Abstract
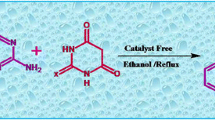
In this report, a facile, operationally, simple and highly efficient one-pot coupling of 2,6-diaminopyrimidin-4(3H)-one and ethyl-2,4-dioxo-4-phenylbutanoate derivatives is reported. This method afforded a novel series of ethyl-2-amino-3,4-dihydro-4-oxo-5-phenyl pyrido[2,3-d] pyrimidine-7-carboxylate heterocycle derivatives in high yields under refluxing AcOH.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrimidines are an important class of nitrogen heterocyclic compounds with a wide range of applications, and these compounds have proven to be convenient building blocks for the synthesis of various fused heterocycles [1,2,3,4]. One of the most important fused heterocycles is pyrido[2,3-d]pyrimidines. These compounds exhibit a wide range of biological properties such as antiviral [5], anti-inflammatory [6], antimicrobial [7], antifungal [8] and anticancer activity [9]. For example, compounds such as 2,4-diamino-6-(thioarylmethyl)pyrido[2,3-d]pyrimidines were shown as inhibitors of dihydrofolate reductases (Fig. 1) [10]. Therefore, the synthesis of diverse structures belonging to this class of compounds is very important. Also, these compounds exist in purine bases of DNA and RNA [11]. There are several synthetic procedures for the preparation of fused pyrimidine systems under different conditions which have opened new horizons in the synthesis of pyridopyrimidines. For example, they can be made with 3-cyano-2-aminopyridines via formamidine formation followed by selective nucleophilic addition with different primary amines [12]. Cyclocondensation of 4,6-dichloro-2-methylsulfanylpyrimidine-5-carbaldehyde with beta-alkyl and beta-aryl-beta-aminoacrylic esters is another route for their preparation [13]. The Michael addition and subsequent cyclodehydration of 2,6-diaminopyrimidin-4-one and butynones provided another method for the synthesis of pyrido [2,3-d]pyrimidines [14]. The three-component reaction of aldehydes, alkyl nitriles and aminopyrimidines in water and in the presence of KF-Al2O3 as catalyst provided the aforementioned compounds in reasonable yields [15]. Some of the reported methods suffer from one or more disadvantages such as multi-step synthesis, use of toxic chemicals, low yields and tedious workup. In addition, these approaches only afford series of anticipated N-fused heterocycle structures. The biological and medicinal character of these compounds inspires us to examine a different and effective method for their preparation. Furthermore, in this paper, we plan to present new pyrido[2,3-d]pyrimidines structures which aims to synthesize tricyclic heterocycles and easily methodologies for the preparation of compounds. Following up on our interest in the synthesis of N-fused heterocycles [16,17,18,19,20,21,22], herein we describe a novel and highly efficient technique for the preparation of new derivatives of pyrido[2,3-d]pyrimidines from 2,6-diaminopyrimidin-4(3H)-one 1 and ethyl-2,4-dioxo-4-phenylbutanoate derivatives 2 (Scheme 1).
Results and discussion
First, the desired starting materials, including 2,6-diaminopyrimidin-4(3H)-one 1 and ethyl-2,4-dioxo-4-phenylbutanoate derivatives 2 (prepared from acetophenones 4 and diethyl oxalate 5), were synthesized by conventional methods according to the literature (Scheme 2) [23, 24].
At first, we tested the reaction of starting materials (1 and 2a) in the presence of different solvents to optimize the reaction conditions (Table 1). As shown in Table 1, the best result (based on the yield of the reaction) was obtained in refluxing AcOH (Table 1, entry 7).
With these results in hand, different ethyl-2-amino-3,4-dihydro-4-oxo-5-phenyl pyrido[2,3-d] pyrimidine-7-carboxylate derivatives 3a–l were prepared using various ethyl-2,4-dioxo-4-arylbutanoates 2 (Table 2, entries 1–12). For all substrates, the reaction could be completed in 5–6 h in high yields.
It was observed that the desired products were obtained in good to excellent yields in almost all cases and their structures were verified by IR, 1H NMR and 13C NMR spectroscopy as well as mass spectrometry. A proposed mechanism for the synthesis of phenylpyrido[2,3-d] pyrimidine-7-carboxylate derivative 3a is shown in Scheme 3. Initially, the acid-catalyzed condensation of amine group from 2,6-diaminopyrimidin-4(3H)-one 1 with the more active carbonyl group of ethyl-2,4-dioxo-4-phenylbutanoates 2a in the presence of acetic acid as solvent gave intermediate 6. In the end, compound 3a can be attained after tautomerization, cyclization and water elimination sequences on intermediate 6 can lead to compound 3a (Scheme 3). We have used acetic acid (50%, solution in water) in the reaction. The acid catalyzes the condensation of an amino group in 2,6-diaminopyrimidin-4(3H)-one 1 with the more active carbonyl group of ethyl-2,4-dioxo-4-phenylbutanoates 2a by donating H+ to the more activated carbonyl group for obtaining intermediate 6. pKa of acetic acid is 4.75 (at 25 °C) and can give a positive proton to lone pair of oxygen atoms of carbonyl compounds to activate them for nucleophilic attack. Furthermore, acetic acid-catalyzed tautomerization, cyclization and water elimination sequences on intermediates 6 and 7 for converting them to target molecule 3a (Scheme 3). Although the glacial acetic acid can be used in this reaction, as it is toxic, we used just acetic acid (50%, solution in water). Furthermore, the acetic acid has higher boiling point than water and can provide higher activation energy for reaction. The temperature is important for providing the activation energy for different steps (especially rate-determining step) of the reaction. However, acetic acid can act as an efficient catalyst and appropriate solvent for the synthesis of entitled fused pyrido[2,3-d]pyrimidine structures.
The nature of the substituents on aromatic ring of compound 2 has a significant effect on the yield of the reaction (Table 2). Aromatic rings of compound 2 bearing electron-withdrawing groups have less electron density than unsubstituted rings or rings containing electron-donating substituents. This electron deficiency renders carbonyl group more susceptible toward nucleophilic attack in the cyclization step, resulting in the desired products in higher yields (Table 2, entries 2–6). From this point of view, one can conclude that the cyclization step (conversion of compound 6 to 7) in Scheme 3 is rate-determining step.
Conclusion
In conclusion, we developed an efficient process for the synthesis of pyrido[2,3-d]pyrimidine-fused heterocycles in good yields (68–90%) in AcOH medium. Prominent among the advantages of this new method are novelty, an easy workup, the absence of a catalyst and operational simplicity.
Experimental
General remarks
All commercially available chemicals and reagents were purchased from Merck and Fluka Chemical Company and were used without further purification. Melting points were measured on a Kofler hot-stage apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker FT-500, using TMS as an internal standard. The abbreviations used are as follows: s, singlet; d, doublet; and m, multiplet. IR spectra were recorded on a Nicolet Magna FTIR 550 spectrophotometer (KBr disks). MS were recorded with an Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. Elemental analysis was Elemental Analysensystem GmbH VarioEL.
General procedure for the synthesis of oxopyrido[2,3-d]pyrimidine derivatives 3
A mixture of 6-diaminopyrimidin-4(3H)-one 1 (1 mmol), ethyl 2,4-dioxo-4-arylbutanoates 2 (1 mmol) in refluxing AcOH (10 mL) was stirred at 115 °C for 7 h. The progress of the reaction was monitored by TLC (ethyl acetate/n-hexane: 1/2). After the completion of the reaction, the mixture was cooled to room temperature, and the precipitate was filtered, washed with ethanol (20 mL) and purified by crystallization or column chromatography to afford pure products 3a–l.
Ethyl-2-amino-3,4-dihydro-4-oxo-5-phenylpyrido[2,3-d] pyrimidine-7-carboxylate (3a)
Yield: 90%; yellow crystals; mp 119–121 °C; IR (KBr): 1685, 1725, 2990, 3019, 3127, 3389 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.3 (t, J = 7 Hz, 3H, CH3), 4.26 (q, J = 7 Hz, 2H, OCH2), 6.850 (s, 2H, NH2), 7.51–7.53 (m, 3H, Ar), 7.67 (s, 1H, pyridine), 8.17–8.19 (m, 2H, Ar). 13C NMR (125 MHz, DMSO): 13.7, 61.3, 106.0, 115.4, 127.3, 129.9, 130.6, 130.9, 131.3, 137.9, 142.4, 154.9, 161.2, 167.0. Anal. Calcd for C16H14N4O3: C, 61.93; H, 4.55; N, 18.06. Found: C, 61.63; H, 4.25; N, 17.76.
Ethyl-2-amino-5-(4-fluorophenyl)-3,4-dihydro-4-oxopyrido [2,3-d]pyrimidine-7-carboxylate (3b)
Yield: 88%; yellow crystals; mp 210–212 °C; IR (KBr): 1668, 1737, 3227, 3253, 3347 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.33 (t, J = 7 Hz, 3H, CH3), 4.24 (q, J = 7 Hz, 2H, OCH2), 6.84 (s, 2H, NH2), 7.33 (t, J = 8.5 Hz, 2H, Ar), 7.68 (s, 1H, pyridine), 8.24 (t, J = 8.5 Hz, 2H, Ar). 13C NMR (125 MHz, DMSO): 13.7, 61.3, 105.8, 111.3, 115.4, 115.8 (d, JC–F = 22 Hz), 129.6 (d, JC–F = 8.7 Hz), 133.7, 135.1, 143.4, 154.8, 159.6, 163.6 (d, JC–F = 247 Hz), 167.2. Anal. Calcd for C16H13FN4O3: C, 58.5; H, 3.99; N, 17.7. Found: C, 58.2; H, 3.49; N, 17.4.
Ethyl-2-amino-5-(4-chlorophenyl)-3,4-dihydro-4-oxopyrido [2,3-d]pyrimidine-7-carboxylate (3c)
Yield: 85%; yellow crystals; mp 215–217 °C; IR (KBr): 1679, 1731, 2994, 3278, 3358 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 4.36 (q, J = 7 Hz, 2H, OCH2), 6.95 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.72 (s, 1H, pyridine), 8.21 (d, J = 8.5 Hz, 2H, Ar). 13C NMR (125 MHz, DMSO): 13.7, 61.2, 105.4, 106.1, 111.6, 128.8, 1128.9, 135.3, 136.0, 143.4, 154.7, 159.4, 161.8, 167.1. Anal. Calcd for C16H13ClN4O3: C, 55.7; H, 3.8; N, 16.2. Found: C, 55.4; H, 3.5; N, 15.9.
Ethyl-2-amino-5-(4-bromophenyl)-3,4-dihydro-4-oxopyrido [2,3-d] pyrimidine-7-carboxylate (3d)
Yield: 86%; yellow crystals; mp: 218–220 °C; IR (KBr): 1693, 1780, 3210, 3245, 3375 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 4.34 (q, J = 7 Hz, 2H, OCH2), 6.83 (S, 2H, NH2), 7.13 (s, 1 H, Pyridine), 7.68 (t, J = 8 Hz, 2H, Ar), 8.13 (d, J = 8 Hz, 2H, Ar).13C NMR (125 MHz, DMSO): 13.7, 61.2, 95.37, 106.01, 111.9, 112.05, 115.2, 121.3, 129.2, 131.7, 132.3, 143.8, 162.6, 167.5. m/z (%) = 389 [M+] (100), 318.0 (50), 237 (30), 165 (20). Anal. Calcd for C16H13BrN4O3: C, 42.38; H, 3.37, N; 14.4. Found: C, 42.33; H, 3.41; N; 13.9.
Ethyl-2-amino-5-(2-chlorophenyl)-3,4-dihydro-4-oxopyrido [2,3-d]pyrimidine-7-carboxylate (3e)
Yield: 84%; yellow crystals; mp: 215–217 °C; IR (KBr): 1680, 1752, 2983, 3333, 3326 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.30 (t, J = 7 Hz, 3H, CH3), 4.34 (q, J = 7 Hz, 2H, OCH2), 6.97 (S, 2H, NH2), 7.28 (S, 1H, Pyridine), 7.47–7.55 (m, 2H, Ar), 7.57–7.63 (m, 2H, Ar), 11.4 (s, NH). 13C NMR (125 MHz, DMSO): 13.7, 61.2, 105.1, 106.04, 127.2, 127.4, 129.9, 130.6, 130.7, 130.9, 131.3, 137.9, 142.4, 154.8, 161.4, 167.0. Anal. Calcd for C16H13ClN4O3: C, 55.7; H, 3.80; N, 16.25; Found: C, 55.2; H, 3.30; N, 15.95.
Ethyl-2-amino-5-(2,4-dichlorophenyl)-3,4-dihydro-4-oxopyrido [2,3-d]pyrimidine-7-carboxylate (3f)
Yield: 81%; yellow crystals; mp: 210–212 °C; IR (KBr): 1679, 1756, 3143, 3292 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.30 (t, J = 7 Hz, 3H, CH3), 4.34 (q, J = 7 Hz, 2H, OCH2), 7.04 (s, 2H, NH2), 7.31 (S, 1H, Pyridine), 7.56 (d, J = 8 Hz, Ar, 1H), 7.64 (d, J = 8 Hz, Ar, 1H), 7.75 (s, 1H, Ar). 13C NMR (125 MHz, DMSO): 13.8, 61.52, 106.35, 115.75, 130.3, 132.17, 132.75, 134.6, 142.6, 155.01, 159.4, 160.4, 167.0, 172. Anal. Calcd for C16H12Cl2N4O3: C, 50.6; H, 3.19; N, 14.78. Found: C, 50.1; H, 2.79; N; 14.26.
Ethyl-2-amino-3,4-dihydro-4-oxo-5-p-tolylpyrido[2,3-d] pyrimidine-7-carboxylate (3g)
Yield: 80%; yellow crystals; mp: 214–217 °C; IR (KBr): 1698, 1738, 3040, 3230, 3330 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 1.3 (S, 3H, CH3), 4.3 (q, J = 7 Hz, 2H, OCH2), 6.89 (s, 2H, NH2), 7.52 (d, J = 6 Hz, 2H, Ar), 7.69 (s, 1H, Pyridine), 8.18 (d, J = 6 Hz, 2H, Ar). 13C NMR (125 MHz, DMSO): 14.30, 16.30, 62.01, 114.3, 114.6, 121.3, 125.6, 128.9, 130.9, 134.2, 139.5, 142.5, 152.2, 156.3, 165.7. Anal. Calcd for C17H16N4O3: C, 62.95; H, 4.97; N, 17.27. Found: C, 62.65; H, 4.77; N, 16.77.
Ethyl-2-amino-3,4-dihydro-4-oxo-5-m-tolylpyrido[2,3-d] pyrimidine-7-carboxylate (3h)
Yield: 75%; yellow crystals; mp: 218–220 °C; IR (KBr): 1676, 1727, 3277, 3376, 3346 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 2.5 (S, 3H, CH3), 4.35 (q, J = 7 Hz, 2H, OCH2), 6.83 (s, 2H, NH2), 7.32 (d, J = 7 Hz, 2H, Ar), 7.40 (t, J = 7 Hz, 1H, Ar), 7.66 (S, 1H, Pyridine), 7.98 d, J = 7 Hz, 1H, Ar), 8.02 (1H, NH). 13C NMR (125 MHz, DMSO): 14.3, 16.2, 62.0, 115.2, 121.3, 124.7, 125.6, 128.1, 128.8, 128.9, 130.6, 138.4, 138.5, 139.4, 142.5, 156.8, 165.7. Anal. Calcd for C17H16N4O3: C, 62.95; H, 4.97; N, 17.27. Found: C, 62.45; H, 4.67; N, 16. 77.
Ethyl-2-amino-3,4-dihydro-5-(4-methoxyphenyl)-4-oxopyrido[2,3-d]pyrimidine-7-carboxylate (3i)
Yield: 71%; yellow crystals; mp: 214–16 °C; IR (KBr): 1682, 1738, 2984, 3270, 3335, 3417 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 3.4 (s, 3H, OCH3), 4.35 (q, J = 7 Hz, 2H, OCH2), 6.89 (s, 2H, NH2), 7.5 (d, J = 6 Hz, 2H, Ar), 7.6 (s, 1H, Pyridine), 8.1 (d, J = 6 Hz, 2H, Ar), 11.3 (s, 1H, NH). 13C NMR (125 MHz, DMSO): 13.7, 60.0, 61.2, 111.6, 127.1, 127.2, 128.7, 128.8, 129.6, 130.5, 137.2, 143.3, 154.8, 160.7, 167.3. Anal. Calcd for: C17H16N4O4: C, 59.99; H, 4.74; N, 16.46. Found: C, 59.69; H, 4.44; N, 16.16.
Ethyl-2-amino-3,4-dihydro-5-(3,4-dimethoxyphenyl)-4-oxopyrido[2,3-d]pyrimidine-7-carboxylate (3j)
Yield: 69%; yellow crystals; mp: 217–219 °C; IR (KBr): 1667, 1734, 2887, 2915, 3253, 3360 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.32 (t, J = 7 Hz, 3H, CH3), 3.84 (s, OCH3), 3.87 (OCH3), 4.35 (q, J = 7 Hz, 2H, OCH2), 7.07 (d, J = 8 Hz, 1H, Ar), 7.68 (s, 1H, Pyridine), 7.79 (d, J = 8 Hz, 2H, Ar), 7.80 (s,1H, NH). 13C NMR (125 MHz, DMSO): 13.7, 51.6, 55.56, 61.2, 105.2, 110.3, 111.1, 111.54, 111.7, 120.5, 129.8, 143.1, 148.9, 151.0, 154.8, 160.4, 167.4, 171.8. Anal. Calcd for: C18H18N4O5: C, 58.37; H, 4.90; N, 15.13. Found: C, 58.07; H, 4.70; N, 14.93.
Ethyl-2-amino-3,4-dihydro-5-(3-methoxyphenyl)-4-oxopyrido[2,3-d]pyrimidine-7-carboxylate (3k)
Yield: 68%; yellow crystals; mp: 220–222 °C; IR (KBr):1689, 1735, 2995, 3220, 3295 cm−1. 1H NMR (500 MHz, DMSO): δH = 1.3 (t, J = 7 Hz, 3H, CH3), 2.4 (s, 3H, OMe), 4.3 (q, J = 7 Hz, 2H, OCH2), 6.8 (s, 1H, Pyridine), 7.32 (d, J = 7.5 Hz, 1H, Ar), 7.4 (t, J = 7.5 Hz, 1H, Ar), 7.66 (s, 1H, NH) 7.97 (d, J = 7.5 Hz, 1H, Ar), 8.02 (s, 1H, Ar). 13C NMR (125 MHz, DMSO): 13.7, 55.5, 61.2, 105.2, 106.1, 111.9, 112.5, 119.5, 120.5, 129.7, 138.7, 143.2, 154.8, 159.6, 160.4, 167.2, 171.8. Anal. Calcd for: C17H16N4O4: C, 59.99; H, 4.74; N, 16.46; Found: C, 59.69; H, 4.44; N, 16.26.
References
Dave Shukla CG, Shukla MC (1997) Diethyl ethoxymethylenemalonate in triheterocycles: a new synthesis of pyrido[3,2-e]pyrimido[1,2-c]pyrimidines. J Heterocycl Chem 34:1805–1808. https://doi.org/10.1002/jhet.5570340627
Lavecchia G, Berteina-Raboin S, Guillaumet G (2005) Selective bifunctionalization of pyrido[2,3-d]pyrimidines in positions 2 and 4 by SNAr and palladium-catalyzed coupling reactions. Tetrahedron Lett 46:5851–5855. https://doi.org/10.1016/j.tetlet.2005.06.141
Mulamba T, Boukili-Garré E, Séraphin D, Noé E, Charlet-Fagnère C, Hénin J, Laronze J, Sapi J, Barret R, Yves Laronze J, Lévy J (1995) Synthesis of compounds with the novel 2,3,7-triazaphenalene ring system. Heterocycles 41:29–36. https://doi.org/10.3987/COM-94-6846
Jones RG (1951) Pyridine syntheses. III. Preparation and reactions of some penta-substituted pyridines. J Am Chem Soc 73:5610–5614. https://doi.org/10.1021/ja01156a034
Darias V, Abdallah S, Tello ML, Delgado LD, Vega S (1994) NSAI activity study of 4-phenyl-2-thioxo-benzo[4, 5]thieno [2,3-d]pyrimidine derivatives. Arch Pharm 237:779–783. https://doi.org/10.1002/ardp.19943271205
Satasia SP, Kalaria PN, Raval DK (2014) Regioselective synthesis of pyrazole based pyrido [2,3-d] pyrimidine-diones and their biological evaluation. Org Biomol Chem Catalytic 12:1751–1758. https://doi.org/10.1039/C3OB42132E
Farghaly TA, Hassaneen HME (2013) Synthesis of pyrido[2,3-d][1, 2, 4]triazolo[4,3-a]pyrimidin-5-ones as potential antimicrobial agents. Arch Pharm Res 36:564–572. https://doi.org/10.1007/s12272-013-0045-2
Gineinah MM, Nasr MNA, Badr SMI, El-Husseiny WM (2013) Synthesis and antitumor activity of new pyrido[2,3-d]pyrimidine derivatives. Med Chem Res 22:3943–3952. https://doi.org/10.1007/s00044-012-0396-0
Palopa JA, Planoa D, Morenoa E, Sanmartin C (2014) Novel quinazoline and pyrido[2,3-d]pyrimidine derivatives and their hydroselenite salts as antitumoral agents. ARKIVOC 2:187–206. https://doi.org/10.3998/ark.5550190.p008.244
Edupuganti R, Wang Q, Tavares CDJ, Chitjian CA, Bachman JL, Ren P, Anslyn EV, Dalby KN (2014) Synthesis and biological evaluation of pyrido[2,3-d]pyrimidine-2,4-dione derivatives as eEF-2 K inhibitors. Bio org Med Chem 22:4910–4916. https://doi.org/10.1016/j.bmc.2014.06.050
Gangjee A, Adair O, Queener FS (2001) Synthesis of 2,4-diamino-6-(thioarylmethyl)pyrido [2,3-d]pyrimidines as dihydrofolate reductase inhibitors. Bio Org Med Chem 9:2929–2935. https://doi.org/10.1016/S0968-0896(01)00223-1
Shen Z, He X, Dai J, Mo W, Hu B, Sun N, Hu X (2011) An efficient HCCP-mediated direct amination of quinazolin-4(3H)- one. Tetrahedron 67:1665–1672. https://doi.org/10.1016/j.tet.2010.12.067
Belhadj F, Kibou Z, Cheikh N, Choukchou-Braham N, Villemin D (2015) Convenient access to new 4-substituted aminopyrido[2,3-d]pyrimidine derivatives. Tetrahedron Lett 56:5999–6002. https://doi.org/10.1016/j.tetlet.2015.09.042
Chizhova ME, Bakulina OY, Ivanov AY, Lobanov PS, Dar’in DY (2015) Facile synthesis of pyrido[2,3-d]pyrimidines via cyclocondensation of 4,6-dichloro-2-methylsulfanylpyrimidine-5-carbaldehyde with β-substituted β-aminoacrylic esters. Tetrahedron 71:6196–6203. https://doi.org/10.1016/j.tet.2015.06.085
Bagley MC, Hughes DD, Lloyd R, Powers VECA (2001) A new and highly expedient synthesis of pyrido[2,3-d]pyrimidines. Tetrahedron Lett 42:6585–6588. https://doi.org/10.1016/S0040-4039(01)01297-7
Ghaedi A, Bardajee GR, Mirshokrayi A, Mahdavi M, Shafiee A, Akbarzadeh T (2015) Facile, novel and efficient synthesis of new pyrazolo [3,4-b] pyridine product from condensation of pyrazole-5-amine derivatives and activated carbonyl group. RSC Adv 5:89652–89658. https://doi.org/10.1039/c5ra16769h
Bardajee GR, Mohammadi M, Yari H, Ghaedi A (2016) Simple and efficient protocol for the synthesis of benzoxazole benzoimidazole and benzothiazole heterocycles using Fe(III)–Schiff base/SBA-15 as a nanocatalyst. Chin Chem Lett 27:265–270. https://doi.org/10.1016/j.cclet.2015.10.011
Mahdavi M, Asadi M, Saeedi M, Ebrahimi M, Rasouli MA, Ranjbar PR, Foroumadi A, Shafiee A (2012) One-pot, four-component synthesis of novel imidazo[2,1-b]thiazol-5-amine derivatives. Synthesis 44:3649–3654. https://doi.org/10.1055/s-0032-1317515
Garmroodi FG, Omidi M, Saeedi M, Sarrafzadeh F, Rafinejad A, Mahdavi M, Bardajee GR, Akbarzadeh T, Firoozpour L, Shafiee A, Foroumadi A (2015) Simple and efficient syntheses of novel benzo[4,5]imidazo[1,2-a]pyridine derivatives. Tetrahedron Lett 56:743–746. https://doi.org/10.1016/j.tetlet.2014.12.099
Khalaj A, Nakhjiri M, Negahbani AS, Samadizadeh M, Firoozpour L, Rajabalian S, Samadi N, Faramarzi MA, AdipourN ShafieeA, Foroumadi A (2011) Discovery of a novel nitroimidazolyl-oxazolidinone hybrid with potent anti Gram-positive activity: synthesis and antibacterial evaluation. Eur J Med Chem 46:65–70. https://doi.org/10.1016/j.ejmech.2010.10.015
Mahdavi M, Asadi M, Saeedi M, Rezaei Z, Moghbel H, Foroumadi A, Shafiee A (2012) Synthesis of novel 1,4-benzodiazepine-3,5-dione derivatives: reaction of 2-aminobenzamides under bargellini reaction conditions. Synlett 23:2521–2525. https://doi.org/10.1055/s-0032-1317297
Bardajee GR (2013) A facile route to functionalized naphthalimide dyes via copper catalyzed C–N, C–O, and C–S cross-coupling reactions in aqueous medium. Tetrahedron Lett 54:4937–4941. https://doi.org/10.1016/j.tetlet.2013.07.010
Zhang J, Didierlaurent S, Fortin M, Lefrançois D, Uridat E, Vevert JP (2000) Potent nonpeptide endothelin antagonists: synthesis and structure-activity relationships of pyrazole-5-carboxylic acids. Bio org Med Chem Lett 10:2575–2578. https://doi.org/10.1016/S0960-894X(00)00513-8
Mallory WR, Morrison RW Jr, Styles VL (1982) Pyrimido[4,5-c]pyridazines. 3. Preferential formation of 8-amino-1H- pyrimido[4,5-c]-1,2-diazepin-6(7H)-ones by cyclizations with alpha, gamma-dioxo esters. J Org Chem 47:667–674. https://doi.org/10.1021/jo00343a013
Acknowledgements
This research was supported by Grants from PNU, Research Council of Tehran University of Medical Sciences and INSF.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghaedi, A., Bardajee, G.R., Mirshokrayi, A. et al. Facile access to new pyrido[2,3-d]pyrimidine derivatives. Mol Divers 23, 333–340 (2019). https://doi.org/10.1007/s11030-018-9852-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9852-1