Abstract
A new class of multi-functional triazole hexahydroquinoline carbohydrazide named 2-amino-7,7-dimethyl-5-oxo-4-phenyl-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide has been synthesized by a novel multi-component process involving the reaction of dimedone, 3-amino-1,2,4-triazole, various benzaldehyde with cyanoacetohydrazide under mild conditions in the stoichiometric melt and chloroform in sequence. The simple one-pot process, straight product isolation without applying tedious purification procedures, progression of the reaction without using any catalyst, the application of diverse aldehydes causing a high molecular diversity, the existence of several nitrogen atoms in the product structure, and the possibility of creating multiple hydrogen bonding in the final compound are attractive specifications of the present strategy.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An important and valuable group among heterocyclic compounds comprising three nitrogen atoms within the five-membered cycle is identified as triazole with molecular formula C2H3N3 acts as isosteres of amide, ester, and carboxylic acid [1, 2]. 1,2,4-Triazole derived compounds are a significant category of heterocyclic products existent in a broad scope of pharmaceuticals and bioactive compounds applied in the drug discovery research against cancer cells, microbes, and different kinds of disease in the human body [3, 4]. The possible therapeutic usages and chemotherapeutical importance of 1,2,4-triazole include anti-cancer [5], antioxidant [6], antibiotic [7], antihypertensive [8], anti-HIV [9], anti-inflammatory [10], and anticonvulsant [11]. This motif is an integral part of a variety of drugs available in clinical therapy including fluconazole, cyproconazole, and triazolam, and also as the third generation of antifungal medicines such as difenoconazole represented in Fig. 1 which are actively used in the pharmacological area [12].
On the other hand, among the important azaheterocycles, quinoline and its derivatives are one of the most significant building blocks in various natural compounds and drugs that due to their diverse usages in the pharmaceutical fields dedicated a remarkable place compounds to themselves [13, 14]. Quinoline displays a library of pharmaceutically valuable products that has a broad range of biological activities of biological activities, including antipsychotic [15], antimalarial [16], antihypertensive [17], anti-inflammatory [18], anti-HIV [19], PDE4B inhibitors [20], and antibiotic activities [21]. Medicines possessing quinolone scaffold, such as mefloquine, and amodiaquine, are applied as useful medicines for the therapy of malaria, ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections, bosutinib is used for the treatment of chronic myelogenous leukemia, neratinib is a tyrosine kinase inhibitor anti-cancer drug applied for the therapy of breast cancer, belotecan is a drug used in chemotherapy, and also, lenvatinib is an anti-cancer medication for the therapy of specified types of thyroid cancer, that the structures of quinoline-based drugs are shown in Fig. 2 [22,23,24,25]. The existence of nitrogen atoms remarkably enhances the fundamental property of products containing quinoline scaffold. In addition, the nitrogen in the quinoline structure may be connected to the target enzymes via hydrogen bonding. An additional important feature of quinoline is the polarity which can cause the reduction in the lipophilic nature, enhancing solubility in water, and subsequently better oral absorption which is required in medication design approaches [26]. In addition, 5-oxo-1,4,5,6,7,8-hexahyroquinolines (5-oxo-HHQs) are a class of heterocycles that have been considered by researchers because of their wide biological and pharmaceutical significance [27,28,29].
Designing new methods for the synthesis of new 5-oxo-HHQ and developing valuable remedial compounds bearing the 5-oxo-HHQ moiety will be useful for pharmaceutical chemists in the area of medication exploration. Accordingly, in the past years, multi-component reactions (MCRs) especially containing aldehydes, activated nitriles and enamines, have been designed for the synthesis of various quinoline and 5-oxo-HHQ compounds. For example, Abdelhamid and coworkers have reported an efficient formation of 1-(4H-1,2,4-triazol-3-yl)hexahydroquinoline-3-carbonitrile products via the condensation between dimedone, 3-amino-4H-1,2,4-triazole, and arylidenemalononitriles under reflux conditions in alcohol for 3 h. In the first step, N-(1,2,4-triazol-3-yl)enamine was created via the condensation of dimedone, and 3-amino-4H-1,2,4-triazole, in the presence of trichloroacetic acid (TCAA) as a catalyst without using a solvent which was used as an β-enaminone intermediate in the next step [30].
Due to the increasing request for convenient and rapid synthesis of bioactive heterocycles, the study of effective MCRs as a simple and potent protocol for the formation of diverse multi-substituted heterocyclic compounds is increasing. Furthermore, the MCR is introduced as an influential and green approach to the synthesis of different biologically active heterocyclic compounds and drug-like motifs from simple preliminary materials that led to eliminating the multiple steps and expensive purification process of the reaction, increasing the productivity, saving energy, and reducing the reaction time [31,32,33,34,35,36].
on the other hand, one of the main protocols for the construction of azaheterocycles is the design of the reactions based on 3-amino-1,2,4-triazole as a beneficial mono-, bi-, and polynucleophile substance with diverse electrophiles in one-pot MCRs. 3-Amino-1,2,4-triazoles contain multiple alternative reaction sites, so these compounds are effective synthetic substrates in controlled multidirectional reactions, and it is possible to synthesize a high molecular diversity of heterocyclic compounds [37, 38].
Results and discussion
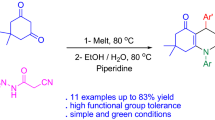
Considering potential medicinal applications of hybrid molecules containing triazole and quinoline moieties, and with regard to our interest in the synthesis of valuable biologically active compounds [39, 40], we described a one-pot four-component reaction of dimedone 1 and 3-amino-1,2,4-triazole 2 carries out under solvent-free conditions, at 120 °C to generate the enaminone intermediate 6 in high yields during 15–20 min. In the following, the addition of various benzaldehydes 3 and cyanoacetohydrazide 4 as valuable synthetic unit in chloroform at room temperature condition successfully gave the multi-functional triazole hexahydroquinoline carbohydrazide 5 as attractive synthetic targets, and the structure of final products was proved by spectroscopic data (Scheme 1). The products were obtained in good yields, with a simple workup technique through straightforward filtration. The synthesized derivatives are new hybrid molecules containing triazole and hexahydroquinoline moieties that it is possible to inherit biological activities of both triazole and hexahydroquinoline moieties.
The acceptable pathway for the synthesis of expected product 5 is illustrated in Scheme 2. Based on 1H NMR spectrum data of intermediate 6, it is logical that at first, the reaction involves the initial generation of enaminone 6 via the reaction of dimedone 1 with 3-amino-1,2,4-triazole 2. Next, the Knoevenagel condensation between benzaldehyde 3 and cyanoacetohydrazide 4 as an active methylene-containing compound leads to Michael acceptor 7. Then the Michael addition between enaminone 6 and Knoevenagel intermediate 7 obtain open-chain intermediate 8, which creates intermediate 9 after sequential imine-enamine tautomerization. This intermediate is transformed into 10 via intramolecular N-cyclization, and then imine-enamine tautomerization gives the desired product 5.
As displayed in Fig. 3, we surveyed the structural variety of this reaction by applying aryl aldehydes 3 with various donor and acceptor substituents in produce products 5a-l. Compounds named 2-amino-7,7-dimethyl-5-oxo-4-phenyl-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide were acquired in moderate to high yields (60–90%) with relatively long reaction time (10–24 h). We also applied aliphatic aldehydes including pyruvic aldehyde and heterocyclic aldehydes such as indole-3-carboxaldehyde to enhance the structural diversity variety of the products, but the process did not proceed. The derivatives in Fig. 3 indicated that aromatic aldehydes carrying electron-withdrawing and one or two electron-donating groups showed reactivity and were involved in the reaction.
The structures of the derivatives were confirmed by their elemental analyses, IR, 1H and 13C NMR spectra. The mass spectra of 5b demonstrated molecular ion peaks at proper m/z values. The 1H NMR spectrum of 5b in DMSO-d6 displayed one singlet (δ 0.98 ppm) for the two methyl groups, two singlet for the two methylene groups (δ 2.03 and 2.34 ppm), a singlet (δ 3.77 ppm) for methoxy, a singlet (δ 3.80 ppm) for methine proton, one singlet for the N–NH2 protons (δ 4.20 ppm, D2O exchangeable), along with special signals for the aromatic moiety (δ 6.96 and 7.61 ppm), one singlet (δ 7.93 ppm) for CH proton of triazole, and three singlet signals for the two NH and one NH2 groups (δ 9.55, 11.66, 13.66 ppm, D2O exchangeable). The 1H decoupled 13C NMR spectrum of 5b displayed 18 separate signals according to the suggested structure. The particular signal of methine carbon was appeared at δ 32.8 ppm and the specific peaks of C=C–NH2, N=CH and N–C=N were assigned at δ 79.6, 159.1 and 161.3 ppm which verified the selective synthesized of 5a. Other signals of the product 5b exhibited characteristic resonances with appropriate chemical shifts.
Conclusion
In the following of introducing novel and efficient protocols to access new scaffolds containing 1,2,4-triazole, which would be very beneficial for the finding of novel therapeutic candidates, we described a new four-component reaction between dimedone, 3-amino-1,2,4-triazole, various benzaldehyde, and cyanoacetohydrazide, for the one-pot synthesis of multi-functional triazole hexahydroquinoline carbohydrazide through sequential enaminone formation/ Knoevenagel condensation/Michael addition/enol-keto tautomerism /intramolecular N-cyclization sequences in chloroform without using any catalyst. The synthesized derivatives are new hybrid molecules containing triazole and hexahydroquinoline moieties that may inherit the biological activities of both triazole and hexahydroquinoline scaffolds.
Experimental
General
The dimedone, 3-amino-1,2,4-triazole, different benzaldehydes, cyanoacetohydrazide and solvents were obtained from Sigma Aldrich and Fluka Co. used without further purification. IR spectra: Bruker Tensor 27 spectrometer. NMR spectra: Bruker DRX-300 Avance instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 as solvents. Chemical shifts are expressed in parts per million (ppm), and coupling constant (J) are reported in hertz (Hz). Mass spectra: Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. Elemental analyses were performed using a PerkinElmer 2004 series [II] CHN elemental analyzer.
General procedure for the formation of 5a-l
The stoichiometric mixtures of dimedone 1 (1.0 mmol, 0.140 g) with 3-amino-1,2,4-triazole 2 (1.0 mmol, 0.084 g) were melted at 120 °C for 15–20 min. Then the reaction mixture was cooled to room temperature and chloroform (5 mL) was added, and the solution was stirred for 10 min at room temperature. Next, benzaldehyde 3 (1.0 mmol) and cyanoacetohydrazide 4 (1.0 mmol, 0.099 g) were added, respectively, and the solution was stirred at room temperature for the time given in Fig. 2. The progress of the reaction was monitored by TLC using ethyl acetate/n-hexane (1:1). After completion of the reaction, the precipitated product was filtered off and washed on the filter funnel with a small amount of chloroform to give pure products 5a-l.
2-Amino-7,7-dimethyl-5-oxo-4-phenyl-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5a)
Yellow solid, m.p.: 201–203 °C, yield 0.322 g (82%); IR (KBr) (νmax /cm−1): 3279, 3048 (NH, NH2), 2867 (C–H), 1694 (C=O), 1588, 1536 (C=C), 1391 (C–N), 1198 (C–O), 753 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.48 (2H, s, CH2), 3.80 (1H, s, CH), 4.19 (2H, s, N–NH2), 7.40–7.43 (3H, m, ArH), 7.66–7.70 (2H, m, ArH), 7.96 (1H, s,=CH), 9.55 (1H, s, O=C–NH), 11.79 (2H, s, NH2), 13.65 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 79.7 (C=C–NH2), 102.7 (O=C–C=C), 116.6, 127.7, 129.3, 130.8 (Ar), 134.2 (O=C–C=C), 144.8 (C-NH2), 157.0 (N=CH), 159.4 (N–C=N), 165.3 (NH–C=O), 197.3 (C=O). Anal. Calcd for C20H23N7O2 (393.19): C, 61.05; H, 5.89; N, 24.92. Found C, 61.84; H, 6.09; N, 25.23.
2-Amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5b)
Yellow solid, m.p.: 203–204 °C, yield 0.380 g (90%); IR (KBr) (νmax /cm−1): 3279, 3215, 3075 (NH, NH2), 2876 (C–H), 1678 (C=O), 1591, 1533 (C=C), 1380, 1258 (C–N), 1185 (C–O), 691 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.34 (2H, s, CH2), 3.77 (3H, s, OCH3), 3.80 (1H, s, CH), 4.20 (2H, s, N–NH2), 6.96 (2H, d, 3JHH = 8.4 Hz, ArH), 7.61 (2H, d, 3JHH = 8.4 Hz, ArH), 7.93 (1H, s, =CH), 9.55 (1H, s, O=C–NH), 11.66 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 55.8 (OCH3), 79.6 (C=C–NH2), 102.7 (O=C–C=C), 114.8, 116.6, 126.8, 129.3 (Ar), 144.7 (O=C–C=C), 156.9 (C–NH2), 159.1 (N=CH), 161.3 (N–C=N), 165.0 (NH–C=O), 197.2 (C=O). MS (EI, 70 eV): m/z (%) = 423 (0.1) [M]+, 217 (28), 133 (100), 77 (15), 51 (9).
2-amino-4-(3-methoxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5c)
Yellow solid, m.p.: 204–205 °C, yield 0.342 g (81%); IR (KBr) (νmax /cm−1): 3280, 3213, 3049 (NH, NH2), 2873 (C–H), 1689 (C=O), 1589, 1536 (C=C), 1369, 1270 (C–N), 1188 (C–O), 731 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.37 (2H, s, CH2), 3.77 (3H, s, OCH3), 3.80 (1H, s, CH), 4.20 (2H, s, N-NH2), 6.97 (2H, d, 3JHH = 7.5 Hz, ArH), 7.25 (1H, s, ArH), 7.24–7.34 (2H, m, ArH), 7.95 (1H, s,=CH), 9.55 (1H, s, O=C–NH), 11.80 (2H, s, NH2), 13.65 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 55.6 (OCH3), 79.7 (C=C–NH2), 102.7 (O=C–C=C), 112.0, 116.6, 120.3, 120.5, 130.35 (Ar), 135.7 (O=C–C=C), 144.6 (C–NH2), 156.9 (N=CH), 160.0 (N–C=N), 165.4 (NH–C=O), 197.3 (C=O). MS (EI, 70 eV): m/z (%) = 423 (0.05) [M]+, 217 (39), 133 (100), 103 (15), 77 (17).
2-Amino-4-(2-chlorophenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5d)
Yellow Solid, m.p.: 205–206 °C, yield 0.362 g (85%); 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.02 (2H, s, CH2), 2.37 (2H, s, CH2), 3.83 (1H, s, CH), 4.22 (2H, s, N–NH2), 7.40–7.47 (3H, s, ArH), 7.97 (1H, d, 3JHH = 7.5 Hz, ArH), 8.37 (1H, s,=CH), 9.56 (1H, s, O=C–NH), 11.96 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.9 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 79.6 (C=C–NH2), 102.7 (O=C–C=C), 116.5, 127.5, 128.0, 130.4, 131.5, 132.0 (Ar), 140.8 (O=C–C=C), 144.1 (C-NH2), 156.9 (N=CH), 159.6 (N–C=N), 165.5 (NH–C=O), 197.3 (C=O). MS (EI, 70 eV): m/z (%) = 429 (0.1) [M + 2]+, 427 (0.3) [M]+, 293 (100), 191 (56), 150 (81), 122 (99), 84 (45).
2-Amino-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5e)
Yellow solid, m.p.: 200–202 °C, yield 0.311 g (73%); 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.39 (2H, s, CH2), 3.80 (1H, s, CH), 4.20 (2H, s, N–NH2, D2O exchangeable), 7.49 (2H, d, 3JHH = 8.4 Hz, ArH), 7.70 (2H, d, 3JHH = 8.7 Hz, ArH), 7.98 (1H, s,=CH), 9.56 (1H, s, O=C–NH, D2O exchangeable), 11.79 (2H, s, NH2, D2O exchangeable), 13.65 (1H, br s, NH, D2O exchangeable). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 102.7 (O=C–C=C), 116.5, 129.1, 129.3, 133.2 (Ar), 135.1 (O=C–C=C), 143.5 (C–NH2), 156.4 (N–C=N), 165.4 (NH–C=O), 197.2 (C=O). MS (EI, 70 eV): m/z (%) = 428 (1) [M + 1]+, 427 (3) [M]+, 216 (27), 165 (34), 104 (74), 86 (100), 75 (40), 45 (59).
2-Amino-4-(4-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5f)
Yellow solid, m.p.: 196–200 °C, yield 0.265 g (65%); 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.38 (2H, s, CH2), 3.76 (1H, s, CH), 4.13 (2H, s, N–NH2), 6.80 (2H, d, 3JHH = 8.4 Hz, ArH), 7.49 (2H, d, 3JHH = 8.1 Hz, ArH), 7.88 (1H, s,=CH), 9.56 (1H, s, O=C–NH), 9.95 (1H, br s, OH), 11.59 (2H, s, NH2), 13.67 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.7 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 102.7 (O=C–C=C), 116.2, 125.2, 129.5, 143.2 (Ar), 145.1 (O=C–C=C), 148.5 (C-NH2), 156.9 (N=CH), 159.1 (N–C=N), 164.8 (NH–C=O), 197.3 (C=O). MS (EI, 70 eV): m/z (%) = 409 (0.5) [M]+, 328 (14), 247 (75), 191 (25), 163 (100), 122 (34).
2-Amino-4-(3-fluorophenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5 g)
Yellow solid, m.p.: 208–209 °C, yield 0.246 g (60%); IR (KBr) (νmax /cm−1): 3280, 3213, 3045 (NH, NH2), 2871 (C–H), 1634 (C=O), 1586, 1537 (C=C), 1385, 1248 (C–N), 1147 (C–O), 785 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.39 (2H, s, CH2), 3.76 (1H, s, CH), 4.22 (2H, s, N–NH2), 7.23 (1H, d, 3JHH = 7.5 Hz, ArH), 7.51 (1H, s, ArH), 7.41–7.57 (2H, m, ArH), 8.00 (1H, s,=CH), 9.55 (1H, s, O=C–NH), 11.90 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 102.7 (O=C–C=C), 113.1, 113.4 (Ar, d, 2JCF = 22.5 Hz), 116.6 (Ar),117.2, 117.5 (Ar, d, 2JCF = 21.7 Hz), 124.1, 131.2 (Ar), 136.8 (O=C–C=C), 143.4 (C–NH2), 156.9 (N=CH), 159.6 (N–C=N), 161.2, 164.5 (Ar, d, 1JCF = 243 Hz), 165.5 (NH–C=O), 197.3 (C=O).
2-Amino-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5 h)
Yellow solid, m.p.: 207–209 °C, yield 0.254 g (62%); IR (KBr) (νmax /cm−1): 3222, 3083 (NH, NH2), 2873 (C-H), 1682 (C=O), 1599, 1532 (C=C), 1278 (C–N), 1152 (C–O), 691 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.02 (2H, s, CH2), 2.36 (2H, s, CH2), 3.80 (1H, s, CH), 4.19 (2H, s, N–NH2), 7.25 (2H, t, ArH), 7.74 (2H, t, ArH), 7.98 (1H, s,=CH), 9.56 (1H, s, O=C–NH), 11.80 (2H, s, NH2), 13.67 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 102.7 (O=C–C=C), 116.2, 116.5 (Ar, d, 2JCF = 22.5 Hz), 129.7, 129.8, 130.9 (Ar), 143.7 (O=C–C=C), 147.1 (C-NH2), 156.9 (N–C=N), 159.4, 162.46 (Ar, d, 1JCF = 227 Hz), 165.3 (NH–C=O), 197.3 (C=O).
2-Amino-4-(3,4-dimethoxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5i)
Yellow solid, m.p.: 196–200 °C, yield 0.403 g (89%); 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.48 (2H, s, CH2), 3.76 (6H, s, OCH3), 3.80 (1H, s, CH), 4.19 (2H, s, N-NH2), 6.96 (1H, d, 3JHH = 8.4 Hz, ArH), 7.14 (1H, d, 3JHH = 8.4 Hz, ArH), 7.31 (1H, s, Ar), 7.90 (1H, s,= CH), 9.56 (1H, s, O=C–NH), 11.67 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 55.9 (OCH3), 79.6 (C=C–NH2), 102.7 (O=C–C=C), 109.1, 111.9, 116.3, 122.0, 126.9 (Ar), 144.9 (O=C–C=C), 148.4 (C-NH2), 151.2 (Ar), 156.9 (N=CH), 159.1 (N–C=N), 165.0 (NH-C = O), 197.3 (C=O). MS (EI, 70 eV): m/z (%) = 453 (0.5) [M]+, 203 (23), 150 (30), 119 (100), 95 (13).
2-Amino-4-(3-chlorophenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5j)
Yellow solid, m.p.: 205–207 °C, yield 0.350 g (82%); 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.37 (2H, s, CH2), 3.81 (1H, s, CH), 4.24 (2H, s, N-NH2), 7.43 (1H, s, ArH), 7.50–7.79 (3H, m, ArH), 7.97 (1H, s,=CH), 9.56 (1H, s, O=C–NH), 11.90 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.9 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 80.0 (C=C–NH2), 102.7 (O=C–C=C), 116.5, 126.5, 126.6, 130.2, 131.1, 134.1 (Ar), 136.5 (O=C–C=C), 143.2 (C-NH2), 156.9 (N–C=N), 165.6 (NH–C=O), 197.2 (C=O). MS (EI, 70 eV): m/z (%) = 427 (0.5) [M]+, 206 (56), 191 (57), 150 (83), 122 (100), 95 (35).
2-Amino-4-(5-bromo-2-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5 k)
Yellow solid, m.p.: 204–206 °C, yield 0.355 g (73%); 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.37 (2H, s, CH2), 3.81 (1H, s, CH), 4.22 (2H, s, N-NH2), 6.86 (1H, d, 3JHH = 6.9 Hz, ArH), 7.36 (1H, d, 3JHH = 6.9 Hz, ArH), 7.74 (1H, s, Ar), 8.30 (1H, s,=CH), 9.55 (1H, s, O=C–NH), 10.40 (OH), 11.77 (2H, s, NH2), 13.66 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.9 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 102.7 (O=C–C=C), 11.3, 116.6, 118.8, 119.1, 122.8, 128.3, 134.1 (Ar), 140.0 (O=C–C=C), 145.4 (C-NH2), 156.1 (N=CH), 159.0 (N–C=N), 165.3 (NH–C=O), 197.3 (C=O).
2-Amino-4-(2-hydroxy-3-methoxyphenyl)-7,7-dimethyl-5-oxo-1-(4H-1,2,4-triazol-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbohydrazide (5 l)
Yellow solid, m.p.: 207–209 °C, yield 0.351 g (80%); 1H NMR (300 MHz, DMSO-d6): δ 0.97 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.37 (2H, s, CH2), 3.76 (3H, s, OCH3), 3.82 (1H, s, CH), 4.16 (2H, s, N-NH2), 6.78–6.84 (1H, m, ArH), 6.96 (1H, d, 3JHH = 8.4 Hz, ArH), 7.30 (1H, d, 3JHH = 7.8 Hz, ArH), 8.35 (1H, s,=CH), 9.31 (1H, br s, OH), 9.56 (1H, s, O=C–NH), 11.72 (2H, s, NH2), 13.68 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 24.8 (C), 28.4 (2CH3), 32.8 (CH), 41.9 (CH2), 50.5 (CH2), 56.3 (OCH3), 102.7 (O=C–C=C), 113.5, 116.1, 118.0, 119.3, 120.6, 142.0, (Ar), 146.5 (O=C–C=C), 148.4 (C-NH2), 157.0 (N=CH), 159.3 (N–C=N), 165.0 (NH–C=O), 197.3 (C=O).
3-((1H-1,2,4-triazol-5-yl)amino)-5,5-dimethylcyclohex-2-en-1-one (6)
White solid, 1H NMR (300 MHz, DMSO-d6): δ 0.98 (6H, s, 2CH3), 2.03 (2H, s, CH2), 2.40 (2H, s, CH2), 6.24 (1H, s=CH), 8.38 (1H, s, N=CH), 9.56 (1H, br s, NH), 13.65 (1H, br s, NH).
(E)-3-(4-chlorophenyl)-2-cyanoacrylohydrazide (7)
Light yellow solid, 1H NMR (300 MHz, DMSO-d6): δ 4.31 (2H, s, NH2), 7.47 (2H, d, 3JHH = 7.2 Hz, ArH), 7.71 (2H, d, 3JHH = 7.5 Hz, ArH), 7.97 (1H, s=CH), 11.83 (1H, s, NH).
References
Sharma V, Shrivastava B, Bhatia R, Bachwani M, Khandelwal R, Ameta J (2011) Exploring potential of 1,2,4-triazole: a brief review. Pharmacol 1:1192–1222
Aggarwal R, Sumran G (2020) An insight on medicinal attributes of 1, 2, 4-triazoles. Eur J Med Chem 205:112652
Peyton LR, Gallagher S, Hashemzadeh M (2015) Triazole antifungals: a review. Drugs Today 51:705–718
Banerjee S, Ganguly S, Sen KK, India BWB (2013) A review on 1,2,4-triazoles. J Adv Pharm Educ Res 3:102–116
Holla BS, Veerendra B, Shivananda MK, Poojary B (2003) Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 38:759–767
Pokuri S, Singla RK, Bhat VG, Shenoy GG (2014) Insights on the antioxidant potential of 1, 2, 4-triazoles: synthesis, screening & QSAR studies. Curr Drug Metab 15:389–397
Eswaran S, Adhikari AV, Shetty NS (2009) Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur J Med Chem 44:4637–4647
Ali KA, Ragab EA, Farghaly TA, Abdalla MM (2011) Synthesis of new functionalized 3 subsitituted[1,2,4]triazolo[4,3-a]pyrimidine dreivatives: Potential antihypertensive agents. Acta Pol Pharm 68:237–247
Küçükgüzel İ, Tatar E, Küçükgüzel ŞG, Rollas S, De Clercq E (2008) Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy) methyl]-4-alkyl/aryl-2, 4-dihydro-3H-1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur J Med Chem 43:381–392
Palaska E, Şahin G, Kelicen P, Durlu NT, Altinok G (2002) Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 57:101–107
Kaproń B, Czarnomysy R, Wysokiński M, Andrys R, Musilek K, Angeli A, Plech T (2020) 1,2,4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. J Enzyme Inhib Med Chem 35:993–1002
Zhou CH, Wang Y (2012) Recent researches in triazole compounds as medicinal drugs. Curr Med Chem 19:239–280
Jin TS, Yin Y, Liu LB, Li TS (2006) Solid state synthesis of 5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives without using solvent and catalyst. ARKIVOC 14:28–34
Dey S, Basak P, Ghosh P (2020) A green synthetic approach towards one pot multi component synthesis of hexahydroquinoline and 9-arylhexahydroacridine-1,8-dione derivatives catalyzed by sulphonated rice husk. ChemistrySelect 5:15209–15217
Zajdel P, Partyka A, Marciniec K, Bojarski AJ, Pawlowski M, Wesolowska A (2014) Quinoline-and isoquinoline-sulfonamide analogs of aripiprazole: novel antipsychotic agents? Future Med Chem 6:57–75
Vanaerschot M, Lucantoni L, Li T, Combrinck JM, Ruecker A, Kumar TR, Fidock DA (2017) Hexahydroquinolines are antimalarial candidates with potent blood-stage and transmission-blocking activity. Nat Microbiol 2:1403–1414
Muruganantham N, Sivakumar R, Anbalagan N, Gunasekaran V, Leonard JT (2004) Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol Pharm Bull 27:1683–1687
El-Feky SA, Abd El-Samii ZK, Osman NA, Lashine J, Kamel MA, Thabet HK (2015) Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg Chem 58:104–116
Bénard C, Zouhiri F, Normand-Bayle M, Danet M, Desmaële D, Leh H, d’Angelo J (2004) Linker-modified quinoline derivatives targeting HIV-1 integrase: synthesis and biological activity. Bioorg Med Chem Lett 14:2473–2476
Pal S, Durgadas S, Nallapati SB, Mukkanti K, Kapavarapu R, Meda CLT, Pal M (2011) Novel 1-alkynyl substituted 1, 2-dihydroquinoline derivatives from nimesulide (and their 2-oxo analogues): A new strategy to identify inhibitors of PDE4B. Bioorg Med Chem Lett 21:6573–6576
Thomas KD, Adhikari AV, Telkar S, Chowdhury IH, Mahmood R, Pal NK, Sumesh E (2011) Design, synthesis and docking studies of new quinoline-3-carbohydrazide derivatives as antitubercular agents. Eur J Med Chem 46:5283–5292
Kaur K, Jain M, Reddy RP, Jain R (2010) Quinolines and structurally related heterocycles as antimalarials. Eur J Med Chem 45:3245–3264
Jain S, Chandra V, Jain PK, Pathak K, Pathak D, Vaidya A (2019) Comprehensive review on current developments of quinoline-based anticancer agents. Arabian J Chem 12:4920–4946
Latha DS, Yaragorla S (2020) C(sp3)-H Functionalization of 2-methyl azaarenes: highly facile approach to aza-heterocyclic compounds. Eur J Org Chem 2020:2155–2179
Mohamed MF, Abuo-Rahma GEDA (2020) Molecular targets and anticancer activity of quinoline–chalcone hybrids: literature review. RSC Adv 10:31139–31155
Aramburu L, Puebla P, Caballero E, González M, Vicente A, Medarde M, Peláez R (2016) Pyridine based antitumour compounds acting at the colchicine site. Curr Med Chem 23:1100–1130
Ranjbar S, Edraki N, Firuzi O, Khoshneviszadeh M, Miri R (2019) 5-Oxo-hexahydroquinoline: An attractive scaffold with diverse biological activities. Mol Divers 23:471–508
Shahraki O, Edraki N, Khoshneviszadeh M, Zargari F, Ranjbar S, Saso L, Miri R (2017) Novel 5-oxo-hexahydroquinoline derivatives: design, synthesis, in vitro P-glycoprotein-mediated multidrug resistance reversal profile and molecular dynamics simulation study. Drug Des Dev Ther 11:407
Kalaria PN, Satasia SP, Raval DK (2014) Synthesis, characterization and pharmacological screening of some novel 5-imidazopyrazole incorporated polyhydroquinoline derivatives. Eur J Med Chem 78:207–216
Ghozlan SA, Abdelmoniem DM, Mady MF, Abdelmoniem AM, Abdelhamid IA (2016) An efficient synthesis of 1-(4H–1,2,4-Triazol-3-yl)-hexahydroquinoline-3-carbonitrile and their spiro derivatives from β-enaminones. Heterocycles 92:637–648
Chang SQ, Zou X, Gong Y, He XW, Liu XL, Zhou Y (2019) Stereocontrolled construction of six vicinal stereogenic centers on a hexahydroxanthone framework through a formal [2+1+3] annulation. Chem Commun 55:14003–14006
Zhang M, He XW, Xiong Y, Zuo X, Zhou W, Liu XL (2021) Asymmetric construction of six vicinal stereogenic centers on hexahydroxanthones via organocatalytic one-pot reactions. Chem Commun 57:6764–6767
Miao HJ, Wang LL, Han HB, Zhao YD, Wang QL, Bu ZW (2020) Regio-and diastereoselective dearomatizations of N-alkyl activated azaarenes: the maximization of the reactive sites. Chem Sci 11:1418–1424
Bai XG, Miao HJ, Zhao Y, Wang QL, Bu ZW (2020) Regioselective and diastereoselective dearomative multifunctionalization of in-situ-activated azaarenes: an access to bridged azaheterocycles. Org Lett 22:5068–5073
Miao H, Bai X, Wang L, Yu J, Bu Z, Wang Q (2021) Diastereoselective construction of cage-like and bridged azaheterocycles through dearomative maximization of the reactive sites of azaarenes. Org Chem Front 8:204–211
Guo J, Miao H, Zhao Y, Bai X, Zhu Y, Wang Q, Bu Z (2019) An unexpected multi-component one-pot cascade reaction to access furanobenzodihydropyran-fused polycyclic heterocycles. Chem Commun 55:5207–5210
Murlykina MV, Morozova AD, Zviagin IM, Sakhno YI, Desenko SM, Chebanov VA (2018) Aminoazole-based diversity-oriented synthesis of heterocycles. Front Chem 6:527
Nasri S, Bayat M, Kochia K (2021) Strategies for synthesis of 1,2,4-triazole-containing scaffolds using 3-amino-1,2,4-triazole. Mol Divers. https://doi.org/10.1007/s11030-021-10197-4
Safari F, Bayat M, Nasri S, Karami S (2020) Synthesis and evaluation of anti-tumor activity of novel triazolo[1,5-a]pyrimidine on cancer cells by induction of cellular apoptosis and inhibition of epithelial-to-mesenchymal transition process. Bioorg Med Chem Lett 30:127111
Bayat M, Safari F, Nasri S, Hosseini FS (2019) A chemoselective synthesis and biological evaluation of novel benzo[g]thiazolo[3,2-a]quinolone derivatives. Monatsh Chem 150:703–710
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amorzesh, H., Bayat, M. & Nasri, S. Catalyst-free synthesis of highly functionalized triazole hexahydroquinoline carbohydrazide scaffolds via four-component cyclocondensation reaction. Mol Divers 28, 51–60 (2024). https://doi.org/10.1007/s11030-022-10592-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10592-5