Abstract
MgCl2 has been reported to be a versatile reagent especially as a Lewis acid catalyst in a variety of organic transformations including the preparation of heterocyclic compounds, the protection of functional groups and condensation reaction. Also the use of MgCl2 in the preparation of metallic magnesium and the application of magnesium chloride in biochemistry such as anesthetic for cephalopods, the separation of serum high-density lipoprotein, effect of MgCl2 on rabbit bronchial smooth muscle, antimicrobial properties of magnesium chloride and effect of MgCl2 on the quality of life for patients with fibromyalgia have been reported. Therefore, in this article the use of MgCl2 in organic chemistry and biochemistry is reviewed.
Graphical abstract
MgCl2 has been reported to be a versatile reagent especially as a Lewis acid catalyst in a variety of organic transformations including the preparation of heterocyclic compounds, the protection of functional groups and condensation reaction. Also the use of MgCl2 in the preparation of metallic magnesium and the application of magnesium chloride in biochemistry such as anesthetic for cephalopods, the separation of serum high-density lipoprotein, effect of MgCl2 on rabbit bronchial smooth muscle, antimicrobial properties of magnesium chloride and effect of MgCl2 on the quality of life for patients with fibromyalgia have been reported. Therefore, in this article the use of MgCl2 in organic chemistry and biochemistry is reviewed.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium chloride, MgCl2, is an inorganic compound including one magnesium and two chloride ions. Magnesium chloride and its variety of hydrate forms, MgCl2·(H2O)x, are dehydrated at higher temperatures [1]. Magnesium chloride salts are highly soluble in water and utilized in medicine as a source of magnesium ions, which are necessary for many cellular activities. Magnesium chloride has also been used as a cathartic and in alloys. Anhydrous MgCl2 is the main source to magnesium metal, which is manufactured on a large scale. Hydrated MgCl2 is the most compound available. Magnesium chloride is utilized for low-temperature deicing of highways, sidewalks and parking lots. When highways are treacherous owing to icy conditions, magnesium chloride helps to prevent the ice bond, allowing snow plows to clear the roads efficiently. Magnesium chloride is employed in nutraceutical and pharmaceutical preparations. It is also an additive in baby formula milk [2].
Also, anhydrous MgCl2 is a low-power Lewis acid. Ziegler–Natta catalysts, used commercially to produce poly olefins, contain MgCl2 as catalyst support [3]. The use of MgCl2 supports raises the activity of traditional catalysts and develops highly stereospecific catalysts for the synthesis of polypropylene [4]. In this communications, herein we interest to review the synthesis and the use of MgCl2 in organic chemistry and biochemistry.
The preparation of magnesium chloride, MgCl2
The hydrated magnesium chloride can be obtained from brine or seawater. In the Dow process, MgCl2 is prepared from magnesium hydroxide using hydrochloric acid (Scheme 1) [5]. It can also be manufactured from magnesium carbonate by a similar procedure.
Hydrated magnesium chloride can be dehydrated via some procedures by heating, and it is impossible to completely dehydrate magnesium chloride by heating in the air due to hydrolytic decomposition. The dehydration should be taken place in hydrochloric acid gas atmosphere accordingly. However, this procedure suffers from many problems, such as hydrochloric acid gas storage, corrosive nature and the use of plenty of hydrochloric acid gas. Many methods have been reported for the synthesis of anhydrous magnesium chloride such as using NH4MgCl3·6H2O (Scheme 2). In this route is reported that anhydrous magnesium chloride is manufactured from NH4MgCl3.6H2O at 50-400 °C in 30 min in air atmosphere [6].
Another method for the preparation of magnesium chloride is the chlorination of the dolomite and obtaining of MgCl2 in titanium sponge production. Osaka Titanium Technologies Co., Ltd (OTC) began manufacturing titanium sponge by the Kroll process in 1952. In this procedure, titanium ore and coke were fed into the chlorinator. Then, chlorine gas was blown into the chlorinator (Scheme 3).
Titanium ore was gotten rid of its oxygen and banded with the chlorine to give crude titanium tetrachloride. Then, the crude TiCl4 was distilled to remove impurities. The highly purified TiCl4 was furnished from ore that contained impurities. The purity standard of pure TiCl4 is 5N (99.999%) grade; therefore, a large amount of impurities is removed in this process. Then molten magnesium is fed into the vessel, and subsequently, liquid titanium tetrachloride is fed into the vessel and a reduction reaction is caused to obtain titanium sponge and magnesium chloride as a by-product is precipitated to the bottom of the vessel due to its heavier density, and is periodically separated by pressurizing the vessel with argon gas [7] (Scheme 4).
The use of MgCl2 in organic chemistry
The synthesis of heterocyclic compounds
The synthesis of 4-chloro-3-hydroxy-2-pyrone
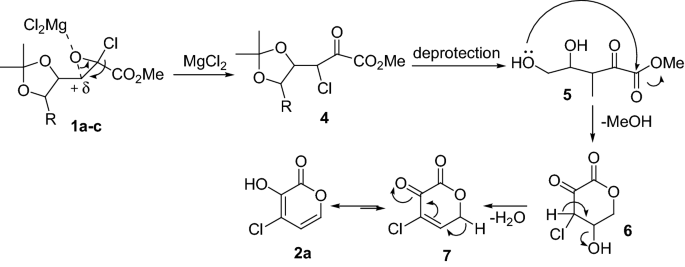
Komiyama et al. [8] reported the preparation of 4-chloro-3-hydroxy-2-pyrones from the treatment of acetonide-protected 4,5-dihydroxy-2-chloroglycidic ester by magnesium chloride in very good yields (Scheme 5). The results revealed that the reaction was carried out by magnesium chloride as a catalyst. Furthermore, the reaction of 5-substituted glycidic esters 1b,c with magnesium chloride also resulted in desired compounds 2b,c in high yields.
The suggested mechanism in the synthesis of pyrone 2a from glycidic ester 1a is shown in Scheme 6. Firstly, 2-chloroglycidic ester 1a is transformed to 3-chloro-2-keto ester 4 in ring opening of oxirane reaction using magnesium chloride, and the subsequent substitution at the chloroglycidic esters 1a is not a simple SN2 mechanism [9]. Nucleophilic addition of hydroxyl group to ester carbonyl carbon 5 gives 6. Lately, dehydration and tautomerization produced 4-chloro-3-hydroxy-2-pyrone 2a.
The synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones
Khaleghi et al. reported facile Biginelli reaction for the production of 3,4-dihydropyrimidin-2(1H)-ones and thiones, in the presence of alkaline earth metal chlorides such as MgCl2 and acetic acid as a solvent in a homogeneous catalytic reaction. The simple separation of the product and the catalyst was the major advantages of this procedure (Scheme 7) [10].
Heravi et al. also reported the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones by MgCl2.6H2O as a catalyst in acetonitrile under reflux condition. Several aromatic and aliphatic aldehydes possessing electron donor and acceptor groups underwent the reaction to provide dihydropyrimidinones/thiones in high yields. The experimental procedure was very practical and was applicable for the other functional groups such as methoxy, nitro, halide and olefins under the reaction conditions. The thiourea was also employed instead of urea with similar success to prepare the corresponding 3,4-dihydropyrimidin-2(1H)-thiones in high yield (Scheme 8) [11].
The suggested mechanism also revealed that the first step of the reaction carried out between aldehyde 8 and urea 9 gave Schiff’s base on the α-olefinic carbon of the tautomer followed by carbonyl attack on imine carbon to obtain a six-membered heterocyclic compound, in which dehydration led to the target DHPMs 11 (Scheme 9).
The synthesis of 3-carboxy-4-oxo-1,8-naphthyridines
Chua et al. introduced the synthesis of 5-oxo-6-carboxy naphthyridines in 2008. Activated 3-nicotinic acids 14 readily were acylated by the magnesium anion of 2-(benzothiazol-2-yl) or 2-(benzimidazol-2-yl) acetates 13. The desired product could next undergo cyclization spontaneously or under very mild conditions to result in the desired naphthyridine products 16. Only near-stoichiometric ratios of reactants were required for this approach, and the products were separated in pure form after a trituration makes this an efficient process (Scheme 10) [12].
The synthesis of 5H-spiro[benzo [7, 8] chromeno [2,3-c]pyrazole-7,3′-indoline]-2′,5,6(9H)-triones
Shen and co-workers presented one-pot synthesis of 5H-spiro[benzo [7, 8] chromeno[2,3-c]pyrazole-7,3′-indoline]-2′,5,6-(9H)-trione derivatives via a multicomponent reaction of hydrazine hydrate, β-keto esters, isatins and 2-hydroxynaphthalene-1,4-dione using MgCl2 in ethanol. This reaction had the advantages of very good yields, environmental-friendly solvent, easy work-up and short reaction time (Scheme 11) [13].
A probable mechanism for the synthesis of products 21 is shown in Scheme 12. Firstly, hydrazine is condensed with β-keto esters 20 to result in the intermediate 22. Then, compound 19 is produced by the coordination of the isatin 17 with MgCl2. Furthermore, 2-hydroxynaphthalene-1,4-dione 18 attacks compound 19 to form unsaturated ketone 25 with the elimination of HCl, MgCl2 and H2O. Next, Michael addition of intermediate 22 to unsaturated ketone 25 takes place to provide intermediate 26, which underwent tautomeric proton shift and intramolecular elimination of H2O to result in the target product 21.
The synthesis of 2-substituted-1H-benzimidazoles
Also, a series of substituted benzimidazoles 29 were prepared by reaction between 1,2-phenylenediamine 27 and aryl, heteroaryl aldehydes 28 using catalytic amount of MgCl2·6H2O. This procedure had some advantages such as high yield, clean reaction profile, the use of green and environmental-friendly catalyst. The protocol was very simple, mild and applicable to aryl as well as hetero aryl aldehydes without significant difference (Scheme 13) [14].
The synthesis of 2-hydroxy-3-((5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) (phenyl) methyl) naphthalene-1,4-diones
Fu et al. described [15] an efficient synthetic method for the preparation of 2-hydroxy-3-((5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(phenyl)methyl)naphthalene-1,4-diones 33 by a one-pot, four-component reaction of aromatic aldehydes 32, 2-hydroxy-1,4-naphthoquinone 18 hydrazine derivatives 31 and ethyl acetoacetate 30 using MgCl2 in ethylene glycol (EG). Easy work-up, short reaction time, very good yield and an environmental-friendly catalyst were the major advantages of this route (Scheme 14).
In another study, the authors examined the reaction in different conditions for the synthesis of pyran derivatives 38, 40 and 42 (Scheme 13). Unfortunately, when the reaction was set up in different solvents at higher temperatures and longer reaction times, no pyran compounds 38, 40 and 42 were obtained and instead formed compounds 37 in good yields. In control experiments, aryl aldehydes were replaced with isatin and acenaphthenequinone under the same reaction conditions. Interestingly, the pyran derivatives 40 and 42 were obtained in high yields (Scheme 13). This anomaly may be obtained from the more stability of compounds 37 in enol form and the hydrogen bond action. So far, these two kinds of reactions all obtained good yields when the EG/MgCl2 system was employed (Scheme 15).
The suggested mechanism for this four-component reaction is shown in Scheme 16. Firstly, aryl hydrazine/hydrazine hydrate 31 was reacted with β-keto esters 30 to yield the pyrazolone ring 43, which was then isomerized to intermediate 44. Additionally, MgCl2 activated aldehyde 32 carbonyl group to attack of 2-hydroxy-1,4-naphthoquinone 18. Nucleophilic attacking of 2-hydroxy-1,4-naphthoquinone 18 to activated MgCl2—aldehyde 32—led to intermediate 45 with C–C bond formation and unsaturated Knoevenagel product 47 was formed with the elimination of MgCl2 and H2O. Then, Michael addition of intermediate 44 to unsaturated Knoevenagel product 47 occurred to afford intermediate 48, which underwent tautomeric proton shift to produce the target product 33.
The synthesis of 1,4-dihydropyridines
Asseri et al. reported the preparation of 1,4-dihydropyridines (1,4-DHPs), 51 in the presence of alkaline earth metal chlorides. Typically, the MgCl2 catalyzed the formation of 1,4-DHPs in (69–87%) yields. The Mg2+ was used as the Lewis acid catalyst in this reaction, and the proposed mechanism of the synthesis of 1,4-DHPs has been depicted in this manuscript. The advantages of serving MgCl2 as a catalyst are including low cost, availability and simple separation, which has sparked considerable interest in the use of this catalyst in promoting organic reactions [16] (Scheme 17).
The functionalization of organic molecules
In 2009, Akselsen and Skattebøl reported a convenient method for the selective ortho-formylation of oxygenated phenols 52 in good yields (73–97%) using MgCl2, paraformaldehyde and triethylamine in refluxing tetrahydrofuran (THF) under an argon atmosphere for 1–4 h (Scheme 18) [17].
Phenols were also converted to their magnesium salts with the MgCl2–Et3N base system and subsequently reacted with Eschenmoser’s salt, affording N,N-dimethyl-substituted benzyl amines in high to excellent yields (Scheme 19). In this method, a series of mono N-substituted benzyl amines 57 were produced in one-pot synthesis by ortho-formylation of phenols 54 to corresponding salicyl aldehydes 55, which in turn reacted with amines to imines 56. The imines were subsequently reduced to mono N-substituted benzyl amines 57 [18].
In another study, an efficient procedure for the synthesis of tetrahydropyranylation of alcohols and phenols were also reported using MgCl2 as a catalyst. In this research, a variety of alcohols 58 and phenols 60 were subjected to synthesize tetrahydropyranyl ethers 59 and 62 (Scheme 20) [19].
MgCl2 was also employed as a heterogeneous catalyst for the acylation of a variety of aryl amines, alcohols and phenols 63 using acetic anhydride 64 under solvent-free conditions at ambient temperature. This procedure described MgCl2.5H2O as an efficient catalyst for the acylation of amines, alcohols and phenols. The others advantages of this protocol included inexpensive, availability of the catalyst, the use of catalytic amount of MgCl2 (0.1 mol%), mild reaction conditions, short reaction time, high yields and solvent-free condition (Scheme 21) [20].
Kim et al. [21] described a facile formation of α-fluoro β-keto esters 68, via diacylation reaction of diethyl (ethoxy carbonyl) fluoromethyl phosphonate 66 with aromatic carboxylic acid chlorides in the presence of magnesium chloride–triethyl amine followed by deacylation of compound 67. Some of the advantages of this procedure were high yields of the product and the mild reaction conditions (Scheme 22).
The isolation of α-fluoro-β-keto esters 68 from the reaction of 69 with NH4Cl in the presence of MgCl2-triethyl amine proved the mechanism shown in Scheme 23.
Condensation reactions
Aldol reaction of α-dimethyl silyl esters with aldehydes, ketones and α-enones
In this method, the aldols 73 were synthesized using a catalytic amount of MgCl2, α-dimethyl silyl esters (α-DMS-esters) 71 smoothly with aldehydes 72 at 30 °C in very good yields (Scheme 24) [22].
Also, the aldol reaction with ketones was effectively accelerated using MgCl2 (Scheme 25).
Also, an equimolar amount of LiCl or CaCl2 induced the aldol reaction; however, the rate-accelerating ability was not high enough to realize a successful aldol reaction. In contrast, MgCl2 was an effective promoter and accelerated addition of 71a at the carbonyl carbon or β-carbon 82 to give β-hydroxy esters 83 (Scheme 26).
The application of MgCl2
Magnesium is used in making a number of alloys which are used in automobile industry, making of aircrafts and optical instruments. Magnesium powder mixed with potassium chlorate is used for flash bulbs in photography. It has also been used as deoxidizer for removing last traces of oxygen from copper, steel, etc. It is utilized in making Grignard reagents which are used for the synthesis of large number of organic compounds. Also, it is applied in the extraction of boron, silicon, etc. [23].
MgCl2 is also employed for the dust control, soil stabilization and wind erosion mitigation. When magnesium chloride is applied to roads and bare soil areas, both positive and negative performance issues occur which are related to many application factors [24].
The preparation of metallic magnesium
Magnesium chloride is the major starting material to produce metallic magnesium. In a protocol, the magnesium is prepared by electrolysis [25, 26] that is applicable on a substantial scale (Scheme 27).
However, approximately 80% of the world demand for magnesium is currently supplied by China and nearly 95% of the primary magnesium output of China is produced using the Pidgeon process mainly due to low labor and energy costs and lax environmental act. In the Pidgeon process, magnesium metal is produced from calcined dolomite under vacuum at high temperatures using ferrosilicon as a reducing agent. In this process, the finely crushed dolomite is fed into kilns where it is calcined and then pulverized in a mill prior to mixing with finely ground ferrosilicon. After weighing and homogenizing, the calcined dolomite and ferrosilicon mixture is made into briquettes which are charged in a retort and placed in the reduction furnace. The reduction operation is a batch process releasing magnesium in vapor form, which is condensed in the cooled end of the retort outside furnace wall. After removal from the furnace, the magnesium “crown” is separated from the sleeves (Scheme 28) [27].
Ion hydration and associated defects in hydrogen bond network of water in the aqueous solutions of MgCl2
In this study, the ion effects, at medium to high concentrations, on dynamical properties of H2O molecules were investigated via classical molecular dynamics simulations by using two well-known non-polarizable water models. Simulations showed that MgCl2-induced perturbations in the hydrogen bond network of water led to the formation of bulk-like domains with “defect sites” on boundaries of such domains; H2O molecules at such defect sites have less number of hydrogen bonds than those in bulk water. Reorientational autocorrelation functions for dipole vectors of such defect water molecules were computed at various concentrations of ions compared with system of pure water. Earlier experimental and simulation studies depicted significant differences in reorientational dynamics for H2O molecules in the first hydration shell of many dissolved ions. The results of this investigation offered that MgCl2 concentration was effective on defect water molecules, which were beyond the ion shells of the first hydration, and experience significant slowing of reorientation times. Also, the addition of cesium chloride to water does not perturb the hydrogen bond network of water even at higher concentrations. This difference in behavior between MgCl2 and CsCl is consistent with the well-known Hofmeister series [28].
The application of MgCl2 in biochemistry
Magnesium chloride as an esthetic for cephalopods
In this study, MgCl2 was as an anesthetic drug for a range of individual cephalopods of different sexes, ages and sizes and relative to five kind genera effectively at varying temperatures from 13 to 22 °C. The results shown that MgCl2 must be rated an excellent “off the shelf” anesthetic for cephalopods. Some advantages of this method were cheap, stable, non-toxic, simple preparation of the solution and usable for worldwide, in the laboratory or on a research vessel, used routinely to narcotize and relax cephalopods prior to fixation. Lately, it appeared to act with minimal trauma to the animal, unlike urethane or ethanol [29].
The separation of serum high-density lipoprotein (HDL) for cholesterol determination
In this investigation, two procedures for the isolation of serum high-density lipoprotein were evaluated: the use of sodium phosphor tungstate and MgCl2 to precipitate selectively and the use of sodium chloride solution under ultracentrifugation condition. Then cholesterol content of fractions resulted from each protocol was compared. Reference intervals for high-density lipoprotein cholesterol in subpopulations were also categorized by age and sex on the basis of data obtained from volunteer blood donors [30].
The effect of MgCl2 on rabbit bronchial smooth muscle
To investigate effect of magnesium ion on the relaxing of rabbit bronchial smooth muscle, a study was designed in vitro model using bronchial rings from New Zealand white rabbits stimulated to contract by electrical stimulation, histamine or bethanechol. In order to show merit, magnesium chloride in various concentrations such as 1, 6, 16, 36 and 86 mM was induced to each tissue bath and resting tension was measured. Electrical stimulation 100 V/100 ms, histamine 10 rnM or bethanechol 6.25 mM was added to wash tissues to induce contraction. Then magnesium chloride 5, 10 and 50 mM was added, and the response of bronchial smooth muscle was measured. The results shown that magnesium chloride 1, 6, 16, 36 and 86 mM decreased the mean ± SEM resting tension of bronchial rings by 40 ± 16, 100 ± 11, 110 ± 10, 170 ± 9 and 275 ± 22 mg, respectively. Electrical stimulation of 100 V/100 ms was raised the mean ± SEM resting tension by 168 ± 52 mg. Magnesium chloride 5, 15 and 50 mM added to the tissue bath decreased the response to 100 V/100 ms to 65 ± 27, 40 ± 23 and 1 ± 0 rag, respectively. Histamine 10 mM increased mean ± SEM resting tension by 490 +/121 mg. Magnesium chloride 5, 15 and 50 mM decreased the histamine response by 80 ± 56, 250 ± 74 and 475 ± 131 mg, respectively. Bethanechol 6.25 mM increased the mean ± SEM resting tension by 495 ± 74 mg. Magnesium chloride (5, 15, 50 mM) decreased bethanechol-induced tension by 52 ± 18, 184 ± 26 and 506 ± 64 mg. Therefore, MgCl2 produced dose-dependent relaxation of bronchial smooth muscle at rest, when stimulated by histamine, bethanechol or electrical impulse. CaCl2 was unable to significantly reverse magnesium-induced relaxation. These data supported the hypothesis that magnesium relaxed smooth muscle and dilates bronchial rings [31].
The comparison of the reaction of bone-derived cells to enhanced MgCl2-salt concentrations
In this study, magnesium-based implants exhibited various advantages such as biodegradability and potential for enhanced in vivo bone preparation. To determine whether high local magnesium concentrations could be osteoconductive and excluded other environmental factors that occur during the degradation of magnesium implants, MgCl2 was utilized as a model system. As cell lines were preferred targets in the studies of non-degradable implant materials, the authors performed a comparative study of three osteosarcoma-derived cell lines (MG63, SaoS2 and U2OS) with primary human osteoblasts. The correlation among cell count, viability, cell size and several MgCl2 concentrations were utilized to study the influence of magnesium on proliferation in vitro. Moreover, bone metabolism alterations during proliferation were investigated by analyzing the expression of genes involved in osteogenesis. It was revealed that for all cell types, the cell count decreased at concentrations above 10 mM MgCl2. However, detailed analysis showed that MgCl2 had a relevant but very diverse influence on proliferation and bone metabolism, depending on the cell type. Only for primary cells was a clear stimulating effect yielded. Therefore, reliable results demonstrating the osteoconductivity of magnesium implants could only be achieved with primary osteoblasts [32].
Multisite ion model in concentrated solutions of divalent cations: osmotic pressure calculations
It is known that precise force field parameters for ions are essential for meaningful simulation studies of proteins and nucleic acids. Currently accepted models of ions, especially for divalent ions, do not necessarily reproduce the right physiological behavior of Ca2+ and Mg2+ ions. Saxena and Sept [33] reported known the multisite ion model, instead of treating the ions as an isolated sphere. The charge was divided into multiple sites with a partial charge. This model provided accurate inner shell coordination of the ion with biomolecules and predicted better free energies for proteins and nucleic acids. In another study, Saxena and García revealed and refined the multisite model to describe the behavior of divalent ions in concentrated MgCl2 and CaCl2 electrolyte solutions, eliminating the unusual ion–ion pairing and clustering of ions which occurred in the original model. The authors also calibrated and improved the parameters of the multisite model by matching the osmotic pressure of concentrated solutions of MgCl2 to the experimental values and then use these parameters to test the behavior of CaCl2 solutions. Also, the concentrated solutions Ca+2 and Mg+2 ions exhibited the experimentally observed behavior with correct osmotic pressure, the presence of solvent-separated ion pairs instead of intimate ion pairs and no aggregation of ions. The improved multisite model for Ca2+ and Mg2+ ions can also be employed in classical simulations of biomolecules at physiologically relevant salt concentrations [34].
Antimicrobial properties of MgCl2 at low pH in the presence of anionic bases
Magnesium is a necessary element for life and is found ubiquitously in all organisms. The various cations play important roles as enzymatic cofactors, as signaling molecules and in stabilizing cellular components. It is not surprising that magnesium salts in microbiological experiments are typically associated with positive effects. In 2014, Oyarzúa and co-workers investigated Listeria monocytogenes as a model organism, on the usefulness of magnesium (in the form of MgCl2) as a stress enhancer. MgCl2 did not affect bacterial viability at near-neutral pH. It was found to strongly compromise culture ability and redox activity when cell suspensions were exposed to the salt at acidic pH. The principle was confirmed with a number of gram-negative and gram-positive species. The magnesium salt dramatically increased the acidity to a level that was antimicrobial in the presence of anionic bases such as phosphate, lactate or acetate. The antimicrobial activity of MgCl2 was much stronger than that of NaCl, KCl or CaCl2. No effect was observed with MgSO4 or when cells were exposed to MgCl2 in phosphate buffer with a pH ≥ 5. Acid stress was reinforced by an additional, salt-specific effect of MgCl2 on microbial viability that needed further examination. Apart from its implications for surface disinfection, this observation might support the commonly stated therapeutic properties of MgCl2 for the treatment of skin diseases and could contribute to understanding why salt from the Dead Sea, where Mg2+ and Cl−1 are the most abundant cation/anion, has healing properties in a microbiological context [35].
The effects of MgCl2 on in vitro cholinesterase and ATPase poisoning by organophosphate
Ajilore et al. studied the benefits of magnesium ion as MgCl2 in organophosphorus poisoning targeting its ability to interact with substrates and membrane enzymes. Blood samples collected from the volunteered healthy adult by venipuncture into anticoagulant test tubes containing EDTA were isolated into plasma and red blood cell and divided into three groups including: normal, pesticide only (0.25–2.0 mom/L chlorpyrifos) and pesticide (0.25–2.0 mmol/L chlorpyrifos) + 0.1 mol/L MgCl2. Acetylcholinesterase, Na+/K+ ATPase and Ca2+ ATPase activities were evaluated. The results showed that chlorpyrifos significantly (P < 0.5) reduced the levels of cholinesterase both in plasma and on red blood cells. Red blood cells Na+/K+ ATPase and Ca2+ ATPase were also significantly (P < 0.5) reduced by chlorpyrifos, while MgCl2 counteracted effects of chlorpyrifos with significant (P < 0.05) increase in the levels of cholinesterase, Na+/K+ ATPase and Ca2+ ATPase. The authors resulted that MgCl2 neutralized the effects of chlorpyrifos by increasing normal ATPase activities and inhibiting release of acetylcholine from the cell [36].
The effects of transdermal MgCl2 on the quality of life for patients with fibromyalgia
Fibromyalgia is a syndrome of chronic pain, fatigue, depression and sleep disturbances symptoms. Its main cause is unclear. Several studies have reported decreased intracellular magnesium levels in patients with fibromyalgia and have found a negative correlation between magnesium levels and fibromyalgia symptoms. Engen and co-workers gathered preliminary data on whether transdermal magnesium could improve the quality of life for women who had fibromyalgia. Forty female patients with the diagnosis of fibromyalgia were enrolled. Each participant was provided a spray bottle containing a transdermal magnesium chloride solution and asked to apply four sprays per limb twice daily for 4 weeks. This pilot study suggested that transdermal magnesium chloride applied on upper and lower limbs might be beneficial to patients with fibromyalgia [37].
The removal of dyestuff
Color removal from dye-containing wastewater
Magnesium salts have been reported to be an efficient alternative to conventional coagulants and can enhance the removal of impurities or pollutants from wastewater [38, 39]. Color removal by MgCl2 when treating synthetic waste containing pure dyes was studied. The color removal efficiency of MgCl2/Ca(OH)2 was compared with that of Al2(SO4)3, polyaluminum chloride (PAC) and FeSO4/Ca(OH)2. The mechanism of color removal by MgCl2 was also investigated. The experimental results showed that the color removal efficiency of MgCl2 was related to the type of dye and depended on the pH of the waste and the dosage of the coagulants used. The treatment of waste containing a reactive dye or dispersed dye with MgCl2 yielded an optimum color removal ratio when the pH of the solution was equal to or above 12.0. For both the reactive and dispersed dye wastes, MgCl2/Ca(OH)2 was shown to be superior to MgCl2/NaOH, Al2(SO4)3, PAC and FeSO4/Ca(OH)2 for color removal. A magnesium hydroxide precipitate formed at pH values greater than 12.0, which provided a large adsorptive surface area and a positive electrostatic surface charge, enabling it to remove the dyes through charge neutralization and an adsorptive coagulating mechanism. So, the MgCl2/Ca(OH)2 system found a viable alternative to some of the more conventional forms of chemical treatment, especially for treating actual textile waste with high natural pH [40].
The removal of dyes and industrial dye wastes
Magnesium chloride, as compared to alum and polyaluminum chloride (PAC), is a less commonly used coagulant in the field of wastewater treatment, with a cost in between alum and PAC. It has been used in this study as a coagulant to investigate the effectiveness of the chemical precipitation method for the removal of coloring matters. The color concentration of dye solutions was measured by visible spectrophotometry. Parameters such as the effect of pH, the effect of coagulant and coagulant aid dosages and the effect of different coagulants have been studied. The results showed that MgCl2 was capable of removing more than 90% of the coloring material at a pH of 11 and a dose of 4 g MgCl2/l of the dye solution. MgCl2 was shown to be more effective in removing reactive dye than alum and PAC in terms of settling time and the amount of alkalinity required. Optimal operating conditions such as pH value, coagulant dose and the effect of polyelectrolyte have been determined. Wastewaters of a dying and printing mill on different days have been treated by MgCl2 aqueous solution in bench scale. The treatment of industrial waste has shown a reduction of 88% in COD and 95% of suspended solids [41].
Conclusions
This review has reported production and the applications of magnesium chloride in organic chemistry and biochemistry including the synthesis of heterocyclic compounds, the protection of functional groups, condensation reaction, the preparation of metallic magnesium, as an anesthetic for cephalopods, the separation of serum high-density lipoprotein, effect of magnesium chloride on rabbit bronchial smooth muscle, antimicrobial properties of magnesium chloride, effect of magnesium chloride on the quality of life for patients with fibromyalgia and color removal from dye-containing wastewater and industrial dye wastes. Therefore, this versatile reagent can be easily prepared, can be used in organic transformation and can be employed in the synthesis of heterocyclic compounds. We believe that magnesium chloride can be examined in the other organic reactions and biochemistry process that have not still searched. Also, this review paper can be used for researchers in all field of sciences, especially for chemistry, biochemistry and sewage treatment researchers.
References
Holleman AF, Wiberg E (2001) Inorganic chemistry. Academic Press, San Diego, pp 1303–1316
Listed under ingredients for Similac hypoallergenic infant formula with iron (Abbott nutrition). abbottnutrition.com. Retrieved 2013
Malpass DB (2010) Commercially available metal alkyls and their use in polyolefin catalysts. In: Hoff R, Mathers RT (eds) Handbook of transition metal polymerization catalysts, chap 1
Kashiwa N (2004) The discovery and progress of MgCl2-supported TiCl4 catalysts. J Poly Sci Part A Poly Chem 42:1–8. https://doi.org/10.1002/pola.10962
Butter E (1985) NN Greenwood, A Earnshaw: Chemistry of the elements. Pergamon Press Oxford 1984, 1542 seiten, 7 anhängePreis. Cryst Res Technol 20:662
Eom HC, Park H, Yoon HS (2010) Preparation of anhydrous magnesium chloride from ammonium magnesium chloride hexahydrate. Adv Powder Technol 21:125–130. https://doi.org/10.1016/j.apt.2010.01.003
Nakamura K, Iida T, Nakamura N, Araike T (2017) Titanium sponge production method by Kroll process at OTC. Mater Trans 58:319–321
Komiyama T, Takaguchi Y, Tsuboi S (2004) Novel synthesis of 4-chloro-3-hydroxy-2-pyrone by the reaction of acetonide protected 4,5-dihydroxy-2-chloroglycidic ester with magnesium chloride. Tetrahedron Lett 45:6299–6301. https://doi.org/10.1016/j.tetlet.2004.06.101
Coutrot P, Grison C, Tabyaoui M, Czernecki S, Valery JM (1988) Novel application of alkyl dihalogenoacetates; chain extension with an α-ketoester unit of carbohydrates. J Chem Soc Chem Commun 23:1515–1516. https://doi.org/10.1039/c39880001515
Khaleghi S, Heravi MM, Khosroshahi M, Behbahani FK, Daroogheha Z (2008) A very high yielding and facile alkaline earth metals homogeneous catalysis of Biginelli reaction: an improved protocol. Green Chem Lett Rev 1:133–139. https://doi.org/10.1080/17518250802342527
Khaleghi S, Heravi MM, Behbahani FK, Daroogheha Z (2006) MgCl2·6H2O: an efficient and economic catalyst for three component one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones. Org Chem Indian J 2:118–120
Chua PC, Nagasawa JY, Pierre F, Schwaebe MK, Vialettes A, Whitten JP (2008) A novel and efficient synthesis of 3-carboxy-4-oxo-1,8-naphthyridines using magnesium chloride. Tetrahedron Lett 49:4437–4442. https://doi.org/10.1016/j.tetlet.2008.05.005
ShenT FuZ, Che F, Dang H, Lin Y, Song Q (2015) An efficient one-pot four-component synthesis of 5H-spiro [benzo [7,8] chromeno [2,3-c] pyrazole-7, 3′-indoline]-2′,5,6 (9H)-trione derivatives catalyzed by MgCl2. Tetrahedron Lett 56(9):1072–1075. https://doi.org/10.1016/j.tetlet.2015.01.062
Ghosh P, Subba R (2015) MgCl2·6H2O catalyzed highly efficient synthesis of 2-substituted-1H-benzimidazoles. Tetrahedron Lett 56:2691–2694. https://doi.org/10.1016/j.tetlet.2015.04.001
Fu Z, Qian K, Li S, Shen T, Song Q (2016) MgCl2 catalyzed one-pot synthesis of 2-hydroxy-3-((5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(phenyl) methyl) naphthalene-1,4-dione derivatives in EG. Tetrahedron Lett 57:1104–1108. https://doi.org/10.1016/j.tetlet.2016.01.089
Asseri SNARM, Tan SH, Mohamad WNKW, Poh SC, Chia PW, Kan S-Y, Chuah TS (2017) MgCl2 as efficient and inexpensive catalyst for the synthesis of 1,4-dihydropyridine derivatives. Malays J Anal Sci 2:13–19. https://doi.org/10.17576/mjas-2017-2101-02
Akselsen ØW, Skattebøl L, Hansen TV (2009) ortho-Formylation of oxygenated phenols. Tetrahedron Lett 50:6339–6341. https://doi.org/10.1016/j.tetlet.2009.08.101
Anwar HF, Skattebøl L, Hansena TV (2007) Synthesis of substituted salicylamines and dihydro-2H-1,3-benzoxazines. Tetrahedron 63:9997–10002. https://doi.org/10.1016/j.tet.2007.07.064
Pasumarthi BR (2010) An efficient synthetic protocol for the tetrahydropyranylation of alcohols and phenols catalyzed by magnesium chloride. Thesis. Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee
Patil VD, Sutar NR, PatiL KP, Gidh PV (2015) Chemoselective acylation of amines, alcohols and phenols using magnesium chloride under solvent free condition. Int J Chem Sci 13:450–458
Kim DY, Rhie DY, Oh DY (1996) Acylation of diethyl (ethoxycarbonyl) fluoromethylphosphonate using magnesium chloride–triethylamine: a facile synthesis of α-fluoro β-keto esters. Tetrahedron Lett 37:653–654. https://doi.org/10.1016/0040-039(95)02224-4
Miura K, Nakagawa T, Hosomi A (2005) Metal chloride-promoted aldol reaction of α-dimethylsilylesters with aldehydes, ketones, and α-enones. Synlett 1917–1921. https://doi.org/10.1055/s-2005-871938
Patel NK, Patel NV College of Pure and Applied Sciences (Industrial chemistry). Chemical Process Industries, UNIT 3B
Bolander P, Yamada A (1999) Dust palliative selection and application guide (No. 9977 1207-SDTDC). https://trid.trb.org/view/713799
Seeger M, Otto W, Flick W, Bickelhaupt F, Akkerman OS (2011) Magnesium compounds (Ullmann’s Encyclopedia of Industrial Chemistry). Wiley, Weinheim. https://doi.org/10.1002/14356007.a15_595
Hill JW, Petrucci RH (2002) General chemistry an integrated approach. Prentice Hall, Upper Saddle River
Mehrabi B, Abdellatif M, Masoudi F (2012) Evaluation of zefreh dolomite (central iran) for production of magnesium via the Pidgeon process. Miner Process Extr Metall Rev 33:316–326. https://doi.org/10.1080/08827508.2011.601478
Baul U, Vemparala S (2015) Ion hydration and associated defects in hydrogen bond network of water: observation of reorientationally slow water molecules beyond first hydration shell in aqueous solutions of MgCl2. Phys Rev E 91:012114(1)–012114(6). https://doi.org/10.1103/PhysRevE.91.012114
Messenger JB, Nixon M, Ryan KP (1985) Magnesium chloride as an anaesthetic for cephalopods. Comp Biochem Physiol C Comp Pharmacol Toxicol 82:203–205. https://doi.org/10.1016/0742-8413(85)90230-0
Seigler L, Wu WT (1981) Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clin Chem 27:838–841
Spivey WH, Skobeloff EM, Levin RM (1990) Effect of magnesium chloride on rabbit bronchial smooth muscle. Ann Emerg Med 19:1107–1112. https://doi.org/10.1016/S0196-0644(05)81513-6
Burmester A, Luthringer B, WillumeitR Feyerabend F (2014) Comparison of the reaction of bone-derived cells to enhanced MgCl2-salt concentrations. Biomatter 4:e967616. https://doi.org/10.4161/21592527
Saxena A, Sept D (2013) Multisite ion models that improve coordination and free energy calculations in molecular dynamics simulations. J Chem Theory Comput 9:3538–3542. https://doi.org/10.1021/ct400177g
Saxena A, García AE (2014) Multisite ion model in concentrated solutions of divalent cations (MgCl2 and CaCl2): osmotic pressure calculations. J Phys Chem B119:219–227. https://doi.org/10.1021/jp507008x
Alarcón PO, Sossa K, Contreras D, Urrutia H, Nocker A (2014) Antimicrobial properties of magnesium chloride at low pH in the presence of anionic bases. Magnes Res 27:57–68. https://doi.org/10.1684/mrh.2014.0362
Kim M, Basharat A, Santosh R, Mehdi SF, Razvi Z, Yoo SK, Dankner R (2019) Reuniting overnutrition and undernutrition, macronutrients, and micronutrients. Diabetes Metab Res Rev 35:e3072. https://doi.org/10.1002/dmrr.3072
Engen DJ, McAllister SJ, Whipple MO, Cha SS, Dion LJ, Vincent A, Wahner-Roedler DL (2015) Effects of transdermal magnesium chloride on quality of life for patients with fibromyalgia: a feasibility study. J Integr Med 13:306–313. https://doi.org/10.1016/S2095-4964(15)60195-9
Judkins JF Jr, Hornsby JS (1978) Color removal from textile dye waste using magnesium carbonate. J Water Pollut Control Fed 50:2446–2456. https://www.jstor.org/stable/25040176
Liao MY, Randtke SJ (1986) Predicting the removal of soluble organic contaminants by lime softening. Water Res 20:27–35. https://doi.org/10.1016/0043-1354(86)90210-1
Gao BY, Yue QY, Wang Y, Zhou WZ (2007) Color removal from dye-containing wastewater by magnesium chloride. J Environ Manag 82:167–172. https://doi.org/10.1016/j.jenvman.2005.12.019
Tan BH, Teng TT, Omar AM (2000) Removal of dyes and industrial dye wastes by magnesium chloride. Water Res 34:597–601. https://doi.org/10.1016/S0043-1354(99)00151-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daloee, T.S., Behbahani, F.K. MgCl2 and its applications in organic chemistry and biochemistry: a review. Mol Divers 24, 463–476 (2020). https://doi.org/10.1007/s11030-019-09947-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09947-2