small-fruit group was significantly lower than that in the large-fruit group at all the three timepoints of sampling (Fig. 5). A cyclin D homolog MdCD11 (MD16G1087700) was located close to the peak region of QTL Z16.1 with a relatively high G′ value (Fig. 4d; Table 1). The expression of MdCD11 was significantly higher at 14 DAA in the large-fruit group than in the small-fruit group of hybrid plants (Fig. 5).
Identification and validation of genetic variations in MdSAUR36
Genomic DNA sequence of MdSAUR36 was 519 bp with only one exon without intron, encoding a protein with 173 amino acids. One non-synonymous single nucleotide polymorphism (SNP) G/T at 379 bp downstream of the ATG codon of MdSAUR36 was identified heterozygous in the maternal parent Zisai Pearl. MdSAUR36 SNP379 G/T caused an amino acid alteration (A-S) on the auxin-inducible functional domain (Fig. 6a). By using a training population including 1835 individuals, MdSAUR36 SNP379 G/T exerted significant genotype effects on fruit weight, i.e., − 91.24 g, − 29.57 g, and 14.12 g for the genotype TT, GT, and GG, respectively (Fig. 6b). Similarly, the genotype effects of MdSAUR36 SNP379 G/T on fruit length/width were estimated as − 34.46/ − 39.86 mm, − 5.24/ − 6.31 mm, and 2.48/2.99 mm for TT, GT, and GG, respectively (Fig. 6b). Genetic composition analysis using the training population revealed that the gene frequency of MdSAUR36 SNP379 T allele was quite low (14.80%) whereas the genotype frequency of TT was as low as 0.76% (14/1835) (Table S5). The TT homozygous genotype of MdSAUR36 SNP379 appeared mainly in M. baccata L. accessions and the descendants, while all the M. domestica cultivars were GG genotype (Table S5). MdSAUR36 was thus proposed to associate closely with fruit weight/size in Malus accessions.
Identification and validation of genetic variations in MdSAUR36. a Diagram showing the genetic variations in the promoter and the coding regions of MdSAUR36 of the maternal parent “Zisai Pearl.” b Box plot demonstrating genotype effects of MdSAUR36 SNP379 G/T on apple fruit weight, length, and width by using a training population including 1922 individuals. c The relative expression of MdSAUR36 during fruit development analyzed via qRT-PCR in small- and large-fruit groups of hybrid plants and the parental cultivars. d GUS reporter assay using transiently transformed tobacco leaves showing the GUS staining (left) and GUS gene relative expression driven by promoters MdSAUR36-Pro-W without altered cis-acting elements or MdSAUR36-Pro-M with altered cis-acting elements
qRT-PCR data confirmed that the expression of MdSAUR36 was higher in the small-fruit hybrid plants and Zisai Pearl than that the in large-fruit hybrid plants and Red Fuji during the early fruit development from anthesis to 28 DAA (Fig. 6c). Two insertion/deletion (InDels) and 10 SNPs were identified in the promoter region of MdSAUR36 in the maternal parent Zisai Pearl altering cis-acting elements (CAE), such as TATA-box, CAAT-box, and G-box (Table S6). Two haplotypes of the promoter in Zisai Pearl were defined as MdSAUR36-Pro-W without altered CAE and MdSAUR36-Pro-M with altered CAE. No CRE-altering variations were found in the promoter region of MdSAUR36 (MdSAUR36-Pro-W) in Red Fuji. GUS histochemical assay and GUS gene expression indicated that the haplotype MdSAUR36-Pro-M exhibited significantly stronger promoter activity than MdSAUR36-Pro-W (Fig. 6d). Together, these data indicated the higher expression levels of MdSAUR36 in small-fruit hybrid plants were attributed to the increased promoter activity by the genetic variations in the promoter region.
Functional validation of MdSAUR36 SNP379 G/T via transgenic apple calli
To investigate whether MdSAUR36 SNP379 G/T may affect cell division, the two allelotypes of MdSAUR36, MdSAUR36-G and MdSAUR36-T, were respectively overexpressed and MdSAUR36 was silenced by RNAi in apple calli (Fig. 7a–d). The fresh weight of transgenic apple calli was significantly reduced by over-expressing MdSAUR36-T compared with the MdSAUR36-G and transformant control with empty vector (Fig. 7a, e). The fresh weight of transformed lines over-expressing MdSAUR36-G was slightly less than that of the transformants with empty vector, which was although not statistically significant (Fig. 7a, e). However, the proliferation of apple calli was significantly increased in MdSAUR36-RNAi lines compared with the empty vector (Fig. 7b, f); the fresh weight of transformed MdSAUR36-RNAi apple calli was significantly higher than that with the empty vector (Fig. 7f). These data suggested that MdSAUR36 negatively affected cell proliferation and SNP379 T allele enhanced the inhibitory effect. Taken together, the variations in both the promoter and the CDS of MdSAUR36 contributed to the small-fruit phenotype in Zisai Pearl and part of the offspring.
Functional validation of MdSAUR36 SNP379 G/T via transgenic apple calli. a, b Appearance of transgenic apple calli derived from mesocarp of the cultivar “Orin” overexpressing MdSAUR36-G, MdSAUR36-T (a), or silencing MdSAUR36 by RNAi (b). c, d Relative expression of MdSAUR36 in transgenic lines of apple calli. e, f Fresh weight of transgenic apple calli overexpressing MdSAUR36-G, MdSAUR36-T (e), or silencing MdSAUR36 by RNAi (f)
Discussion
Phenotype diversity or segregation of apple fruit size is attributed to the variations in the mesocarp cell number
It has been reported that both cell division and expansion rates determine the number and size of mesocarp cells and thus the size of ripe fruit in an apple. However, cell division was once proposed as key to the differences in fruit size among cultivars, because the cell areas of each cultivar, “Twenty Ounce” (large-sized), “Royal Gala” (medium-sized), and crab apple (small-sized), were similar at pollination or at ripening (Karim 2015; Karim et al. 2022). By using an interspecific hybrid population, we confirmed that no considerable differences were found in mesocarp cell size between large-fruit and small-fruit groups but the mesocarp cell number of the large-fruit group was significantly higher than that of the small-fruit group (Fig. 2a, b). Large- and small-fruit groups shared similar mesocarp cell expansion rates and time course. The fruit weight of Sue was 3.0 g at ripening and the mesocarp cell size was almost the same as the large-fruit group and Red Fuji; thus, the mesocarp cell number contributed to the diversity of apple fruit size. Reduction of fruit load during early fruit development led to a rapid increase in MdANT1 expression, which caused an increase in cell production and early fruit growth (Dash et al. 2013). Similarly in Japanese pear (Pyrus pyrifolia Nakai), late ripening cultivars usually have larger fruit than early ripening cultivars owing to a longer period of cell division and a greater cell number (r = 0.9739) but not cell length (r = − 0.0170) (Zhang et al. 2006). In sweet cherry (Prunus avium L.) cultivars, it was cell number (r = 0.72) not cell length as the major contributor to the differences in fruit size (Olmstead et al. 2007). Consistently in 20 rabbiteye blueberry (Vaccinium ashei Read.) genotypes with the fruit diameter varied by over 1.6-fold, cell number was significantly related to fruit diameter (R2 = 0.79), but no correlation was found between cell area and fruit diameter (Johnson et al. 2011). Furthermore, we found that during the rapid cell division period from zero to 28 DAA, the mesocarp cell size exhibited a negative correlation with final fruit weight, and the cell size of Sue was significantly larger than both large- and small-fruit hybrid plants.
Active mesocarp cell division occurs at an early stage of apple fruit development, followed by continuous cell expansion
In this study, rapid mesocarp cell division was observed from 7 to 28 DAA in both large- and small-fruit groups, but the cell number of large-fruit hybrid plants increased more rapidly than that of small-fruit hybrid plants. On the contrary, no obvious increase in mesocarp cell number was found in M. baccata L. accession Sue throughout the fruit development and the final fruit weight was 3.0 g at ripening. After 28 DAA, cell division ceased, and cell expansion became the dominant source for fruit growth irrespective of genotypes. These data indicated that not only the rate of cell division but also the duration of cell division influence final fruit size. In apple cultivar “Royal Gala,” mesocarp cell division ceased at 35 DAA and thereafter the rate of cell expansion increased and maintained a high level of cell expansion during 60–87 DAA (Janssen et al. 2008). The increases in cell number index (CNI) sustained until 10, 28, and 35 DAA in M. floribunda Sieb. (11.1 mm), M. coronaria Mill. (51.9 mm), M. domestica cultivar “Fuji” (87.7 mm), respectively. In addition, the CNI of M. floribunda Sieb. was smaller than the other species (Harada et al. 2005). Consistently in pears, the duration of cell division was cultivar-dependent, the critical points of cell division were 34, 36, and 49 DAA in “Shinsui” (200 g), “Kousui” (350 g), and “Shinsetsu” (1000 g), respectively (Zhang et al. 2005). Histological studies indicated that the fruit of “Shinsetsu” had significantly more cells than the other two cultivars (Zhang et al. 2006).
Genetic variations in cell division related genes are closely associated with apple fruit size
GO and WGCNA revealed that genes involving cell division, phytohormone metabolism, and cell wall catabolism were differentially expressed during early fruit development (Figure S2). Auxin has been shown to be involved in the initial signal for fertilization, cell division, and cell expansion in fruit development (Devoghalaere et al. 2012). Two auxin signaling-related genes, MdSAUR36 and MdILL2, were predicted as candidate genes controlling fruit size in this study. ILL2 can hydrolyze certain amino acid conjugates of IAA to buffer free IAA level in plant organ (LeClere et al. 2002). SAUR was one of the early auxin-responsive genes with multiple biological functions such as hypocotyl growth, stem elongation, auxin transport, and light signaling (Deng et al. 2019). EjSAUR22 played an important role in regulating fruit size via affecting cell expansion, silencing EjSAUR22 led to small fruit size and small cell size in loquat (Eriobotrya japonica L.) (Peng et al. 2022). In this study, however, we found that MdSAUR36 exhibited an inhibitory effect on mesocarp cell division and thus final fruit size. The genetic variations in the promoter of MdSAUR36 were associated with increased gene expression in small-fruit hybrid plants and maternal Zisai Pearl than that in large-fruit hybrid plants and paternal Red Fuji from anthesis to 28 DAA. MdSAUR36 SNP379 T allele led to a lower cell proliferation rate in transgenic apple calli. The genotype effect of MdSAUR36 SNP379 TT on fruit weight was estimated as low as − 91.24 g. Additionally, the homozygous MdSAUR36 SNP379 TT genotype appeared only in small-fruit species like M. baccata L. The average fruit weight of accessions and hybrid plants with MdSAUR36 SNP379 GG genotype was 107.3 g but the variance was quite large owing to major effect variations other than those on MdSAUR36 (Fig. 6b; Table S5) (Yao et al. 2015; Shen et al. 2022). These data demonstrated that both SNP379 G/T and the functional variation(s) in the promoter of MdSAUR36 contributed to the small-fruit trait in Malus accessions. Multiple genetic variations in one gene like MdSAUR36 further broaden the phenotypic segregation spectrum of the target trait (Zheng et al. 2020; Yang et al. 2022).
Three cell cycle-related genes, MdCYCB1-2, MdSMR3, and MdCD11, were identified as candidate genes based on QTL and WGCNA data. In eukaryotes, the cell cycle progression is determined by the complexes of cyclin-dependent kinases (CDKs) and cyclin. A-type cyclins control S-phase and the G2-M transition, B-type cyclins controls G2-M and intra-mitotic transitions, and D-type cyclins controls the G1-S transition (Dante et al. 2014). Leaves of SMR2 loss-of-function mutant smr2 in Arabidopsis were noticeably larger than that of wild-type (WT), and the cell division rate of the mutant was approximately double that of WT (Kumar et al. 2015). In this study, the expression of both MdCYCB1-2 and MdCD11 in large-fruit hybrid plants was significantly higher than that in small-fruit plants, while the expression of MdSMR3 was lower in large-fruit hybrid plants than that in small-fruit plants. MdCYCB1-2, MdSMR3, and MdCD11 were also located in the confidential region of QTLs for apple fruit weight; however, the genetic variations in these candidate genes are not yet identified nor validated.
Data availability
The apple genome used was a version of the Malus × domestica genome GDDH13_v1.1 (GDDH13, https:// iris.angers. inra. fr/ gddh13/). All the sequencing data of RNA-seq are available in the National Center for Biotechnology Information Sequence Read Archive database, with the accession number PRJNA998177 (https://www.ncbi.nlm.nih.gov/sra/PRJNA998177).
References
Bain JM, Robertson RN (1951) The Physiology of growth in apple fruits. 1. Cell size, cell number, and fruit development. Australian J Sci Res Ser B-Biol Sci 4:75–91
Bu H, Yu W, Yuan H, Yue P, Wei Y, Wang A (2020) Endogenous auxin content contributes to larger size of apple fruit. Front Plant Sci 11:592540. https://doi.org/10.3389/fpls.2020.592540
Chen XL, Li SM, Zhang D, Han MY, Jin X, Zhao CP, Wang SB, Xing LB, Ma JJ, Ji JJ, An N (2019) Sequencing of a wild apple (Malus baccata) genome unravels the differences between cultivated and wild apple species regarding disease resistance and cold tolerance. G3-Genes Genom Genet 9:2051–2060. https://doi.org/10.1534/g3.119.400245
Cho MS, Kim JH, Yamada T, Maki M, Kim SC (2021) Plastome characterization and comparative analyses of wild crabapples (Malus baccata and M. toringo): insights into infraspecific plastome variation and phylogenetic relationships. Tree Genet Genomes 17:41. https://doi.org/10.1007/s11295-021-01520-z
Cong B, Liu JP, Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. P Natl Acad Sci USA 99:13606–13611. https://doi.org/10.1073/pnas.172520999
Cong B, Barrero LS, Tanksley SD (2008) Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat Genet 40:800–804. https://doi.org/10.1038/ng.144
Cornille A, Antolin F, Garcia E, Vernesi C, Fietta A, Brinkkemper O, Kirleis W, Schlumbaum A, Roldan-Ruiz I (2019) A multifaceted overview of apple tree domestication. Trends Plant Sci 24:770–782. https://doi.org/10.1016/j.tplants.2019.05.007
Dante RA, Sabelli PA, Nguyen HN, Leiva-Neto JT, Tao YM, Lowe KS, Hoerster GJ, Gordon-Kamm WJ, Jung R, Larkins BA (2014) Cyclin-dependent kinase complexes in developing maize endosperm: evidence for differential expression and functional specialization. Planta 239:493–509. https://doi.org/10.1007/s00425-013-1990-1
Dash M, Malladi A (2012) The AINTEGUMENTA genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus x domestica Borkh.). BMC Plant Biol 12:98. https://doi.org/10.1186/1471-2229-12-98
Dash M, Johnson LK, Malladi A (2013) Reduction of fruit load affects early fruit growth in apple by enhancing carbohydrate availability, altering the expression of cell production-related genes, and increasing cell production. J Am Soc Hortic Sci 138:253–262. https://doi.org/10.21273/jashs.138.4.253
Deng G, Huang X, Xie L, Tan SB, Gbokie T, Bao YN, Xie ZL, Yi KX (2019) Identification and expression of SAUR genes in the CAM plant agave. Genes 10:555. https://doi.org/10.3390/genes10070555
Denne MP (1963) Fruit development and some tree factors affecting it. New Zeal J Bot 1:265–294. https://doi.org/10.1080/0028825X.1963.10428999
Devoghalaere F, Doucen T, Guitton B, Keeling J, Payne W, Ling TJ, Ross JJ, Hallett IC, Gunaseelan K, Dayatilake GA, Diak R, Breen KC, Tustin DS, Costes E, Chagne D, Schaffer RJ, David KM (2012) A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol 12:7. https://doi.org/10.1186/1471-2229-12-7
Disch S, Anastasiou E, Sharma VK, Laux T, Fletcher JC, Lenhard M (2006) The E3 ubiquitin ligase BIG BROTHER controls Arabidopsis organ size in a dosage-dependent manner. Curr Biol 16:272–279. https://doi.org/10.1016/j.cub.2005.12.026
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8:155–168. https://doi.org/10.1105/tpc.8.2.155
Frary A, Nesbitt TC, Grandillo S, van der Knaap E, Cong B, Liu JP, Meller J, Elber R, Alpert KB, Tanksley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289:85–88. https://doi.org/10.1126/science.289.5476.85
Gao Y, Wang DJ, Wang K, Cong PH, Li LW, Piao JC (2021) Analysis of genetic diversity and structure across a wide range of germplasm reveals genetic relationships among seventeen species of Malus Mill. native to China. J Integr Agr 20:3186–3198. https://doi.org/10.1016/s2095-3119(20)63421-9
Gardiner SE, Norelli JL, de Silva N, Fazio G, Peil A, Malnoy M, Horner M, Bowatte D, Carlisle C, Wiedow C, Wan YZ, Bassett CL, Baldo AM, Celton JM, Richter K, Aldwinckle HS, Bus VGM, de Silva N, Wan YZ (2012) Putative resistance gene markers associated with quantitative trait loci for fire blight resistance in Malus “Robusta 5” accessions. BMC Genet 13:25. https://doi.org/10.1186/1471-2156-13-25
Harada T, Kurahashi W, Yanai M, Wakasa Y, Satoh T (2005) Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci Horti 105:447–456. https://doi.org/10.1016/j.scienta.2005.02.006
Hu YX, Xie O, Chua NH (2003) The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15:1951–1961. https://doi.org/10.1105/tpc.013557
Janssen BJ, Thodey K, Schaffer RJ, Alba R, Balakrishnan L, Bishop R, Bowen JH, Crowhurst RN, Gleave AP, Ledger S, McArtney S, Pichler FB, Snowden KC, Ward S (2008) Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol 8:16. https://doi.org/10.1186/1471-2229-8-16
Johnson LK, Malladi A, NeSmith DS (2011) Differences in Cell Number Facilitate Fruit Size Variation in Rabbiteye Blueberry Genotypes. J Am Soc Hortic Sci 136:10–15. https://doi.org/10.21273/jashs.136.1.10
Karim SKA, Allan AC, Schaffer RJ, David KM (2022) Cell division controls final fruit size in three apple (Malus x domestica) cultivars. Horticulturae 8:7. https://doi.org/10.3390/horticulturae8070657
Karim SKA, The study of apple, Malus × domestica, fruit cell division and expansion in vivo and in vitro (2015). PhD Thesis, The University of Auckland
Klucher KM, Chow H, Reiser L, Fischer RL (1996) The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8:137–153. https://doi.org/10.1105/tpc.8.2.137
Krizek BA (1999) Ectopic expression AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Dev Genet 25:224–236. https://doi.org/10.1002/(sici)1520-6408(1999)25:3%3c224::aid-dvg5%3e3.0.co;2-y
Kumar N, Harashima H, Kalve S, Bramsiepe J, Wang K, Sizani BL, Bertrand LL, Johnson MC, Faulk C, Dale R, Simmons LA, Churchman ML, Sugimoto K, Kato N, Dasanayake M, Beemster G, Schnittger A, Larkin JC (2015) Functional conservation in the SIAMESE-RELATED family of cyclin-dependent kinase inhibitors in land plants. Plant Cell 27:3065–3080. https://doi.org/10.1105/tpc.15.00489
LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277:20446–20452. https://doi.org/10.1074/jbc.M111955200
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327. https://doi.org/10.1093/nar/30.1.325
Malladi A, Hirst PM (2010) Increase in fruit size of a spontaneous mutant of “Gala” apple (Malus x domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J Exp Bot 61:3003–3013. https://doi.org/10.1093/jxb/erq134
Malladi A, Johnson LK (2011) Expression profiling of cell cycle genes reveals key facilitators of cell production during carpel development, fruit set, and fruit growth in apple (Malus x domestica Borkh.). J Exp Bot 62:205–219. https://doi.org/10.1093/jxb/erq258
Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. P Natl Acad Sci USA 97:942–947. https://doi.org/10.1073/pnas.97.2.942
Olmstead JW, Lezzoni AF, Whiting MD (2007) Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J Am Soc Hortic Sci 132:697–703. https://doi.org/10.21273/jashs.132.5.697
Peil A, Garcia-Libreros T, Richter K, Trognitz FC, Trognitz B, Hanke MV, Flachowsky H (2007) Strong evidence for a fire blight resistance gene of Malus robusta located on linkage group 3. Plant Breeding 126:470–475. https://doi.org/10.1111/j.1439-0523.2007.01408.x
Peng Z, Li WX, Gan XQ, Zhao CB, Paudel D, Su WB, Lv J, Lin SQ, Liu ZL, Yang XH (2022) Genome-wide analysis of SAUR gene family identifies a candidate associated with fruit size in loquat (Eriobotrya japonica Lindl.). Int J Mol Sci 23:13271. https://doi.org/10.3390/ijms232113271
Shen F, Bianco L, Wu B, Tian ZD, Wang Y, Wu T, Xu XF, Han ZH, Velasco R, Fontana P, Zhang XZ (2022) A bulked segregant analysis tool for out-crossing species (BSATOS) and QTL-based genomics-assisted prediction of complex traits in apple. J Adv Res 42:149–162. https://doi.org/10.1016/j.jare.2022.03.013
Wang GL, Zhang CL, Huo HQ, Sun XS, Zhang YL, Hao YJ, You CX (2022) The SUMO E3 ligase MdSIZ1 sumoylates a cell number regulator MdCNR8 to control organ size. Front Plant Sci 13:836935. https://doi.org/10.3389/fpls.2022.836935
Westwood MN, Batjer LP, Billings HD (1967) Cell size cell number and fruit density of apples as related to fruit size position in cluster and thinning method. Proc Am Soc Hortic Sci 120:802–807
Yan ZY, Sousa-Gallagher MJ, Oliveira FAR (2008) Shrinkage and porosity of banana, pineapple and mango slices during air-drying. J Food Eng 84:430–440. https://doi.org/10.1016/j.jfoodeng.2007.06.004
Yang Z, Wu Z, Zhang C, Hu EM, Zhou R, Jiang FL (2016) The composition of pericarp, cell aging, and changes in water absorption in two tomato genotypes: mechanism, factors, and potential role in fruit cracking. Acta Physiol Plant 38:215. https://doi.org/10.1007/s11738-016-2228-1
Yang XL, Wu B, Liu J, Zhang ZY, Wang X, Zhang HE, Ren XJ, Zhang X, Wang Y, Wu T, Xu XF, Han ZH, Zhang XZ (2022) A single QTL harboring multiple genetic variations leads to complicated phenotypic segregation in apple flesh firmness and crispness. Plant Cell Rep 41:2379–2391. https://doi.org/10.1007/s00299-022-02929-z
Yao JL, Xu J, Cornille A, Tomes S, Karunairetnam S, Luo ZW, Bassett H, Whitworth C, Rees-George J, Ranatunga C, Snirc A, Crowhurst R, de Silva N, Warren B, Deng C, Kumar S, Chagne D, Bus VGM, Volz RK, Rikkerink EHA, Gardiner SE, Giraud T, MacDiarmid R, Gleave AP (2015) A microRNA allele that emerged prior to apple domestication may underlie fruit size evolution. Plant J 84:417–427. https://doi.org/10.1111/tpj.13021
Zhang CX, Tanabe K, Tamura F, Matsumoto K, Yoshida A (2005) C-13-photosynthate accumulation in Japanese pear fruit during the period of rapid fruit growth is limited by the sink strength of fruit rather than by the transport capacity of the pedicel. J Exp Bot 56:2713–2719. https://doi.org/10.1093/jxb/eri264
Zhang CX, Tanabe K, Wang S, Tamura F, Yoshida A, Matsumoto K (2006) The impact of cell division and cell enlargement on the evolution of fruit size in Pyrus pyrifolia. Ann Bot-London 98:537–543. https://doi.org/10.1093/aob/mcl144
Zheng CX, Shen F, Wang Y, Wu T, Xu XF, Zhang XZ, Han ZH (2020) Intricate genetic variation networks control the adventitious root growth angle in apple. BMC Genomics 21:852. https://doi.org/10.1186/s12864-020-07257-8
Zhou Z, Zhu YM, Zhang HT, Zhang RP, Gao QM, Ding TY, Wang H, Yan ZL, Yao JL (2021) Transcriptome analysis of transgenic apple fruit overexpressing microRNA172 reveals candidate transcription factors regulating apple fruit development at early stages. Peer j 9:e12675. https://doi.org/10.7717/peerj.12675
Acknowledgements
We would like to thank the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (Nutrition and Physiology) of the Ministry of Agriculture, People’s Republic of China, for providing the experimental platform.
Funding
This work was funded by the Modern Agricultural Industry Technology System (CARS-27) and the Key Research and Development Program of Hebei (21326353D; 21326308D). The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ZT, BW, and XZ conceived and designed the experiments. JL, LZ, YW, TW, XZ, and ZH contributed to the plant materials. ZT performed the experiments. ZT, BW, and XZ wrote or revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, Z., Wu, B., Liu, J. et al. Genetic variations in MdSAUR36 participate in the negative regulation of mesocarp cell division and fruit size in Malus species. Mol Breeding 44, 1 (2024). https://doi.org/10.1007/s11032-024-01441-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-024-01441-4