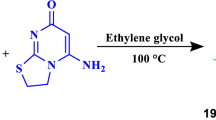
Abstract
A one-pot three-component cascade reaction for the green synthesis of a new class of 2-amino-5,8-dihydro-3H-pyrido[2,3-d]pyrimidin-4-ones was developed from the condensation of aromatic aldehydes with 2,6-diaminopyrimidin-4(3H)-one and acetophenone derivatives or various cyclic ketones in the presence of a catalytic amount of sodium carbonate in a mixture of water and ethanol at 60 \({^{\circ }}\hbox {C}\). This reaction led to the construction of two carbon–carbon bonds and one carbon–nitrogen bond in a single synthetic step.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pyrido[2,3-d]pyrimidine scaffold is present in a number of compounds showing a variety of biological properties, such as anticancer [1, 2], antitumor [3], antimicrobial [4] and antibacterial activities [5, 6]. Pterin and folic acid, a natural pigment and an essential vitamin, respectively, have a pyrazino[2,3-d]pyrimidine core. Folic acid (I) has a role in many prominent biological processes including DNA synthesis, DNA repair and cell division [7]. Additionally, several derivatives having this scaffold (e.g., II) were found to induce inhibition of cyclin-dependent kinase and induce apoptosis and/or reduce cell proliferation in different solid tumors and leukemia cell lines [8] (Fig. 1).
The most direct synthetic routes to pyrido[2,3-d]pyrimidines are via condensation of heterocyclic amines such as 2,6-diaminopyrimidin-4(3H)-one (1) and 6-amino-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one and aldehydes (or isatin) with various cyclic and acyclic ketones under a variety of reaction conditions. Some other methods are reported based on two-component reaction of 2,6-diaminopyrimidin-4(3H)-ones and chained conjugated aldehydes or ketones [9,10,11,12,13,14,15,16,17]. However, most of the methods have one or more limitations that may include moderate yields, harsh reaction conditions, long reaction times and use of nongreen organic solvents or catalysts [18,19,20,21,22,23]. Furthermore, all of these methods are reported for ketones containing an active \(\upalpha \)-hydrogen such as various \(\upbeta \)-dicarbonyl compounds and diphenylethanones in the presence of metal catalysts under microwave irradiation which give products only in moderate yields [24,25,26,27,28,29].
The use of toxic and hazardous solvents and catalysts in organic synthesis is considered a huge problem for health, safety of workers and environmental pollution. Green chemistry aims to eliminate the use of toxic solvents and catalysts and replace them with greener alternatives by re-engineering the selection approach of new substrates, innovative changes of the catalyst, greener solvents or using of solvent- and catalyst-free conditions [30, 31].
In continuation of our interest in using multi-component reactions (MCRs) in organic synthesis [32,33,34,35], herein we describe a complementary approach toward the synthesis of new 2-amino-5,8-dihydro-3H-pyrido[2,3-d]pyrimidin-4-ones via a new three-component condensation reaction using an aromatic aldehyde, 2,6-diaminopyrimidin-4(3H)-one and acetophenone derivatives or cyclic ketones in the presence of a catalytic amount of an inorganic base in aqueous medium at low temperature and short reaction times. This process is a straightforward approach for the synthesis of fused dihydropyridine and pyrimidine rings due to the suitable change in selection of substrates which simplified the reaction procedure and includes the use of a green solvent and a catalyst (Scheme 1).
Results and discussion
In order to investigate the optimal reaction conditions, we chose the reaction of benzaldehyde, acetophenone and 2,6-diaminopyrimidin-4(3H)-one as a model system. At first, the reaction rate was compared in different solvents using isolated yields of products with identical amounts of reactants where different catalysts are used with respect to the nature of the solvent (i.e., \(\hbox {Na}_{2}\hbox {CO}_{3}\) in protic and \(\hbox {Et}_{3}\hbox {N}\) in aprotic) at 60 \({^{\circ }}\hbox {C}\) (entries 1–9). The expected product 4a was scarcely obtained in nonpolar and aprotic polar solvents. Additionally, the reaction failed in the absence of catalyst in ethanol. It was found that the addition of water to the ethanol solution in the presence of \(\hbox {Na}_{2}\hbox {CO}_{3}\) can improve the reaction yield significantly (entries 10–12). As seen in entry 12, 4a was qualitatively obtained in water/ethanol (1:3). Next, the model reaction was studied in water/ethanol at various temperatures. As indicated in Table 1, when the temperature raised the reaction rate increased (entries 13–15). At 60 \({^{\circ }}\hbox {C}\), the maximum yield (98%) was obtained after 4 h. The model reaction was examined in water/ethanol (1:3) at 60 \({^{\circ }}\hbox {C}\) using different bases (entries 16–20). The best results were obtained using 10 mol % of \(\hbox {Na}_{2}\hbox {CO}_{3}\) or \(\hbox {K}_{2}\hbox {CO}_{3}\), and \(\hbox {Na}_{2}\hbox {CO}_{3}\) was chosen since it is more affordable than \(\hbox {K}_{2}\hbox {CO}_{3}\). As seen in Table 1, it is clear that the best results were obtained when the reaction was carried out at 60 \({^{\circ }}\hbox {C}\) for 4 h in water/ethanol (1:3) using 10 mol % of \(\hbox {Na}_{2}\hbox {CO}_{3}\) (entry 12).
With the optimized conditions established above, we next attempted to extend the process to six different ketones (3a-f) such as acetophenone, 4-nitroacetophenone, barbituric acid, 1-indanone, indendione and dimedone, and various types of aromatic aldehydes 2 such as benzaldehyde, 4-chlorobenzaldehyde, 4-nitrobenzaldehyde, 4-methylbenzaldehyde, 4-pyridinecarbaldehyde, 2-naphthaldehyde and 2-furfuraldehyde. The results in Table 2 show that all reactions proceeded smoothly to afford the expected 2-amino-5,8-dihydro-3H-pyrido[2,3-d]pyrimidin-4-one derivatives in good to excellent yields, and no undesirable side-reactions were observed under the reported conditions. The obtained products are shown in Fig. 2. All new compounds and some of the known compounds were characterized on the basis of their spectroscopic data (\({}^{1}\hbox {H}\) and \({}^{13}\hbox {C}\) NMR, FTIR, CHN analysis) and by comparison with those reported in the literature.
A possible mechanism for the formation of products 4a is shown in Schemes 2, 3. Firstly, a condensation between 2,6-diaminopyrimidin-4(3H)-one 1 and a benzaldehyde 2 would give the imine intermediate 5. The intermediate 5 undergoes nucleophilic addition of enolate 3 furnishing the intermediate product 6, which upon intramolecular cyclization and dehydration afforded 2-amino-5,8-dihydro-3H-pyrido[2,3-d]pyrimidin-4-one 4.
To clarify the proposed mechanism, the imine intermediate 5 was synthesized by reacting 2,6-diaminopyrimidin-4(3H)-one 1 and benzaldehydes 2 in water/ethanol mixture in 5 min in the absence of catalyst (as indicated by TLC). Next, acetophenone 3 and a base were added to the reaction mixture to afford product 4a. In addition, due to the instability of the intermediate 5, it was reacted with secondary 2,6-diaminopyrimidin-4(3H)-one 1 producing 5,5\(^{\prime }\)-(phenylmethylene)bis(2,6-diaminopyrimidin-4(3H)-one) 8 in absence of acetophenone after 30 min. These results support the proposed mechanism.
Conclusion
In conclusion, we have developed an environmental and economical three-component approach for the synthesis of a wide range of 2-amino-5,8-dihydro-3H-pyrido[2,3-d]pyrimidin-4-ones from aromatic aldehydes, ketones and 2,6-diaminopyrimidin-4(3H)-one in aqueous medium under mild conditions and short reaction times. This approach provides a high yielding reaction for wide functional group tolerance with very straightforward product isolation. We hope that this approach can be used in the synthesis of bioactive compounds.
Supplementary information
Supplementary data (copies of \({}^{1}\hbox {H}\) and \({}^{13}\hbox {C}\) NMR spectra for unknown compounds) associated with this article can be found in the online version.
Experimental
All starting materials in this work were purchased from Merck and Fluka Chemical Companies and used without purification. Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded on a Shimadzu IR-470 spectrometer. \({}^{1}\hbox {H}\) NMR and \({}^{13}\hbox {C}\) NMR spectra were recorded on a Bruker DRX-300 Avance spectrometer using DMSO-\(d_{6}\) as deuterated solvent. Chemical shifts are given in \(\updelta \) relative to tetramethylsilane (TMS) as an internal standard, and coupling constants J are given in Hz. Abbreviations used for \({}^{1}\hbox {H}\) NMR signals are s = singlet, d = doublet, m = multiplet and b = broad. Elemental analyses were performed with an Elementar Analysensysteme GmbH VarioEL.
General procedure for synthesis of 2-(1-(alkylcarbamoyl)-2,2-dicyanoethyl)-N-alkylbenzamides 4a-l
A solution of 2,6-diaminopyrimidin-4(3H)-one (0.13 g, 1 mmol), an aldehyde (1 mmol) and a ketone (1 mmol) in the presence of \(\hbox {Na}_{2}\hbox {CO}_{3}\) (0. 01 g, 0.1 mmol) in water/ethanol (1:3) (5 mL) was stirred at 60 \({^{\circ }}\hbox {C}\) for 4–6 h. After completion of the reaction, as indicated by TLC with ethyl acetate/n-hexane (4:1), the solution was diluted with water. The resulting solid was filtered and washed with water (10 mL). Then, the solid was crystalized from ethanol to afford pure product.
2-Amino-5,7-diphenyl-5,8 -dihydropyrido[2,3- d]pyrimidin-4(3H) -one ( 4a )
Pale brown solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.59 (d, 1H, J 3.0, CH), 5.07 (d, 1H, J 3.0, CH), 5.77 (OH), 6.20 (s, 2H, \(\hbox {NH}_{2})\) 7.1–7.5 (m, 10H, H-Ar), 8.36 (s,1H, NH), 10.20 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 38.1, 88.4, 103.5, 126.0, 127.8, 128.5, 128.6, 128.8, 136.0, 148.8, 154.2, 156.7, 162.3; Anal. Calcd. for \(\hbox {C}_{19}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}\): C, 72.13; H, 5.10; N, 17.71; found C, 72.11; H, 5.15; N, 17.65.
2-(Amino-\(\hbox {d}_{2}\) )-5,7-diphenyl-5,8-dihydropyrido[2,3-d]pyrimidin-4(3H)-one-3,8-\(d_{2}\) (4a, after addition of \(D_{2}O\))
\({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6}+ \hbox {D}_{2}\hbox {O})\, \delta \) 4.63 (d, 1H, J 3.0, CH), 5.13 (d, 1H, J 3.0, CH), 7.16–7.50 (m, 10H, H–Ar), 8.36 (s,1H, NH), 10.20 (s, 1H, NH).
2-Amino-5-(4 -chlorophenyl)-7- phenyl-5,8- dihydropyrido[2,3-d]pyrimidin-4(3H)-one (4b)
Pale brown solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1515, 780; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.88 (d, 1H, J 1.8, CH), 5.34 (d, 1H, J 1.8, CH), 5.87 (OH), 6.42 (2H, \(\hbox {NH}_{2}\)), 7.16-7.64 (m, 9H, H–Ar), 8.43 (s,1H, NH), 10.07 (s,1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 38.0, 87.8, 103.3, 126.5, 126.7, 127.6, 128.2, 128.9, 136.0, 149.4, 150.1, 152.9, 158.8, 160.0, 163.2; Anal. Calcd. for \(\hbox {C}_{19}\hbox {H}_{15}\hbox {ClN}_{4}\hbox {O}\): C, 65.05; H, 4.31; N, 15.97; found C, 64.98.11; H, 4.28; N, 15.95.
2-Amino-5-(4-nitrophenyl) -7-phenyl-5,8- dihydropyrido[2,3 -d]pyrimidin-4(3H) -one (4c)
Yellow solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1515, 1350; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\, \delta \) 4.77 (d, 1H, J 3.0, CH), 5.12 (d, 1H, J 3.0, CH), 5.82 (OH), 6.27 (s, 2H, \(\hbox {NH}_{2}\)), 7.35–7.99 (m, 9H, H-Ar), 8.24 (s,1H, NH), 10.24 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 38.3, 88.8, 103.7, 126.0, 126.3, 127.7, 128.8, 129.0, 136.2, 148.2, 150.8, 154.3, 157.1, 164.8; Anal. Calcd. for \(\hbox {C}_{19}\hbox {H}_{15}\hbox {N}_{5}\hbox {O}_{3}\): C, 63.15; H, 4.18; N, 19.38; found C, 63.10; H, 4.15; N, 19.40.
2-Amino-7-phenyl- 5-(p-tolyl)-5,8-dihydropyrido[2,3- d]pyrimidin- 4(3H)-one (4d)
Yellow solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr), \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 2962, 1650, 1609, 1550,1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\, \delta \) 2.50 (s, 3H, \(\hbox {CH}_{3}\)), 4.55 (d, 1H, J 4.8, CH), 5.05 (d, 1H, J 4.8, CH), 5.82 (OH), 6.23 (s, 2H, \(\hbox {NH}_{2}\)), 7.03–7.50 (m, 10H, H–Ar) 8.37 (s, 1H, N–H), 10.21 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\,\delta \) 21.1, 38.5, 88.5, 103.6, 125.9, 127.7, 128.8, 129.0, 147.1, 154.2, 156.8, 162.5, 164.6; Anal. Calcd. for \(\hbox {C}_{20}\hbox {H}_{18}\hbox {N}_{4}\hbox {O}\): C, 72.71; H, 5.49; N, 16.96; found C, 72.68; H, 5.45; N, 16.92.
2-Amino-7-phenyl- 5-(pyridin-4-yl)-5,8- dihydropyrido[2,3-d]pyrimidin -4(3H)-one (4e)
Yellow solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.64 (d, 1H, J 3.0, CH), 5.03 (d, 1H, J 3.0, CH), 6.24 (OH), 6.69 (s, 2H, \(\hbox {NH}_{2})\), 7.26–7.50 (m, 8H, H–Ar), 8.14 (s,1H, H–Ar), 8.50 (m, 2H, H–Ar), 10.48 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\,\delta \) 37.9, 86.8, 101.8, 123.2, 126.2, 128.8, 129.2, 135.8, 137.0, 148.9,149.9, 154.8, 156.7, 162.8; Anal. Calcd. for \(\hbox {C}_{18}\hbox {H}_{15}\hbox {N}_{5}\hbox {O}\): C, 68.13; H, 4.76; N, 22.07; found C, 68.2; H, 4.80; N, 22.02.
2-Amino-5-(naphthalen -2-yl)-7-phenyl-5,8- dihydropyrido[2,3- d]pyrimidin-4(3H)-one (4f)
Yellow solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.61 (d, 1H, J 3.0, CH), 5.03 (d, 1H, J 3.0, CH), 5.89 (OH), 6.36 (s, 2H, \(\hbox {NH}_{2})\), 7–7.78 (12H, m, H–Ar), 8.41 (s, 1 H, NH), 10.42 (s, 1 H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\,\delta \) 40.9, 89.4, 104.2, 125.5, 127.7, 128.6, 128.7, 128.9, 136.4, 137.2, 138.8, 152.8, 157.6, 162.2, 167.0; Anal. Calcd. for \(\hbox {C}_{23}\hbox {H}_{18}\hbox {N}_{4}\hbox {O}\): C, 73.39; H, 4.95; N, 15.29; found C, 73,45.2; H, 4.89; N, 15.20.
2-Amino-5-(furan-2-yl)-7-phenyl-5,8-dihydropyrido [2,3-d]pyrimidin-4(3H)-one (4g)
Yellow solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.89 (d, 1H, J 4.8, CH), 5.16 (d, 1H, J 4.8, CH), 5.89 (OH), 6.33 (s, 2H, \(\hbox {NH}_{2}\)), 7.06–7.75 (m, 8H, H–Ar), 8.42 (s, 1H, NH), 10.23 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 37.7, 89.6, 103.5, 110.0, 117.8, 127.3, 127.7, 128.9, 137.9, 142.1, 146.0, 146.9, 152.2, 158.0, 164.1; Anal. Calcd. for \(\hbox {C}_{17}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{2}\): C, 66.66; H, 4.61; N, 18.29; found C, 66.56; H, 4.71; N, 18.35.
2-Amino-7-(4-nitrophenyl)-5-phenyl-5,8-dihydropyrido [2,3-d]pyrimidin-4(3H)-one (4h)
Yellow solid; mp 295–297 \({^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3420, 3073, 1655, 1612, 1552, 1517, 1353; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 4.62 (d, 1H, J 5.1, CH), 5.31 (d, 1H, J 5.1, CH), 5.98 (OH) 6.64 (s, 2H, \(\hbox {NH}_{2})\), 7.13–7.40 (m, 6H, H–Ar), 8.18 (d, 2H, J 8.4, H–Ar), 8.32 (d, 2H, J 8.4, H–Ar), 8.44 (s, 1H, NH), 10.35 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\,\delta \) 37.8, 92.8, 103.3, 126.1, 126.4, 126.6, 128.5, 128.9, 133.0, 150.1, 154.2, 154.2, 159.9, 162.3; Anal. Calcd. for \(\hbox {C}_{19}\hbox {H}_{15}\hbox {N}_{5}\hbox {O}_{3}\): C, 63.15; H, 4.18; N, 19.38; found C, 63.25; H, 4.08; N, 19.40.
2-Amino-5-phenyl-3,5,6,11-tetrahydro-4H-indeno [2’,1’:5,6]pyrido[2,3-d]pyrimidin-4-one (4i)
Light brown solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\,\delta \) 2.73 (d, 2H, J 6.0, \(\hbox {CH}_{2})\), 2.89 (d, 2H, J 6.0, \(\hbox {CH}_{2}\)), 4.77 (s, 1H, CH), 5.94 (OH), 6.31 (s, 2H, \(\hbox {NH}_{2}\)) 7.13– 7.57 (m, 9H, H–Ar), 8.00 (s, 1H, NH), 10.02 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 34.5, 35.9, 103.8, 105.4, 126.1, 127.7, 128.5, 128.6, 128.8, 132.4, 149.0, 154.5, 156.8, 162.4; Anal. Calcd. for \(\hbox {C}_{20}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}\): C, 73.15; H, 4.91; N, 17.06; found C, 73.05; H, 4.85; N, 17.20.
2-Amino-5-phenyl-5,11-dihydro-3H-indeno [2’,1’:5,6]pyrido[2,3-d]pyrimidine-4,6-dione (4j)
White solid; mp \({>}300\, {^{\circ }}\hbox {C}; {}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6}) \,\delta \) 4.89 (s, 1 H, CH), 5.91(OH), 6.27(s, 2H, \(\hbox {NH}_{2})\), 7.09–7.80(m, 9H, H–Ar), 8.13 (s, 1H, NH), 10.03(s, 1H, NH).
2-Amino-8,8-dimethyl-5-phenyl-5,8,9,10-tetrahydropyrimido[4,5-b]quinoline-4,6(3H,7H)-dione (4k)
White solid; mp \(>300\,{^{\circ }}\hbox {C}; {}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6}) \,\delta \) 0.84 (s, 3 H, Me), 1.18 (s, 3 H, Me), 1.68–1.92 (m, 2 H, \(\hbox {CH}_{2}\)), 2.69 (s, 2H, \(\hbox {CH}_{2}\)), 4.89 (s, 1H), 5.59 (OH), 6.37 (s, 2 H), 7.18–7.52 (m, 5 H), 8.12 (s, 1 H), 9.87 (NH).
8-Amino-5-phenyl-5,10-dihydropyrido[2,3-d:6,5-d’] dipyrimidine-2,4,6(1H,3H,7H)-trione (4l)
White solid; mp \({>}300\,{^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3071, 1650, 1609, 1550, 1512; \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\, \delta \) 4.61 (s, 1 H, CH), 5.63 (OH), 6.33 (s, 2H, \(\hbox {NH}_{2}\)), 6.91 (s, NH), 7.17–7.36 (m, 5H, H-Ar), 8.38 (s, 1H, NH), 10.32 (s, 1H, NH), 10.78 (s, 1H, NH); \({}^{13}\hbox {C}\) NMR (75.47 MHz, DMSO-\(d_{6})\, \delta \) 43.8, 88.9, 109.2, 127.6, 128.3, 128.4, 133.4, 147.9, 148.9, 150.0, 162.5, 167.0, 167.8; Anal. Calcd. for \(\hbox {C}_{15}\hbox {H}_{12}\hbox {N}_{6}\hbox {O}_{3}\): C, 55.56; H, 3.73; N, 25.91; found C, 55.46; H, 3.83; N, 25.82.
5,5\(^\prime \)-(Phenylmethylene)bis(2,6-diaminopyrimidin -4(3H)-one) (8)
White solid; mp 198 \({^{\circ }}\hbox {C}\); IR (KBr) \(\upnu /\hbox {cm}^{-1}\) 3400, 3068, 1652, 1610, 1552, 1515, \({}^{1}\hbox {H}\) NMR (300.13 MHz, DMSO-\(d_{6})\, \delta \) 5.72 (s, 1 H, CH), 6.13 (OH), 7.1–7.5 (m, 5H, H–Ar), 8.12–8.21 (8H, \(NH_{2}\) ), 9.38–9.42 (b, 2H, NH).
References
Mahale S, Bharate SB, Manda S, Joshi P, Bharate SS, Jenkins PR, Vishwakarma RA, Chaudhuri B (2014) Biphenyl-4-carboxylic acid [2-(1H-Indol-3-yl)-ethyl]-methylamide (CA224), a nonplanar analogue of fascaplysin, inhibits Cdk4 and tubulin polymerization: evaluation of in vitro and in vivo anticancer activity. J Med Chem 57:9658–9672. doi:10.1021/jm5014743
Nandi S, Bagchi MC (2009) QSAR of aminopyrido[2,3-d]pyrimidin-7-yl derivatives: anticancer drug design by computed descriptors. J Enzyme Inhib Med Chem 24:937–948. doi:10.1080/14756360802519327
Barvian M, Boschelli DH, Cossrow J, Dobrusin E, Fattaey A, Fritsch A, Fry D, Harvey P, Keller P, Garrett M (2000) Pyrido[2,3-d]pyrimidin-7-one inhibitors of cyclin-dependent kinases. J Med Chem 43:4606–4616. doi:10.1021/jm000271k
Chen Y, Wu S, Tu S, Shi F, Li C (2008) An efficient synthesis of new benzo[\(1^{\prime },2^{\prime }:6,7\)]quinolino[2,3-d]pyrimidine derivatives via three-component microwave-assisted reaction. J Heterocycl Chem 45:1243–1246. doi:10.1002/jhet.5570450452
Reck F, Alm RA, Brassil P, Newman JV, Ciaccio P, McNulty J, Barthlow H, Goteti K, Breen J, Comita-Prevoir J (2012) Novel N-linked aminopiperidine inhibitors of bacterial topoisomerase type II with reduced pKa: antibacterial agents with an improved safety profile. J Med Chem 55:6916–6933. doi:10.1021/jm2008826
Chaudhari PK (2011) Synthesis and biological studies of trihydropyrido[2,3-d]pyrimidines-6-carbonitrile. Int J Life Sci Pharma Res 1:71–76
Ibrahim DA, Ismail NS (2011) Design, synthesis and biological study of novel pyrido[2,3-d]pyrimidine as anti-proliferative CDK2 inhibitors. Eur J Med Chem 46:5825–5832. doi:10.1016/j.ejmech.2011.09.041
Srinivasan A, Broom AD (1981) Synthesis of 3-deaza analogs of aminopterin and folic acid. J Org Chem 46:1777–1781. doi:10.1021/jo00322a004
Pastor A, Alajarin R, Vaquero JJ, Alvarez-Builla J, de Casa-Juana MF, Sunkel C, Priego JG, Fonseca I, Sanz-Aparicio J (1994) Synthesis and structure of new pyrido[2,3-d]pyrimidine derivatives with calcium channel antagonist activity. Tetrahedron 50:8085–8098. doi:10.1016/S0040-4020(01)85291-1
Bagley MC, Dale JW, Bower J (2002) A new one-pot three-component condensation reaction for the synthesis of 2,3,4,6-tetrasubstituted pyridines. Chem Commun 1682–1683. doi:10.1039/B203900A
Bagley MC, Singh N (2002) Microwave-assisted synthesis of 5-deaza-5,8-dihydropterins. Synlett 2002:1718–1720. doi:10.1055/s-2002-34246
Tu S, Zhang J, Zhu X, Xu J, Zhang Y, Wang Q, Jia R, Jiang B, Zhang J (2006) New potential inhibitors of cyclin-dependent kinase 4: Design and synthesis of pyrido[2,3-d]pyrimidine derivatives under microwave irradiation. Bioorg Med Chem Lett 16:3578–3581. doi:10.1016/j.bmcl.2006.03.084
Quiroga J, Cisneros C, Insuasty B, Abonía R, Cruz S, Nogueras M, de la Torre JM, Sortino M, Zacchino S (2006) Microwave-assisted three-component synthesis and in vitro antifungal evaluation of 6-cyano-5,8-dihydropyrido[2,3-d]pyrimidin-4(3H)-ones. J Heterocycl Chem 43:299–306. doi:10.1002/jhet.5570430208
Tu S, Wang Q, Xu J, Zhu X, Zhang J, Jiang B, Jia R, Zhang Y, Zhang J (2006) An efficient one-pot synthesis of 5-aryl substituted 2-amino-5,8-dihydropyrido[2,3-d]pyrimidin-4,7-diones under microwave irradiation without catalyst. J Heterocycl Chem 43:855–858. doi:10.1002/jhet.5570430407
Shi D, Ji S, Niu L, Shi J, Wang X (2007) One-pot synthesis of pyrido[2,3-d]pyrimidines via efficient three-component reaction in aqueous media. J Heterocycl Chem 44:1083–1090. doi:10.1002/jhet.5570440517
Tu SJ, Zhang Y, Jiang H, Jiang B, Zhang JY, Jia RH, Shi F (2007) A simple synthesis of furo[\(3^{\prime },4^{\prime }:5,6\)]pyrido[2,3-d]pyrimidine derivatives through multicomponent reactions in water. Eur J Org Chem 2007:1522–1528. doi:10.1002/ejoc.200600913
Wang X-S, Zhang M-M, Li Q, Yao C-S, Tu S-J (2008) Simple procedure for the synthesis of 5,7-diarylpyrido[2,3-d]pyrimidine derivatives catalyzed by KF-alumina. Synth Commun 38:1896–1908. doi:10.1080/00397910801997488
Tu S, Li C, Shi F, Zhou D, Shao Q, Cao L, Jiang B (2008) An efficient chemoselective synthesis of pyrido[2,3-d]pyrimidine derivatives under microwave irradiation. Synthesis 369–376. doi:10.1055/s-2008-1032031
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Spirooxindoles: reaction of 2,6-diaminopyrimidin-4(3H)-one and isatins. Tetrahedron 65:2005–2009. doi:10.1016/j.tet.2009.01.013
Shi F, Ding J, Zhang S, Hao W-J, Cheng C, Tu S (2011) Substrate-controlled chemoselective synthesis and potent cytotoxic activity of novel 5,6,7-triarylpyrido[2,3-d]pyrimidin-4-one derivatives. Bioorg Med Chem Lett 21:1554–1558. doi:10.1016/j.bmcl.2010.09.114
Imani Shakibaei G, Feiz A, Reza Khavasi H, Abolhasani Soorki A, Bazgir A (2010) Simple three-component method for the synthesis of spiroindeno[1,2-b]pyrido[2,3-d]pyrimidine-5,\(3^{\prime }\)-indolines. ACS Comb Sci 13:96–99. doi:10.1021/co1000053
Shakibaei GI, Feiz A, Bazgir A (2011) A simple and catalyst-free three-component method for the synthesis of spiro[indenopyrazolopyridine indoline]diones and spiro[indenopyridopyrimidine indoline]triones. Comptes Rendus Chim 14:556–562. doi:10.1016/j.crci.2010.10.001
Jadidi K, Ghahremanzadeh R, Mirzaei P, Bazgir A (2011) Three-component synthesis of spiro[indoline-3,\(5^{\prime }\)-pyrimido[4,5-b]quinoline]-triones in water. J Heterocycl Chem 48:1014–1018. doi:10.1002/jhet.655
Shahrzad A, Saeed B (2012) An efficient synthesis of pyrido[2,3-d]pyrimidine derivatives via one-pot three-component reaction in aqueous media. Int J Org Chem 2:7–14. doi:10.4236/ijoc.2012.21002
Du BX, Li YL, Wang XS, Shi DQ (2013) Ionic liquid as an efficient and recyclable reaction medium for the synthesis of pyrido[2,3-d]pyrimidines. J Heterocycl Chem 50:534–538. doi:10.1080/00397911.2016.1165254
Naeimi H, Rashid Z, Zarnani A-H, Ghahremanzadeh R (2014) \(\text{ MnFe }_{2}\text{ O }_{4}@\text{ NH }_{2}@2\text{ AB-Ni }\): a novel, highly active, stable and magnetically recoverable nanocatalyst and use of this heterogeneous catalyst in green synthesis of spirooxindoles in water. New J Chem 38:5527–5535. doi:10.1039/C4NJ01182A
Naeimi H, Rashid Z, Zarnani AH, Ghahremanzadeh R (2014) Efficient synthesis of novel spiro-furo-pyrido-pyrimidine-indolines by manganese ferrite nanoparticles as a highly active magnetically reusable nanocatalyst in water. New J Chem 38:348–357. doi:10.1039/C3NJ00940H
Yao C, Lu T, Yu C, Wang X, Li T (2014) A Catalyst-free aqueous synthesis of 2-amino-7,9-dihydrothieno[\(3^{\prime },2^{\prime }\):5,6]pyrido[2,3-d]pyrimidine-4,6(3H,5H)-dione derivatives. J Heterocycl Chem 51:163–166. doi:10.1002/jhet.1915
Wang J, Li J, Liu H, Xu Z, Zhu S (2015) A facile and efficient synthesis of spiro[indoline-3,5-pyrido[2,3-d]pyrimidine] derivatives via microwave-assisted multicomponent reactions. Lett Org Chem 12:62–66. doi:10.2174/157017861201150112124526
DeSimone JM (2002) Practical approaches to green solvents. Science 297:799–803. doi:10.1126/science.1069622
Sheldon RA (2005) Green solvents for sustainable organic synthesis: state of the art. Green Chem 7:267–278. doi:10.1039/B418069K
Shaabani A, Sepahvand H, Nejad MK (2016) A re-engineering approach: synthesis of pyrazolo[1,2-a]pyrazoles and pyrano[2,3-c]pyrazoles via an isocyanide-based four-component reaction under solvent-free conditions. Tetrahedron Lett 57:1435–1437. doi:10.1016/j.tetlet.2016.02.051
Shaabani A, Hooshmand SE, Tabatabaei AT (2016) Synthesis of fully substituted naphthyridines: a novel domino four-component reaction in a deep eutectic solvent system based on choline chloride/urea. Tetrahedron Lett 57:351–353. doi:10.1016/j.tetlet.2015.12.017
Shaabani A, Mofakham H, Maleki A, Hajishaabanha F (2010) Novel isocyanide-based one-pot multicomponent syntheses of tetrahydrobenzo[b][1,4]oxazepine and malonamide derivatives. J Comb Chem 12:630–632. doi:10.1021/cc100032d
Shaabani A, Rezayan AH, Keshipour S, Sarvary A, Ng SW (2009) A novel one-pot three-(in situ five-) component condensation reaction: an unexpected approach for the synthesis of tetrahydro-2,4-dioxo-1H-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives. Org Lett 11:3342–3345. doi:10.1021/ol901196z
Acknowledgements
We gratefully acknowledge financial support of the Iran National Elites Foundation (INEF) and the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaabani, A., Sepahvand, H., Boroujeni, M.B. et al. A green one-pot three-component cascade reaction: the synthesis of 2-amino-5,8-dihydro-3H-pyrido[2,3-D]pyrimidin-4-ones in aqueous medium. Mol Divers 21, 147–153 (2017). https://doi.org/10.1007/s11030-016-9712-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9712-9