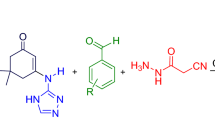
Abstract
Two new categories of fused pyridines include 2H-thiazolo[3,2-a]pyridine-6-carbohydrazides and 2H-oxazolo[3,2-a]pyridine-6-carbohydrazides have been successfully synthesized via five-component cascade reactions using 9-fluorenone, cyanoacetohydrazide, 1,1-bis(methylthio)-2-nitroethene, aromatic aldehydes and cysteamine hydrochloride or ethanol amine as starting materials. This new approach involves a subsequence of key steps: N,S-acetal or N,O-acetal formation, Knoevenagel condensation, Michael addition, tautomerization and N-cyclization. It also has some advantages, such as convenience of operation, tolerance of a wide diversity of functional groups, use of green solvent and ease of purification by washing the crude products with ethanol.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiazolopyridines have shown a broad spectrum of pharmacological and biological activities. Among the various derivatives, thiazolo[3,2-a]pyridines are an important framework with antifungal and antibacterial activity [1]. Their other significant biological properties contain betaamyloid production inhibitor [2], antihypertensive, muscle relaxant [3], potent CDK2-cyclin A inhibitor [4] and potential uterus stimulant [5]. They also have applications in chemotherapy of various cancers, such as lung cancer, leukemia and melanoma [6,7,8]. Some bioactive thiazolo[3,2-a]pyridines are shown in Fig. 1 [9,10,11]. Oxazolopyridine cores have also been demonstrated to possess significant biological properties such as inhibitors for dual thrombin/factor Xa [12], monoamine oxidase B for Parkinson’s disease treatment [13], SIRT1 activators [14], antihypertensive agents [15], antibacterial agents [16] and selective sphingomyelin synthase 2 inhibitors [17].
Minimizing the number of synthetic steps is one of the goals of modern chemistry. One way to achieve this aim is to design multicomponent reactions. MCRs have emerged as an economical, efficient and eco-friendly substitute to the conventional multistep synthesis for the construction of complex heterocyclic structures, especially various biologically active compounds [18,19,20,21]. Among these, multicomponent cascade reactions are double important due to in situ formation of intermediates with reactive sites for subsequent variations [22, 23].
In recent years, the synthesis of thiazolo[3,2-a]pyridines and oxazolo[3,2-a]pyridines using various multicomponent reactions has been reported. In this work, we used cyclic ketene acetals to synthesize the target compounds. So first we review the previous methods based on five-membered nitroketene N,S- or N,O-acetals (Scheme 1). In 2010, a three-component synthesis of fused pyridines was developed with diverse ketene aminals, trifluorooxobutanoate and triethoxymethane as starting components (A) [24]. In 2011, the one-pot reaction between 2-(nitromethylene)thiazolidine, various CH-acids (ethyl 2-cyanoacetate, malononitrile or 2-(phenylsulfonyl)acetonitrile) and aromatic aldehydes was reported that led to the formation of thiazolo[3,2-a]pyridines (B) [25]. In 2014, Zou and his group synthesized thiazolo[3,2-a]pyridinones via a two-step procedure using phthalic anhydride, ethyl cyanoacetate and 2-(nitromethylene)thiazolidine (C) [26]. During the last four years, our research group synthesized various thiazolo/oxazolo[3,2-a]pyridines utilizing the five-membered cyclic nitroketene acetals. In 2019, benzo[g]thiazolo[3,2-a]quinolones were synthesized using 2-hydroxy-1,4-naphthoquinone, 2-(nitromethylene)thiazolidine and aromatic aldehydes (D) [27]. Also, the desired products were formed via a one-pot reaction between nitroketene acetals, aromatic aldehydes and cyanoacetamide or cyanoacetohydrazide (E and F) [28, 29]. In addition, we were able to produce functionalized thiazolo[3,2-a]pyridines in a one-pot reaction between acetophenones, cyanoacetohydrazide, aromatic aldehydes and 2-(nitromethylene)thiazolidine (G) [30].
As a part of our studies on the synthesis of novel fused heterocyclic structures, we describe herein a five-component synthesis of highly functionalized 2H-thiazolo/oxazolo[3,2-a]pyridines by in situ formation of ketene acetals. The synthesis of these structures is reported for the first time.
Results and discussion
We have designed a convenient synthesis of 3,7-dihydro-2H-thiazolo/oxazolo[3,2-a]pyridine-6-carbohydrazide derivatives via a one-pot five-component reaction. To optimize the reaction conditions, initially 9-fluorenone 1, cyanoacetohydrazide 2, 4-chlorobenzaldehyde 3, 1,1-bis(methylthio)-2-nitroethene 4 and cysteamine hydrochloride 5a were used as substrates in the model reaction to achieve the best results. Generally, due to the five-component nature of the designed reactions as well as the variable reactivity of cyanoacetohydrazide in different conditions, many attempts have been made to synthesize the target products with desirable purity. It should be noted that for the synthesis of 2-(nitromethylene)thiazolidine (from 1,1-bis(methylthio)-2-nitroethene and cysteamine hydrochloride), triethylamine is used to release cysteamine from its salt, and it is not working on the rate-limiting step [28, 31]. Therefore, it is not considered as a catalyst for the whole reaction. (The use of catalysts in Table 1 is related to the whole reaction.)
Firstly, the model reaction was investigated in five different solvents under refluxing conditions. When ethanol was examined as solvent without any catalyst, it was observed that desired product 6c was not produced (Table 1, entry 1). The use of other solvents such as chloroform, acetonitrile, methanol and water did not lead to the formation of the product (Table 1, entry 2–5). The experimental results showed that the use of ethanol in the presence of acetic acid under reflux conditions leads to the formation of product 6c with an efficiency of 85% (Table 1, entry 6). With acetic acid in a mixture of water and ethanol, the efficiency decreased significantly, and with p-TSA, the five-component product did not form (Table 1, entry 7,8). The use of basic catalysts resulted in no product formation (Table 1, entry 9,10). So the reactions proceeded with good yields to formation of 3,7-dihydro-2H-thiazolo/oxazolo[3,2-a]pyridine-6-carbohydrazides in ethanol as solvent and acetic acid as catalyst at reflux conditions (entry 6). In these reactions initially, a two-component product of 9-fluorenone and cyanoacetohydrazide is formed in acidic medium, and then, subsequent reactants (aldehyde and nitro ketene acetal) were added without the need for separation.
Based on the optimal reaction conditions, we could synthesize target compounds 6a–l using 9-fluorenone 1, cyanoacetohydrazide 2, various aromatic aldehydes 3, 1,1-bis(methylthio)-2-nitroethene 4 and cysteamine hydrochloride 5a or ethanol amine 5b as starting materials (Scheme 2). The processes were completed in a total of 24 h and resulted in the formation of desired products 6a–l with good yields (65–92%). The relevant results are presented in Table 2. Observations showed that when the reaction is performed with ortho derivatives (2-chloro and 2-nitro benzaldehyde) under similar conditions, it does not lead to the formation of the product. The reaction was also tested with aliphatic ketones instead of 9-fluorenone, which did not yield the product. In addition, anthrone was used instead of 9-fluorenone, but again the favorable result was not observed.
The results revealed that substitutions on aldehyde has only a slight influence on the yield, which did not lead us to find the obvious rules.
The structures of proposed structures 6a–l were deduced from their 1H NMR, 13C NMR, IR and mass spectra (see the supporting information).
As an example, the main chemical shifts of 1H and 13C NMR of 5-amino-N′-(9H-fluoren-9-ylidene)-7-(3-methoxyphenyl)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide 6a are shown in Fig. 2. In 1H NMR spectrum of 6a, the NH and NH2 groups were observed at δ 10.56 and 8.39 ppm, respectively. The proton of CH at pyridine ring appeared at δ 5.90 ppm. Two multiplet signals at δ 4.25–4.34 and 4.40–4.48 ppm were assigned to two methylene groups. The signal in δ 3.69 was assigned to the methoxy group. The 13C NMR spectrum of 6a showed 28 separate signals in accordance with the expected product. As seen in Fig. 2, the characteristic signals of three aliphatic carbons (CH2S, CH and CH2N groups) appeared at δ 27.8, 37.0 and 50.9 ppm, respectively. The signals at δ 81.1 and 120.2 ppm were determined as C–C=O and C–NO2. Carbonyl signal was observed at δ 166.7 ppm. Methoxy group was detected at δ 54.9 ppm (Fig. 2).
The IR spectrum of 6a showed absorption bands at 3155 and 3275 cm−1 related to NH and NH2 groups and stretching bands due to aliphatic C–H at 2924 cm−1. The carbonyl group showed strong absorption at 1632 cm−1. Two absorption bands due to nitro group were observed at 1499 and 1308 cm−1, and two stretching vibrational bands of C=C of aromatic ring and C–N were seen at 1451 and 1241 cm−1, respectively.
An acceptable mechanism for the synthesis of 5-amino-N′-(9H-fluoren-9-ylidene)-7-aryl-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazides 6a–h is shown in Scheme 3. As expected from the well-known chemistry of 1,1-bis(methylthio)-2-nitroethene, firstly, the addition of cysteamine hydrochloride 5a to 1,1-bis(methylthio)-2-nitroethene 4 in the presence of an equivalent amount of triethylamine, for releasing cysteamine salt, leads to the formation of ketene N,S-acetal C. On the other hand, condensation of 9-fluorenone 1 with cyanoacetohydrazide 2 in acidic medium leads to hydrazone A formation. Further, with increasing aldehyde 3 to A, the Knoevenagel product B is formed. In the following, Michael addition of nitro ketene acetal C to B affords intermediate D, which leads to the formation of intermediate E by imine-enamine tautomerization and followed by N-cyclization via intramolecular addition of –NH group to nitrile group. At the end, the second imine-enamine tautomerization in F affords the desired products 6a–h (Scheme 3). In the synthesis of oxazolo[3,2-a]pyridine-6-carbohydrazides 6i–l, the mechanism of the reaction is similar. The only difference is the formation of 2-(nitromethylene)oxazolidine as ketene acetal, which is synthesized by the reaction of 1,1-bis(methylthio)-2-nitroethene 4 and ethanolamine 5b.
According to our studies, the most important side product in these reactions is a four-component product that is formed with participation of two aldehydes [26]. To prevent the formation of this by-product, at first, the two-component product, hydrazone A, was completely synthesized and then the aldehyde and ketene acetal were added simultaneously.
Conclusion
In summary, we have developed an efficient five-component domino reaction for the preparation of novel 3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazides and 3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazides using annulation of heterocyclic ketene acetals (2-(nitromethylene)thiazolidine/oxazolidine) and a three-component product of 9-fluorenone, cyanoacetohydrazide and aromatic aldehydes. This approach minimizes solvent consumption by using only a filtration and washing and avoiding traditional purification techniques, such as chromatography. The mild reaction conditions, easy workup procedure, experimental simplicity, high regioselectivity and good-to-high yields make this methodology attractive for synthesizing a variety of highly substituted thiazolo/oxazolo[3,2-a]pyridines.
Experimental
Materials
1,1-Bis(methylthio)-2-nitroethene, aromatic aldehydes, 9-fluorenone, cyanoacetohydrazide, cysteamine hydrochloride, ethanol amine and solvents were purchased from Aldrich and Merck chemical Co. and used with no further purification. IR spectra were registered with Bruker Tensor 27 spectrometer (ῡ in cm−1). The NMR spectra were recorded in DMSO-d6 as solvent with a Bruker DRX-300 AVANCE instrument (300 MHz for 1H and 75.4 MHz for 13C). Chemical shift values are given in ppm (δ), and coupling constant (J) is reported in Hertz (Hz). All melting points were measured with an electrothermal 9100 apparatus. Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV.
General procedure of the synthesis of 3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide derivatives 6a–h
A mixture of 1,1-bis(methylthio)-2-nitroethylene (0.165 g, 1 mmol), cysteamine hydrochloride (0.113 g, 1 mmol), Et3N (140 µL, 1 mmol) and 10 mL EtOH in a 50-mL round-bottom flask was heated with stirring at reflux temperature for 5 h. In another 50-mL round-bottom flask, the mixture of 9-fluorenone (1 mmol, 0.180 g) and cyanoacetohydrazide (1 mmol, 0.99 g) in EtOH (10 ml) and AcOH (1 mmol) was stirred at reflux conditions for 5 h. After this time, TLC shows the consumption of the starting materials. At this point, aromatic aldehyde (1 mmol) and the first solution (ketene N,S-acetal) were added to the hydrazone mixture simultaneously. After reaction completion (monitored by TLC using ethyl acetate/n-hexane (1:1), the formed solid was filtered and washed with warm ethanol to isolate the pure products 6a–h.
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(3-methoxyphenyl)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6a)
Yellow solid; yield: 0.473 g (90%); mp: 220–222 °C; IR (KBr) (νmax /cm−1): 3426, 3275, 3155, 2924, 1632, 1499, 1451, 1308, 1241, 1177, 1128, 777, 729; 1H NMR (300 MHz, DMSO-d6): δ 3.69 (3H, s, OCH3), 4.25–4.34 (2H, m, CH2), 4.40–4.48 (2H, m, CH2), 5.90 (1H, s, CH), 6.84 (1H, d, J = 9 Hz, ArH), 7.07–7.16 (2H, m, ArH), 7.17–7.30 (1H, m, ArH), 7.34–7.39 (3H, m, ArH), 7.40–7.47 (2H, m, ArH), 7.73 (1H, d, J = 9 Hz, ArH), 7.80 (1H, d, J = 6 Hz, ArH), 7.85 (1H, d, J = 6 Hz, ArH), 8.39 (2H, s, NH2), 10.56 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 27.8 (CH2S), 37.0 (CH), 50.9 (CH2N), 54.9 (OCH3), 81.1 (C–C=O), 111.6, 114.4, 119.9 (Ar), 120.2 (C–NO2), 120.5, 121.4, 123.8, 126.7, 127.7, 128.1, 129.6, 129.6, 130.0, 130.8, 136.9, 139.2, 140.9, 145.7 (Ar), 150.6 (C=C–S), 151.4 (C=N), 156.8 (C–NH2), 159.2 (CAr–OMe), 166.7 (C=O); m/z (%) = 523 (0.05) [M-2]+, 482 (0.3), 433 (0.3), 379 (0.2), 355 (100), 327 (42), 288 (42), 257 (28), 220 (19), 194 (21), 180 (13), 164 (73), 102 (5), 61 (7), 41 (2).
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(4-methoxyphenyl)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6b)
Yellow solid; yield: 0.483 g (92%); mp: 245–247 °C; IR (KBr) (νmax /cm−1): 3270, 3151, 1632, 1489, 1448, 1306, 1240, 1185, 1129, 778, 726; 1H NMR (300 MHz, DMSO-d6): δ 3.71 (3H, s, OCH3), 4.24–4.34 (2H, m, CH2), 4.39–4.47 (2H, m, CH2), 5.82 (1H, s, CH), 6.94 (2H, d, J = 6 Hz, ArH), 7.17–7.49 (5H, m, ArH), 7.41 (2H, d, J = 6 Hz, ArH), 7.73 (1H, d, J = 6 Hz, ArH), 7.80 (1H, d, J = 9 Hz, ArH), 7.85 (1H, d, J = 9 Hz, ArH), 8.34 (2H, s, NH2), 10.50 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 27.7 (CH2S), 36.3 (CH), 50.8 (CH2N), 55.0 (OCH3), 81.4 (C–C=O), 113.7 (Ar), 120.2 (C–NO2), 120.5, 121.4, 124.3, 126.7, 127.7, 128.1, 128.9, 129.6, 130.0, 130.8, 136.1, 136.9, 139.2, 140.8 (Ar), 150.7 (C=C–S), 151.2 (C=N), 156.4 (C–NH2), 158.3 (CAr–OMe), 166.6 (C=O); m/z (%) = 357 (2), 356 (7), 355 (8), 326 (2), 288 (14), 257 (14), 220 (5), 194 (5), 180 (6), 164 (30), 139 (3), 77 (5), 40 (100).
5-Amino-7-(4-chlorophenyl)-N′-(9H-fluoren-9-ylidene)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6c)
Orange solid; yield: 0.450 g (85%); mp: 244–246 °C; IR (KBr) (νmax /cm−1): 3467, 1632, 1498, 1451, 1308, 1215, 1133, 854, 727, 652; 1H NMR (300 MHz, DMSO-d6): δ 4.25–4.33 (2H, m, CH2), 4.40–4.45 (2H, m, CH2), 5.91 (1H, s, CH), 7.14–7.17 (2H, m, ArH), 7.31–7.52 (7H, m, ArH), 7.71–7.86 (3H, m, ArH), 8.35 (2H, s, NH2), 10.62 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 27.8 (CH2S), 36.7 (CH), 50.9 (CH2N), 81.0 (C–C=O), 120.2 (C–NO2), 120.5, 121.5, 123.5, 126.7, 127.5, 128.1, 128.3, 129.7, 129.8, 130.1, 130.9, 131.6, 136.8, 139.3, 140.9, 143.1 (Ar), 151.2 (C=C–S), 151.8 (C=N), 157.0 (C–NH2), 166.6 (C=O);); m/z (%) = 523 (0.03), 509 (0.1), 482 (0.1), 435 (0.2), 361 (0.4), 287 (1), 213 (6), 180 (100), 152 (4), 126 (6), 80 (53), 44 (47).
5-Amino-7-(3,4-dimethoxyphenyl)-N′-(9H-fluoren-9-ylidene)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6d)
Yellow solid; yield: 0.444 g (80%); mp: 248–250 °C; IR (KBr) (νmax /cm−1): 3410, 3162, 1631, 1502, 1447, 1311, 1242, 1183, 1128, 1025, 773, 619; 1H NMR (300 MHz, DMSO-d6): δ 3.66 (3H, s, OCH3), 3.71 (3H, s, OCH3), 4.29–4.35 (2H, m, CH2), 4.38–4.45 (2H, m, CH2), 5.83 (1H, s, CH), 6.95 (2H, s, ArH), 7.12–7.46 (6H, m, ArH), 7.73 (1H, d, J = 6 Hz, ArH), 7.80 (1H, 0d, J = 6 Hz, ArH), 7.85 (1H, d, J = 6 Hz, ArH), 8.36 (2H, s, NH2), 10.46 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 28.2 (CH2S), 37.0 (CH), 51.3 (CH2N), 55.8 (OCH3), 56.0 (OCH3), 81.7 (C–C=O), 112.5 (Ar), 120.1 (C–NO2), 120.7, 121.0, 121.9, 124.6, 128.0, 128.6, 130.0, 130.4, 131.3, 137.0, 137.3, 139.6, 141.3, 148.4, 148.8 (Ar), 150.9 (C=C–S), 151.8 (C=N), 157.0 (C–NH2), 167.0 (C=O); m/z (%) = 554 (0.02) [M-1]+, 509 (0.2), 435 (0.3), 361 (0.8), 287 (2), 213 (9), 180 (100), 152 (37), 126 (6), 80 (81), 48 (37).
5-Amino-N′-(9H-fluoren-9-ylidene)-8-nitro-7-(4-nitrophenyl)-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6e)
Orange solid; yield: 0.421 g (78%); mp: 261–264 °C; 1H NMR (300 MHz, DMSO-d6): δ 4.30–4.36 (2H, m, CH2), 4.41–4.45 (2H, m, CH2), 6.06 (1H, s, CH), 7.11 (2H, d, J = 6 Hz, ArH), 7.33–7.44 (4H, m, ArH), 7.70–7.84 (4H, m, ArH), 8.24 (2H, d, J = 6 Hz, ArH), 8.39 (2H, s, NH2), 10.76 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 27.9 (CH2S), 37.4 (CH), 51.0 (CH2N), 80.7 (C–C=O), 120.3 (C–NO2), 120.5, 122.7, 123.5, 124.0, 126.6, 127.5, 127.9, 128.2, 129.2, 129.7, 131.0, 136.8, 139.3, 141.0, 146.4, 151.2 (Ar), 151.7 (C=C–S), 152.5 (C=N), 157.6 (C–NH2), 166.6 (C=O).
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(3-fluorophenyl)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6f)
Brick red solid; yield: 0.374 g (73%); mp: 280–282 °C; 1H NMR (300 MHz, DMSO-d6): δ 4.11 (2H, s, CH2), 4.44 (2H, s, CH2), 5.94 (1H, s, CH), 7.13–7.31 (1H, m, ArH), 7.36–7.51 (5H, m, ArH), 7.75–7.89 (5H, m, ArH), 8.25 (1H, d, J = 6 Hz, ArH), 8.38 (2H, s, NH2), 10.62 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 25.6 (CH2S), 37.8 (CH), 51.4 (CH2N), 81.2 (C–C=O), 120.7 (C–NO2), 121.2, 121.9, 122.2, 123.7, 124.4, 127.1, 127.7, 128.0, 128.2, 128.6, 129.0, 130.1, 130.9, 131.3, 132.0, 136.9, 139.7, 141.4, 142.0 (Ar), 151.7 (C=C–S), 157.6 (C–NH2), 167.1, 167.8 (C=O).
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(4-fluorophenyl)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6g)
Dark orange solid; yield: 0.359 g (70%); mp: 290–293 °C; 1H NMR (300 MHz, DMSO-d6): δ 4.24–4.35 (2H, s, CH2), 4.41–4.46 (2H, s, CH2), 5.90 (1H, s, CH), 7.10–7.36 (4H, m, ArH), 7.40–7.49 (4H, m, ArH), 7.70–7.86 (4H, m, ArH), 8.34 (2H, s, NH2), 10.61 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 28.2 (CH2S), 36.8 (CH), 51.3 (CH2N), 81.7 (C–C=O), 115.3, 115.6 (Ar), 120.7 (C–NO2), 120.9, 121.9, 124.2, 125.5, 127.1, 128.0, 128.6, 128.8, 128.9, 130.1, 130.2, 130.3, 130.5, 131.3, 137.3, 139.7, 140.8, 141.3, 151.6 (Ar), 152.1 (C=C–S), 157.3 (C–NH2), 160.8 (C–F), 167.0 (C=O).
5-Amino-N′-(9H-fluoren-9-ylidene)-8-nitro-7-phenyl-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide (6h)
Brown solid; yield: 0.361 g (73%); mp: 243–246 °C; 1H NMR (300 MHz, DMSO-d6): δ 4.05–4.15 (2H, s, CH2), 4.54–4.56 (2H, s, CH2), 5.89 (1H, s, CH), 7.07 (1H, d, J = 6 Hz, ArH), 7.22–7.32 (4H, m, ArH), 7.37–7.48 (4H, m, ArH), 7.71–7.94 (4H, m, ArH), 8.36 (2H, s, NH2), 10.59 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 27.8 (CH2S), 37.9 (CH), 50.8 (CH2N), 81.3 (C–C=O), 119.7, 119.8 (Ar), 120.2 (C–NO2), 122.2, 125.0, 126.4, 127.0, 127.4, 127.6, 127.9, 128.4, 128.8, 130.8, 136.8, 138.1, 138.6, 139.2, 141.6, 151.0 (Ar), 151.4 (C=C–S), 156.8 (C–NH2), 160.4, 166.7 (C=O).
General procedure of the synthesis of 3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazide derivatives 6i–l
A mixture of ethanolamine (60 µL, 1 mmol), 1,1-bis(methylthio)-2-nitroethylene (0.165 g, 1 mmol) and 10 mL EtOH in a 50-mL flask was refluxed for 5 h. In another 50-mL flask, the stoichiometric mixture of 9-fluorenone (1 mmol, 0.180 g) and cyanoacetohydrazide (1 mmol, 0.99 g) in EtOH (10 ml) and AcOH (1 mmol) was refluxed for 5 h. After this time, TLC shows the consumption of the starting materials. At this point, aromatic aldehyde (1 mmol) and the first solution (ketene N,O-acetal) were added to the hydrazone mixture simultaneously. The progress of the reaction was monitored by TLC using ethyl acetate/n-hexane (1:1). After completion of the reaction, the mixture was cooled to room temperature and the solid product was filtered and washed with hot ethanol to obtain the products 6i–l.
5-Amino-N′-(9H-fluoren-9-ylidene)-8-nitro-7-(4-nitrophenyl)-3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazide (6i)
Brick red solid; yield: 0.434 g (83%); mp: 212–214 °C; IR (KBr) (νmax /cm−1): 3409, 1661, 1513, 1463, 1341, 1254, 1185, 732, 628; 1H NMR (300 MHz, DMSO-d6): δ 4.16–4.25 (2H, m, CH2), 4.87–4.95 (2H, m, CH2), 6.03 (1H, s, CH), 7.02–7.10 (2H, m, ArH), 7.31–7.36 (2H, m, ArH), 7.43 (1H, t, J = 6 Hz, ArH), 7.70–7.84 (5H, m, ArH), 8.23 (2H, d, J = 9 Hz, ArH), 8.30 (2H, s, NH2), 10.67 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 38.4 (CH), 44.3 (CH2N), 71.6 (CH2O), 80.5 (C–C=O), 108.7 (C–NO2), 120.7, 120.9, 121.9, 123.8, 127.1, 127.9, 128.6, 129.7, 130.2, 131.3, 137.2, 139.7, 141.4, 146.7, 150.4 (Ar), 152.9 (C=N), 153.1 (C=C–O), 157.1 (C–NH2), 166.7 (C=O); m/z (%) = 523 (0.04) [M-1]+, 509 (0.09), 493 (0.08), 435 (0.2), 355 (100), 327 (49), 280 (5), 254 (4), 205 (10), 180 (36), 151 (26), 126 (4), 64 (64), 44 (22).
5-Amino-7-(4-chlorophenyl)-N′-(9H-fluoren-9-ylidene)-8-nitro-3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazide (6j)
Orangish yellow solid; yield: 0.348 g (68%); mp: 217–220 °C; IR (KBr) (νmax /cm−1): 3411, 2924, 1711, 1617, 1510, 1443, 1187, 1094, 825, 726, 625; 1H NMR (300 MHz, DMSO-d6): δ 4.79–4.86 (2H, m, CH2), 5.08–5.15 (2H, m, CH2), 6.56 (1H, s, CH), 7.38–7.50 (4H, m, ArH), 7.60–7.92 (4H, m, ArH), 8.10 (4H, m, ArH), 8.41 (2H, s, NH2), 10.18 (1H, s, NH); m/z (%) = 512 (0.07) [M-1]+, 435 (1), 361 (2), 287 (6), 213 (19), 180 (86), 152 (34), 126 (7), 98 (31), 80 (100), 48 (67).
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(4-fluorophenyl)-8-nitro-3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazide (6k)
Brick red solid; yield: 0.323 g (65%); mp: 189–193 °C; 1H NMR (300 MHz, DMSO-d6): δ 4.14–4.22 (2H, m, CH2), 4.83–4.92 (2H, m, CH2), 6.56 (1H, s, CH), 7.38–7.57 (3H, m, ArH), 7.84–7.91 (4H, m, ArH), 7.90 (1H, d, J = 9 Hz, ArH), 8.16–8.18 (4H, m, ArH), 8.42 (2H, s, NH2), 10.52 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 37.4 (CH), 48.1 (CH2N), 71.3 (CH2O), 81.0 (C–C=O), 97.7, 109.7 (C–NO2), 116.9, 117.2, 121.0, 121.3, 122.6, 128.8, 128.9, 130.1, 130.5, 131.6, 132.5, 133.6, 133.7, 140.3, 141.3, 142.1 (Ar), 150.4 (C=N), 156.7 (C–NH2), 166.7 (C=O).
5-Amino-N′-(9H-fluoren-9-ylidene)-7-(3-methoxyphenyl)-8-nitro-3,7-dihydro-2H-oxazolo[3,2-a]pyridine-6-carbohydrazide (6l)
Brick red solid; yield: 0.356 g (70%); mp: 172–175 °C; 1H NMR (300 MHz, DMSO-d6): δ 3.84 (3H, s, OCH3), 4.80–4.91 (2H, m, CH2), 5.10–5.15 (2H, m, CH2), 6.56 (1H, s, CH), 7.22 (1H, d, J = 9 Hz, ArH), 7.35–7.67 (6H, m, ArH), 7.84–7.87 (3H, m, ArH), 7.93 (2H, d, J = 9 Hz, ArH), 8.39 (2H, s, NH2), 10.19 (1H, s, NH); 13C{1H} NMR (75.4 MHz, DMSO-d6): δ 55.8 (OCH3), 105.1 (C–NO2), 115.2, 116.5, 118.7, 120.5, 120.9, 122.2, 122.8, 128.3, 128.5, 130.4, 133.2, 139.9, 141.7 (Ar), 156.2 (C–NH2), 159.5 (CAr–OMe).
References
El-Hag Ali G, Khalil AK, Lamphon RQ, El-Maghraby AA (2005) Studies of thiazolopyridines, Part 6: Synthesis and antimicrobial evaluation of some novel thiazolo[3,2-a]pyridine and thiazolo[2′,3′:6,1]-pyrido[2,3-d]pyrimidine derivatives. Phosphor Sulfur Silicon Relat Elem 180:1909–1919
El-Maghraby AA, El-Hag Ali G, Ahmed AHA, El-Gaby MSA (2002) Studies on thiazolopyridines. Part 1: antimicrobial activity of some novel fluorinated thiazolo[3,2-a]pyridines and thiazolo[2′,3′:1,6]pyrido[2,3-d]pyrimidines. Phosphor Sulfur Silicon Relat Elem 177:293–302
Terzidis MAJ, Stephanatou S, Tsoleridis CA, Terzis A, Raptopoulou CP (2010) Expeditious one-pot synthesis of highly substituted thiazolo[3,2-a]pyridines involving chromones. Tetrahedron 66:947–954
Vadivelan S, Sinha BN, Irudayam SJ, Jagarlapudi SA (2007) Virtual screening studies to design potent CDK2-cyclin A inhibitors. J Chem Inf Model 47:1526–1533
Manhi FM, Soliman GA (1993) Studies with 2-thiazoline-4-one derivatives as potential uterus stimulants. Bull Fac Pharm 31:265–271
El-Gaby MS, Al-Sehemi AG, Mohamed YA, Ammar YA (2006) Recent trends in chemistry of thiazolopyridines. Phosphor Sulfur Silicon Relat Elem 181:631–674
Acheson RM (1963) Reactions of acetylenecarboxylic acids and their esters with nitrogen-containing heterocyclic compounds. Adv Heterocycl Chem 1:125–165
Acheson RM, Elmore NF (1978) Reactions of acetylenecarboxylic esters with nitrogen-containing heterocycles. Adv Heterocycl Chem 23:263–482
El-Hag Ali GAM, Khalil A, Ahmed AHA, El-Gaby MSA (2002) Studies on thiazolopyridines. Part 2. Synthesis and antimicrobial activity of novel thiazolo [3,2-a] pyridine and thiazolo [3,2-a][1,8]-naphthyridine derivatives. Acta Chim Slov 49:365–376
Park H, Hwang KY, Oh KH, Kim YH, Lee J, Kim YK (2008) Discovery of novel alpha-glucosidase inhibitors based on the virtual screening with the homology-modeled protein structure. Bioorg Med Chem 16:284–292
Aberg V, Sellstedt M, Hedenstro M, Pinkner JS, Hultgrenband JS, Almqvista R (2006) Design, synthesis and evaluation of peptidomimetics based on substituted bicyclic 2-pyridones-Targeting virulence of uropathogenic E. coli. Bioorg Med Chem 14:7563–7581
Deng JZ, McMasters DR, Rabbat PMA, Williams PD, Coburn CA, Yan Y (2005) Development of an oxazolopyridine series of dual thrombin/factor Xa inhibitors via structure-guided lead optimization. Bioorg Med Chem Lett 15:4411–4416
Park HR, Kim J, Kim T, Jo S, Yeom M, Moon B, Choo IH (2013) Oxazolopyridines and thiazolopyridines as monoamine oxidase B inhibitors for the treatment of Parkinson’s disease. Bioorg Med Chem 21:5480–5487
Bemis JE, Vu CB, Xie R, Nunes JJ, Ng PY, Disch JS, Milne JC (2009) Discovery of oxazolo[4,5-b]pyridines and related heterocyclic analogs as novel SIRT1 activators. Bioorg Med Chem Lett 19:2350–2353
Martin E, Moran A, Martin ML, Roman LS, Puebla P, Medarde M (2000) Antihypertensive activity of substituted 2,3,8,8a-tetrahydro-7H-oxazolo[3,2-a]pyridinedicarboxylate enantiomers. Bioorg Med Chem Lett 10:319–322
Yalcin A, Sener E (1993) QSARs of some novel antibacterial benzimidazoles, benzoxazoles and oxazolopyridines against an enteric gram-negative rod; K. pneumonia. Int J Pharm 98:1–8
Qi XY, Cao Y, Li YL, Mo MG, Zhou L, Ye DY (2017) Discovery of the selective sphingomyelin synthase 2 inhibitors with the novel structure of oxazolopyridine. Bioorg Med Chem Lett 27:3511–3515
Sunderhaus D, Martin SF (2009) Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem Eur J 15:1300–1308
Ryzhkova YE, Elinson MN, Maslov OI, Fakhrutdinov AN (2021) Multicomponent synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic acids in DMSO. Molecules 26:6839
Karami M, Hasaninejad A, Mahdavi H, Iraji A, Mojtabavi S, Faramarzi MA, Mahdavi M (2021) One-pot multi-component synthesis of novel chromeno[4,3-b]pyrrol-3-yl derivatives as alpha-glucosidase inhibitors. Mol Divers 25:1–13
Shaabani A, Mohammadian R, Afshari R, Hooshmand SE, Nazeri MT, Javanbakht S (2021) The status of isocyanide-based multi-component reactions in Iran (2010–2018). Mol Divers 25:1145–1210
Nicolaou C, Edmonds DJ, Bulger PG (2006) Cascade reactions in total synthesis. Angew Chem Int Ed 45:7134–7186
Dong S, Fu X, Xu X (2020) [3+2]-Cycloaddition of catalytically generated pyridinium ylide: a general access to indolizine derivatives. Asian J Org Chem 9:1133–1143
Yan SJ, Chen YL, Liu L, He NQ, Lin J (2010) Three-component solvent-free synthesis of highly substituted bicyclic pyridines containing a ring-junction nitrogen. Green Chem 12:2043–2052
Altug C, Burnett AK, Caner E, Dürüst Y, Elliott MC, Glanville RPJ, Gu C, Westwell AD (2011) An efficient one-pot multicomponent approach to 5-amino-7-aryl-8-nitrothiazolo[3,2-a]pyridines. Tetrahedron 67:9522–9528
Zou MM, Zhu FJ, Tian X, Ren LP, Shao XS, Li Z (2014) A novel and facile synthesis of 2-oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycles via heterocyclic ketene aminals and Konevenagel adducts formed by phthalic anhydride and ethyl cyanoacetate. Chin Chem Lett 25:1515–1519
Bayat M, Safari F, Nasri S, Hosseini FS (2019) A chemoselective synthesis and biological evaluation of novel benzo[g]thiazolo[3,2-a]quinolone derivatives. Monatsh Chem 150:703–710
Nasri S, Hosseini FS, Bayat M (2018) Solvent-controlled dehydration and diastereoselective formation of indenone-fused thiazolo[3,2-a]pyridines via a one-pot four-component reaction. Tetrahedron 74:4409–4417
Hosseini FS, Nasri S, Bayat M (2018) Simple synthesis of 5-amino-8-nitro-7-aryl-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carboxamide derivatives. J Sulfur Chem 39:622–632
Razavi Z, Bayat M, Hosseini H (2020) Synthesis of highly functionalized thiazolo[3,2-a]pyridine derivatives via a five-component cascade reaction based on nitroketene N, S-acetal. RSC Adv 10:31039–31048
Bayat M, Hosseini FS, Nasri S (2018) An efficient one-pot synthesis of tetrahydrothiazolo[3,2-a]quinolin-6-one derivatives. J Sulfur Chem 39:99–111
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rouzban, H., Bayat, M. & Hosseini, H. Efficient regioselective five-component synthesis of novel thiazolo[3,2-a]pyridine carbohydrazides and oxazolo[3,2-a]pyridine carbohydrazides. Mol Divers 27, 667–678 (2023). https://doi.org/10.1007/s11030-022-10446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10446-0