Reduce, reuse, and recycle are important techniques for waste management. These become significant for improving environmental and economic condition of industries. Integrated steel industries are generating huge amounts of steel slag as waste through the blast furnace and Linz–Donawitz (LD) process. Presently, these wastes are disposed by dumping in an unplanned manner, which causes many environmental problems. The generation rate of slag produced from steel industries is found to be in the range of 150–200 kg per ton of steel production. The LD slag generated by the basic oxygen converter, is one of the waste which can be reused due to the presence of a considerable amount of valuable minerals. In order to recycle and reuse the waste, assessment of their physicochemical, mineralogical and geotechnical characterization is imperative. This paper addresses the characterization and possible utilization of LD slag.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction. Steel production in all integrated steel plants requires five components namely air, water, fuel, power, and raw materials like iron ore, limestone, etc. During the steel production process, considerable amounts of solid wastes are being generated in steelmaking units such as blast furnace (BF) slag, tar sludge, flue dust, Linz–Donawitz (LD) converter slag, etc. The basic oxygen furnace (BOF) steelmaking slag also known as LD slag, is one of the important industrial byproducts generated in the steelmaking process or pig iron refining process from integrated steel plants. In this process the hot metal is converted to steel by blowing of oxygen in the LD converters [1–4]. The LD slag contains a considerable amount of metals and chemical components that can be useful for various applications. The chemical composition and major phases of typical LD slag samples generated at integrated steel plants in India [1] are depicted in Table 1.
The average rate of generation of LD slag is approximately 150–180 kg per ton of crude steel in India [5]. Researchers also found that this rate of production of LD slag somtimes reaches a high of 200 kg per ton of crude steel [6]. In India, over 12.15–14.58 metric tons of steelmelting slag has been generated in the financial year 2013, and this rate is still increasing with the escalation of steel production [7]. However, it depends on the quality of raw materials and yield efficiency of the steel plants. In India, the utilization of LD slag is a meagre 25% compared to a high of 70–100% in other countries [6]. Therefore, disposal of this huge mass of waste slag has become a problem due to environmental and space constraints in the steel plants. The utilization of integrated steel plant waste by an economical and environmental friendly technique will decrease a major portion of the production cost. If these steel plant wastes are reused as a raw material substitute then it is possible to conserve valuable natural resources like dolomite that are used as fluxing material to reduce the iron in steel making thereby reduce the environmental hazards of mining the ore and reducing slag dumping space [8]. Special processing of slag can produce similar aggregate products originating from the rock and mineral industries. Therefore, much effort has been exerted on utilization of slags as raw materials for infrastructure sector. The LD slag is utilized as a raw material for concrete production, road construction, railway ballast, plastering, and other civil engineering works [9]. Such utilization of the slags will bring in the 3R (reduce, reuse, and recycle) concept to provide an effective and sustainable disposal system for this industrial waste.
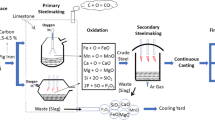
Steelmaking process by Basic Oxygen Furnace (BOF) or Linz–Donawitz (LD) converter and slag generation: In all integrated steel plants, basic oxygen furnaces are charged with the molten iron produced in the blast furnace along with steel scraps. Usually, the BOF charge mainly consists of about 10–20% of steel scrap and 80–90% of molten iron [10, 11]. A typical iron and steelmaking process is depicted in (Fig. 1) [12]. The BOF/LD has to be maintained at a temperature of approximately 1600–1650°C. Figure 2 shows a schematic diagram of the BOF process and its slag generation.
Flowchart of iron and steelmaking processes and slag generation in modern steel plant, modified from [12].
Schematic representation of the basic oxygen furnace process, modified from [12].
Initially in BOF, the steel scrap is charged and a ladle of molten iron (∼200 tons) is poured over it with a crane. An oxygen lance is then lowered into the furnace, which blows 99% pure oxygen on the charge at supersonic speed. The blowing cycle, continues for 20–25 min, wherein intense oxidation reaction removes the impurities of the charge. The carbon impregnated in the steel is burnt to form carbon monoxide, causing the temperature to rise to 1600–1700°C. The scrap gets melted and the carbon content of the molten iron is lowered [10, 11]. The furnace is also charged with fluxing agents, such as lime (CaO) or dolomite (MgCa(CO3)2), to remove the unwanted chemical elements of the melt during the oxygen blowing cycles. Molten metal samples are collected and tested for their chemical composition near the end of the blowing cycle. Once the chemical composition is achieved, the oxygen lance is pulled up from the furnace. The BOF is then tilted in one direction in order to tap the steel into ladles. The steel produced either undergoes further refining in a secondary refining unit or sent directly to a continuous caster where semifinished shapes are solidified. After the molten steel is removed from the BOF, it is again tilted in the opposite direction to pour the liquid slag into ladles. From the steelmaking cycle, the slag is later processed, and the final product is referred to as LD slag [10, 11, 13].
Chemical and mineralogical composition of LD slag. The chemical and mineralogical characteristics of LD slag are mainly determined by using the ICP-AES and C-H-N-S analyzer, and the main mineral phases by using x-ray diffraction (XRD), scanning electron microscope (SEM), electron probe microanalysis (EPMA) and energy dispersive x-ray spectroscopy (EDS). It was observed that, the slag mainly contains various components like CaO, Fe, SiO2, and Mn. The lime content measurements were carried out by using three different analytical techniques, namely the Leduc test, thermos-gravimetric analysis, and Bernard calcinatory analysis [14]. The major phases present in LD slag were studied and found to contain two phases, one that contains some reactive mineral phases such as 2CaO.SiO2, 3CaO.SiO2, and free CaO and MgO, while the other is the metal phase [15]. The EPMA study of LD slag sample was carried out to determine the association of phosphorus in LD slag, and there was some variation found with respect to Fe, Ca, Al, and Mn content. However, it is evident that the P content in the slag is more attached to the calcium phase than to the iron phase [1]. The chemical composition and several phases of LD slag are summarized in Tables 2 and 3, respectively.
Morphological properties of LD slag. Different studies have been carried out on the morphological characteristics of LD slag. From SEM studies it is observed that LD slag samples are rough textured, cubical and angular in external appearance. Internally, each particle is vesicular in nature with many noninterconnected cells. The cellular structures are formed by the gases entrapped in the hot slag at the time of cooling and solidification. Since these cells do not form connecting passages, the term cellular or vesicle is more applicable to steel slag than the term porous [35]. Other SEM studies showed that the sand and silt size BOF slag particles have subrounded to angular shapes. Distinct asperities and edges are visible in angular, bulky particles. Most of the sand and silt size particles examined under the SEM have rough surface textures as depicted in (Fig. 3) [12, 33].
SEM micrographs of LD slag samples: a) LD slag from Mittal Steel, Indiana Harbor Works West Plant, Indiana. Image shows that the sand and silt size LD slag particles are subrounded to angular shapes [12]; b) LD slag from Bokaro Steel Plant, Jharkhand, India. Image shows that LD slag particles are rough textured, cubical and angular in external appearance and internally vesicular in nature [35].
Geotechnical properties of LD slag. The applications of LD slag samples are closely related to their geotechnical properties. Some of the geotechnical properties of steel slags were investigated and compared with those of natural aggregates [40]. The results are summerized in Table 4. Based on the above geotechnical properties, it can be observed that
-
1)
the densities of steel slag are higher than that of the natural aggregates due to the high content of iron;
-
2)
steel slags are hard materials, as their compressive strengths are close to the strength of granite; and
-
3)
the resistance of steel slags to impact is approximately similar to the natural aggregates as they are difficult to crush, and the Los Angeles test values are also high, indicating that they are difficult to grind.
Additionally, steel slags contain some reactive minerals like 2CaO.SiO2, 3CaO.SiO2, and free CaO and MgO, which are water hardenable materials [41, 42]. When certain amounts of free CaO or MgO are present in steel slag, the slag is not stable in volume due to the hydration of these free compounds [40, 42, 43].
Environmental issues of LD slag: It has been noted by some researchers that the discharge of metals and elements from LD slag may cause environmental problems such as air, water, and soil pollution. These dry LD slag dumps represent a toxicological risk to the human body through inhalation of small slag particles i.e., those less than 10 μm [44–49]. The leachates pollute the soil, groundwater, as well as surface water bodies. To confirm this statement, the leaching parameters were studied by conducting toxicity characteristic leaching procedure (TCLP) test of iron and steel slags in the USA. It was observed that the trace elements that may be present include nickel, chromium, lead, zinc, titanium, and vanadium. Chromium is found in higher amounts in the slag, but the concentrations in the leachate are low because the chromium ions are bound within stable crystalline phases. It has been observed that none of the metals in the leachates exceed the TCLP criterion concentrations. LD slag can be highly alkaline due to the presence of free lime. The runoff or leachate through exposed steel slag stockpiles, embankment fills or granular bases can exhibit high pH values, which may impact the local aquatic ecology. Organic chemicals or substances do not exist in the slag because of their high melting temperatures. The dumping of slags causes a shortage of land and other associated problems. Thus, it can be concluded that iron and steel slags should not be considered a hazardous waste and may be used for various purposes [49].
Utilizations of LD Slag
Use for soil stabilization and as a soil conditioner. LD slag is used as a soil additive to improve its physicochemical properties and in situ stabilization of Cu and other trace metals in a sandy Cu contaminated soil. It was found that soil pH increases with a higher incorporation rate of LD slag and allows bean growth, foliar Ca concentration, and further reduced foliar Cu concentration below its upper critical value, thus avoiding excessive soil EC and Zn deficiency [50]. Experiments have found that the soil pH increases from 5.3 to 6.4 with the use of 7500 kg of slag per hectare, the second year response being higher, i.e., 41% increase in soil pH with 3000 kg slag per hectare [51]. This is in the natural range and shows a positive effect on mustard and wheat seedling growth. Thus, this can be used in rural agriculture for better plant growth [52]. The Government of India, Ministry of Finance has issued a circular (Circular No 553/49/2000-CX New Delhi, October 18, 2000) notifying that LD slag may be used as a soil conditioner after certain processing such as crushing, washing, and addition of rock phosphates. LD slag contains 29% calcium in the form of CaO. LD slag also contains phosphorous in the form of P2O5. Thus use of LD slag has a limiting to ground limestone and is used regularly to reduce the need of liming on acidic soil [53]. Steel slag has also been used as amendment for metal-contaminated soils after proper environmental assessment [54–56].
Use as a fertilizer. Attempts have been made in Tata Steel, India, to use LD slag after grinding to 300 mesh as a soil conditioner in paddy fields, tea gardens, etc. [57]. Nippon Kokan Corporation (NKK) Japan has developed a process to produce eco-friendly slow release potassium silicate fertilizer from the slag that shows less release effect rather than conventional fertilizers [58]. Many experimental works have been conducted for the production of fertilizers from LD slag, semi-calcined dolomite, and ammonium sulfate, and their agricultural applications for agro-forestry and pasture farming. The potential economic benefits of applying this new fertilizer to the soil were also evaluated [59]. According to soil type and agricultural use, by adding a concentration of LD slags between 1.5 and 5.0 tons/ha, it is possible to achieve an increase in soil pH and hence to improve the soil quality and productivity. Experimental works were also carried out using pulverized LD slag for growing vegetables and crops like tomato, potato, onion, spinach and wheat in acidic soil [60].
Use for road making and floor preparation. With increasing environmental awareness, the waste utilization of steel plants has become an attractive alternative to disposal. For the sustainable development of the steel industry innovative environmental solutions should be applied. Nippon Slag Association in Japan is using LD slag in port and harbor construction [61]. LD slag bolders are used for floor preparation and in road making for its high hardness and cementing properties. Many steel plants are selling more than 50% of LD slag for construction and ground filling. The LD slag has proved to be an excellent railway ballast material and is being used by Indian Railways. In the Durgapur Steel Plant (Steel Authority of India) the LD slag is sold in the form of boulders for road making. Due to presence of lime and magnesia, LD slag absorbs moisture and CO2 from atmosphere to form hydroxides and carbonates which leads to volume expansion or swelling in road or building materials. This problem can be overcome by weathering the slag for a duration of 6 to 9 months for the hydration of free lime before its use [62].
Use in cement making for replacement of clinker. In India, cement manufacturers are unwilling to take advantage of the LD slag as a low cost raw material for cement manufacture, whereas its use in cement making is commercialized in China. At Tata Steel, India, a project was initiated in collaboration with Lafarge India for use of LD slag for cement making [57]. In a comparison of LD slag and BF slag, LD slag has higher CaO content and acts as an activator and gives better strength, though the presence of P2O5 creates corrosion in reinforced concrete structure. If only 10% LD slag is used in cement, then the P2O5 content will be around 0.3% which is not so harmful in Portland slag cement (PSC), because low P2O5 in PSC reacts with alkali contributing additional strength of cement [63]. Thus, it is not possible tu use than 10% LD slag in PSC. Due to the presence of iron oxide in LD slag it forms a phase, tetracalcium aluminoferrite which has an adverse effect on cement quality. This ferruginous part of LD slag can be separated by magnetic separation [64].
Use in sinter plant after removal of phosphorus. Removal of phosphorus from LD slag is a better option for recyclable iron making processes. Many authors prefer physical processes like magnetic separation, flotation, dual phase separation, etc. for the same. In magnetic separation study, particles with high magnetic susceptibility are separated by a light magnetic field, and weaker magnetic particles will be separated by a strong magnetic field. Therefore, heterogeneous magnetic fields of different intensities can separate different particles. However, this is not a very effective way of dephosphorization [65, 66]. A numbers of workers have performed chemical treatment (pyrometallurgical) of LD slag [67]. Phosphorus is also removed through evaporation by adding silicon to the Fe–P–C alloy obtained slag with carbon. During reduction of converter slag in an electric furnace it is possible to form two different phases: one metallic and other nonmetallic [68]. The reduction of phosphorus in molten slag containing 8.3% Al2O3 and 3.5% P2O5 with basicity of CaO/SiO2 of 1.1 at 1735 K, 1823 K, and 1893 K has been studied. It shows that temperature has an effect on the reduction of phosphate by graphite. The possibility of reduction of phosphorus by CO gas at 1823 K and the increase in reduction rate with increasing CO gas flow rate has also been investigated. It was concluded that the reaction rate of P2O5 was not controlled by diffusion of the P2 gas phase product but by chemical reaction [69].
At Tata Steel a two-stage crushing facility at the raw material bedding yard has been found to be adequate to crush to the raw material to the desired size (90% of the material to –3mm) for sinter making [70]. It has been reported that high gradient magnetic separation (HGMS) was able to separate around 50% of the P from the LD slag. It was also possible to remove around 70% of phosphorus from slow cooled slag by HGMS. Slow cooling of LD slag promotes mineral grain growth and formation of calcium phosphate as fine grained crystal [71]. Hot metal with a phosphorus content of 0.09–0.16% was treated in a ladle with a dephosphorization agent consisting of LD slag, iron oxide or lime and the phosphorus content of hot metal was reduced to 0.020–0.060 or 0.003–0.020%, respectively. By refining the dephosphorized hot metal in the LD converter, extra low phosphorus steel having a phosphorus content of less than 0.005% was obtained [72]. A study on bacterial leaching for the removal of P from LD slag was carried out by using specific bacteria for P dissolution, namely Frateuria aurantia. The studies were undertaken at 10% (v/v) inculcation. It was possible to remove around 72.17% P from the LD slag [73]. The phosphorus solubilization efficiency was studied at different pulp densities, incubation periods, NaCl concentrations, and at different initial pH of the medium. About 46.87% of P could be solubilised as P2O5 from LD slag at 5% pulp density after 24 days of incubation [74].
Use in recovery of different metal values. The metal values recovery from LD slag was carried out by different techniques. Out of these techniques, the smelting reduction technique was carried out for valuable metals recovery, i.e., vanadium and chromium using a Tamman furnace. The recoveries of metal from LD slag are as high as 98% at 1600°C in 30 min [75]. This process was carried out by addition of a small quantity of mineral additive to the molten slag followed by crystallization of the slag. The additive acts as nuclei for crystallization of dicalcium silicate in the slag, and the breaking of slag produces 65–80% slag and 10–15% chips [76]. It was also reported that the most harmful components in LD slag are phosphorus and sulfur, which are to be removed before use either in the sintering plant or the blast furnace. In the Bhilai Steel Plant, India, LD slag was used in the blast furnace but later discontinued due to its high sulfur and phosphorus content [77]. However, the slag is not suitable in cement making due to the presence of a high percentage of iron oxide. The study has been carried out by magnetic separation techniques, and it was found that the combination of low and high intensity magnetic separation of ground LD slag at 63μm in the wet proces is effective [42].
Use for wastewater treatment. The use of steel slag in industrial wastewater treatment has received intensive attention in recent years. Due to the porous structure and large surface area of steel slag, it is easy to separate from water due to its high density. A high adsorption capacity of steel slag was observed in the treatment of mercury-containing seawater [78]. The steel slag has been used as a low cost adsorbent for arsenic in aqueous systems, showing 95–100% removal efficiency at pH 2. The removal mechanism included the coprecipitation of the analyte from the solvent and its adsorption in CaCO3 [79]. The removal of copper from wastewater using steel slag was conducted by adsorption and precipitation [80]. Additionally, steel slag can be used as a separate adsorbent to remove aqueous ammonium nitrogen [81], phosphorous [82], and phenol [83].
Use in CO 2 capture and flue gas desulfurization. CO2 is one of the primary greenhouse gases and a large contributor to climate change. Thus, carbon capture and storage (CCS) research has been the focus of CO2 reduction technology. With the current CO2 sequestration routes, mineral CO2 sequestration is regarded as a potential important technology due to its benefits such as environmental freindliness, and permanent trapping of CO2 in the form of carbonate and without the need for post-storage surveillance for CO2 leakage [84]. CO2 gas is stored by allowing magnesium or calcium oxides in silicate minerals to react with carbon dioxide and form carbonates in mineral carbonation [85]. It is possible to store CO2 in carbonate form using steel slag slurry under mild conditions of temperature and CO2 pressure though steel slag contains a large amount of CaO [86]. The method of flue gas desulfurisation includes wet, dry, and semidry processes, among which the wet limestone/lime method is most widely used. It was also concluded that aglomeration gas desulfurization with steel slag was feasible [87]. The experiment was carried out on wet flue gas desulfurization with scrap slag powder residue. It was inferred that a more than 60% wet desulfurization rate could be achieved with a reasonable design and suitable operation by using steel slag. However, this technology is still limited to the laboratory research stage [88]. From this study, it can be confirmed that LD slag may be used in different fields due to its various properties. The uses, advantages, and disadvantages are shown in Table 5 and Fig. 4.
Conclusions. From the above study it can be concluded that the steel industry is now focused on increasing the recycling of LD slags day by day to conserve energy and natural resources and ultimately improve production. New technologies and/or the improvement of existing technologies have been investigated and developed in order to achieve the ambitious target of “zero-waste” in the incoming years. The effective utilization of LD slags turns into a high-value added product and allows improving the steel plant competitiveness. On other hand, the sustainable use of slags contributes to natural resource saving. Thus the use of LD slag in different fields produces not only economic but also ecological advantages, since their use has led to resource conservation and thus solves the disposal problems. This paper demonstrates the significance of LD slag and its utilization.
Due to its high lime content, LD slag can replace lime addition in steelmaking. It can also be used for road making and floor preparation, cement making for replacement of clinker, and recovery of metal values. The phosphorus-enriched slag can also be used as a fertilizer for agricultural purposes and as a soil conditioner for acidity correction of soil. The presence of endemic phosphorus in LD slag is sometimes high, which restricts its reutilization in iron production and steelmaking. The amount of phosphorus can be reduced by physical, chemical, and biological means. As phosphorus is intimately associated with other elements in slag, bioleaching may be an ideal approach for its removal from the LD slag. Also it is possible to use LD slag for recovery of metal values, CO2 capture and flue gas desulfurization and wastewater treatment through LD slag by applying alternative techniques. However, dephosphorization consumes fluxes and produces a high phosphorus slag, which again needs to be disposed off. Therefore, reuse and recycling of LD slag after removal of phosphorus seems to be a better approach. This will not only solve the present-day problem of LD slag but will also lead to the achievement of zero waste status and sustainable utilization of byproducts of the steel industry.
A detailed study on steel manufacturing with waste management of LD slag reveals that it can be cost effective if handled properly. For reuse of LD slag, after tapping of heat, the slag can be treated under a slow cooling process. The three phases (dicalcium silicate, dicalcium ferrite, and wustite) can be separated. After separation, the iron rich portion (wustite) can be recycled for iron manufacture or steelmaking, and the lime-rich portion (dicalcium ferrite) can be converted to Mg–Mn-wustite by adding a mixture of metallic powder and coke breeze. If the P content is within tolerable limits in dicalcium ferrite, it can be used directly in iron manufacture or steelmaking. The P-rich portion (dicalcium silicate) can be used for fertilizer making. On the other hand, LD slag can be used for landfilling after checking its long-term leaching properties.
References
B. Das, S. Prakash, P. S. R. Reddy, and V. N. Mishra, “An overview of utilisation of slag and sludge from steel industries,” Resour. Conserv. Recycl., 50, 40–57 (2007).
J. Alexandre and J. Y. Boudonnet, “Les laitiers d'aciérie LD et leurs utilisations routières,” Laitier sidérurgiques, 75, 57–62 (1993).
S. Wu, Y. Xue, Q. Ye, and Y. Chen, “Utilisation of steel slag as aggregates for stone mastic asphalt (SMA) mixtures,” Build Environ., 42, 2580–5 (2007).
D. H. Shen, C. M. Wu, and J. C. Du, “Laboratory investigation of basic oxygen furnace slag for substitution of aggregate in porous asphalt mixture,” Constr. Build Mater., 23, 453–61(2009).
U. S. Yadav, B. K. Das, A. Kumar, and H. S. Sandhu, “Solid waste recycling through sinter status at Tata Steel,” Proc. Int. Environment and Waste Management., NML, Jamshedpur, India (2002), pp. 81–94.
T. Umadevi, S. P. Rao, Pankaj. Roy, et al., “Influence of LD slag on iron ore sinter properties and productivity,” 6th International Seminar on Mineral Processing Technology, NML Jamshedpur, 747–757 (2010).
An Overview of Steel Sector, http://steel.gov.in/Annual%20Report%20(201314)/English/Annual%20Report%20(English).pdf.
J. Pal, P. N. Chaudhary, and M. C. Goswami, “Utilisation of LD slag – An overview,” J. Met. Mater. Sci., 45, No. 2, 61–72 (2003).
H. Kolb and W. Leipold, “Slag for the building industry,” Redex Rundschau, No. 1–2, 261–9 (1993).
H, Schoenberger, “Final draft: best available techniques reference document on the production of iron and steel,” Publications of EC: European Commission, Joint Research Centre, IPTS, European IPPC Bureau, (2001).
D. Brandt and J. C. Warner (3rd ed.), Metallurgy Fundamentals, Goodheart-Willcox, Tinley Park, Ill, USA (2005).
I. Z. Yildirim and M. Prezzi, “Chemical, mineralogical, and morphological properties of steel slag,” Adv. Civ. Eng., 13 (2011).
S. Seetharaman, Fundamentals of Metallurgy, Woodhead Publishing and CRC Press, Boca Raton, Florida, USA (2005).
J. Waligora, D. Bulteel, P. Degrugilliers, et al., “Chemical and mineralogical characterisations of LD converter steel slag: A multi-analytical techniques approach,” Mater. Charact., 61, 39–48 (2010).
D. C. Goldring and L. M. Juckes, “Petrology and stability of LD slag,” Iron Making Steel Making, 24, No. 6, 447–56 (1997).
J. N. Murphy, T. R. Meadowcroft, and P. V. Barr, “Enhancement of the cementitious properties of steelmaking slag,” Can. Metall. Q., 36, 315–331(1997).
A. Monshi and M. K. Asgarani, “Producing Portland cement from iron and steel slags and limestone,” Cem. Concr. Res., 29, 1373–1377(1999).
W. Xuequan, Z. Hong, H. Xinkai, and L. Husen, “Study on steel slag and fly ash composite Portland cement,” Cem Concr Res., 29, No. 7, 1103–1106 (1999).
Y. S. Li, “The use of waste basic oxygen furnace slag and hydrogen peroxide to degrade 4-chlorophenol,” Waste Manage., 19, 495–502 (1999).
H. Motz and J. Geiseler, "Products of steel slags an opportunity to save natural resources,” Waste Manage., 21, 285–293 (2001).
S. K. Kawatra, S. J. Ripke, “Pelletising steel mill desulphuurisation slag,” Int. J. Miner. Process., 65, 165–175 (2002).
I. A. Altun and I. Yilmaz, “Study on steel furnace slags with high MgO as additive in Portland cement,” Cem. Concr. Res., 32, 1247–1249 (2002).
K. Morita, M, Guo, N, Oka, and N, Sano, “Resurrection of the iron and phosphorous resource in steel-making slag,” J. Mater. Cycles. Waste Manage., 4, 93–101 (2002).
L. M. Juckes, “The volume stability of modern steelmaking slags,” Miner. Process. Extr. Metall. Rev., 112, No. 3, 177–197 (2003).
H. Shen and E. Forssberg, “An overview of recovery of metals from slags,” Waste Manage., 23, 933–949 (2003).
Y. Topkaya, N. Sevinc, and A. Gunaydin, “Slag treatment at Kardemir integrated iron and steel works,” Int. J. Miner. Process., 74, 31–40 (2004).
C. Shi, “Steel slag–its production, processing, characteristics, and cementitious properties,” J. Mater. Civ. Eng., 16, No. 3, 230–236 (2004).
H. Y. Poh, G. S. Ghataora, and N. Ghazireh, “Soil stabilisationusing basic oxygen steel slag fines,” J. Mater. Civ. Eng., 18, No. 2, 229–240 (2006).
M. Tossavainen, F. Engstrom, Q. Yang, et al., “Characteristics of steel slag under different cooling conditions,” Waste Manage., 27, No. 10, 1335–1344 (2007).
P. Y. Mahieux, J. E. Aubert, and G. Escadeillas, “Utilisation of weathered basic oxygen furnace slag in the production of hydraulic road binders,” Construct. Build. Mater., 23, 742–747 (2009).
N. Menad, M. Save, and M. Gamet, “Broyage et tris des laitiers d'aciérie de conversion,” Technical Report, BRGM, Orléans, France, 33 (2010).
M. Aarabi-Karasgani, F. Rashchi, N. Mostoufi, and E. Vahidi, “Leaching of vanadium from LD converter slag using sulphuric acid,” Hydromettallurgy, 102, 14–21 (2010).
N. P. Mahural, N. Pradhan, N. C. Mohanta, et al., “Dephosphorisation of LD slag by phosphorus solubilising bacteria,” Int. Biodeterior. Biodegrad., 65, 404–409 (2011).
D. Wang, M. Jiang, C. Liu, et al., “Enrichment oe Fe-containing phases and recovery of iron and its oxides by magnetic separation from BOF slags,” Steel Res. Int., 83, No. 2, 189–196 (2012).
R. Singh, A. K. Gorai, and R. G. Segaran, “Characterisation of LD slag of Bokaro Steel Plant and its feasibility study of manufacturing commercial fly ash-LD slag bricks,” Int. J. Environ. Technol. Manage., 16, No. 1/2, 129–145 (2013).
N. Menad, N. Kanari, and M. Save, “Recovery of high grade iron compounds from LD slag by enhanced magnetic separation techniques,” Int. J. Miner. Process., 126, 1–9 (2014).
M. Nicolae, I. Vılciu, and F. Zaman, “x-Ray diffraction analysis of steel slag and blast furnace slag viewing their use for road construction,” UPB Sci. Bull. Ser. B, 69, No. 2, 99–108 (2007).
A. S. Reddy, R. K. Pradhan, and S. Chandra, “Utilisation of Basic Oxygen Furnace (BOF) slag in the production of a hydraulic cement binder,” Int. J. Miner. Process., 79, No. 2, 98–105 (2006).
F. Wachsmuth, J. Geiseler, W. Fix, et al., “Contribution to the structure of BOF-slags and its influence on their volume stability,” Can. Metall. Q., 20, No. 3, 279–284 (1981).
J. Geiseler, “Use of steel works slag in Europe,” Waste Manage., 16, No. 1–3, 59–63 (1996).
“Recycling of steel waste materials,” Steel Today and Tomorrow (Japan), 124, 11–12 (in English) (1993).
X. Huang and F. Wang, An overview of steel slag processing and utilisation, Manganese Ore of China 3 (in Chinese) (2001).
V. Ghai and W. H. Noseworthy, “Resource recovery programs at Lake Erie Steel,” Iron Steel Eng., (USA), 75, No. 5, 24–29 (1998).
K. M. Goodson and N. Donaghy, R. O. Russsel, Steelmaking Conference Proc., ISS (1995), p. 481.
C. J. Liu, X. Zhuy, and M. F. Jiang, Iron Steelmaking, 36 (2003).
T. Emi, “Molten slags, fluxes and salts,” Proc. 6th Int. Conf., Stockholm (2000), p. 001.
R. Dippenaar, “Industrial uses of slag (the use and re-use of iron and steelmaking slags),” Ironmaking and Steelmaking, 32, No. 1, 35–46 (2005).
T. M. Miller, J. Jimenez, A. Sharan, and D. A. Goldstein, “In Making, shaping and treating of steel," Steelmaking and Refining, 514, AISE Steel Foundation (1998).
D. M. Proctor, K. A. Fehling, E. C. Shay, et al.,“Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags,” Environ. Sci. Technol., 34, No. 8, 1576–1582 (2000).
O. Negim, M. Mench, C. Bes, et al.,“In situ stabilisation of trace metals in a copper-contaminated soil using P-spiked Linz–Donawitz slag,” Environ. Sci. Pollut. R., 19, 847–857 (2012).
M. Pinto, M. Rodrigvez, and G. Besga, “Effects of Linz–Donawitz (LD) slag as soil properties and pasture production in the Basque country (Northern Spain),” New Zealand J. Agri. Res., 38, 143–155 (1995).
N. Pradhan, R. N. Kar, L. B. Sukla, et al., “Use of steel plant waste (LD Slag) as soil conditioner,” Proc. International Seminar on Downsizing Technology for Rural Development (2003), pp. 224–229.
M. Mench, V. Didier, M. Löffler, et al., “A mimicked in-situ remediation study of metal-contaminated soils with emphasis on cadmium and lead,” J. Environ. Qual., 23, 58–63 (1994a).
D. M. Proctor, E. C. Shay, K. A. Fehling, and B. L. Finley, “Assessment of human health and ecological risks posed by the uses of steel industry slags in the environment,” Hum. Ecol. Risk. Assess., 8, 681–711 (2002).
C. S. Gahan, M. L. Cunha, and A. Sandstrom, “Comparative study on different steel slags as neutralising agent in bioleaching,” Hydrometallurgy, 95, 190–197 (2009).
D. Yilmaz, L. Lassabatere, R. Angulo-Jaramillo, et al., “Hydrodynamic characterisation of basic oxygen furnace slag through an adapted BEST method,” Vadose Zone J., 9, 107–116 (2010).
G. S. Basu, R. P. Sharma, and A. S. Dhilon, “Solid waste management in steel plants challenges and opportunities,” Tata Search., 39–42 (2002).
“Promoting effective utilisation of steel making slag,” NKK Monthly, Dec. 25, 2000.
F. A. Lopez Gomez, R. Aldecoa, M. A. Fernandez Prieto, et al., “Preparation of NPK fertilisers from ferrous-metallurgy,” Simoes. C. Eur. Commun. [Rep.], 18616, 1–57 (1999).
M. Maslehuddin, A. M. Alfarabi, M. Sharif, et al., “Comparison of properties of steel slag and crushed limestone aggregate concretes,” Construct. Build. Mater., 17, No. 2, 105–12 (2003).
S. Ozeki, “Properties and usage of steel plant slag. Encosteel: steel for sustainable development,” International Iron and Steel, Stockholm, Sweden (1997), pp. 16–17.
A. K. Mukherjee and T. K. Chakraborty, “Towards zero waste concept and possibilities in Indian iron and steel industry,” Proc. Int. Environment and Waste Management, NML, Jamshedpur, India (2000), pp. 37–49.
R. P. Sharma, G. S. Basu, M. D. Maheshawari, et al., “Utilisation of LD-slag in cementmaking-experience at Tata Seel,” Proc. ASIA Steel Int. Conf., Jamshedpur, India (2003), pp. 1.i.7.1–1.i.7.7.
H. N. Banerjee (ed.), The Technology of Portland Cement and Blended Cement, A.H. Wheeler and Co., Bengalore (1980), pp. 8–15.a.
H. Suito, Y. Hayashida, and Y. Takahashi “Minaralogical study of LD converter slags,” Tetsu-to-Hagane., 63, No. 8, 1252–1259 (1977).
B. Das, S. Prakash, P. S. R. Reddy, et al., “Effective utilisation of blast furnace flue dust of integrated steel plants,” Eur. J. Miner. Process. Environ. Prot., 2, No. 2, 61–7 (2002b).
S. Shiomi, N. Sano, and Y. Matsushita, “Removal of phosphorus in BOF slag,” Tetsu-to-Hagane., 63, No. 9, 1520–1528 (1977).
M. Dziarmagowski, M. Karboniczek, M. Pyzalsky, and J. Okon, “Reduction of converter slag in electric arc furnace,” Ironmaking and Steelmaking., 19, No. 1, 45–49 (1992).
Z. Guo, D. Wang, and Z. Xu, “Fundamental research on phosphorus removal in the smelting reduction process,” Steel Res., 65, No. 2, 47–52 (1994).
T. K. Roy, B. B. Sinha, B. Singh, and A. K. Das, “The metallurgy of solid waste recycling in integrated steel plant,” Tata Search., 123–126 (1998).
E. Fregeau-Wu, S. Pignolet-Brandom, and I. Iwasaki, “Liberation analysis of slow cooled steel making slags: implications for phosphorus removal," Proc. 1st International Conference on Processing Materials for Properties, sponsored by TMS, MMIJ Punl by Minerals, Metals & Materials Society (TMS) (1993), pp. 153–6.
G. H. Thomas, “Investigations on LD slag with particular reference to its use for road construction (pamphlet),” Commission of the European Communities, Boite Postale, Luxembourg, 1003, 75 (1983).
N. Pradhan, B. Das, S. Acharya, et al., “Removal of phosphorus from LD slag using a heterotrophic bacterium,” Miner. Metall. Process., 3, No. 21, 149–52 (2005).
R. Panda, R. N. Kar, and C. R. Panda, “Dephosphorisation of LD slag by penicillium citrinum,” The Ecoscan: Int. Quart. J. Envir. Sci., 3, 247–250 (2013).
H. S. Park, B. C Ban, and K. S. Cho, “Smelting reduction for vanadium-recovery from LD-slag (I),” J Korean Inst. Met. Mater., 32, No. 8, 982–8 (1994).
F. J. Weiss, M. A. Goksel, J. L. Coburn, G. E. Metius, “The recycling of steel plant waste oxides using the PTC (Pellet Technology Corporation) cold bond carbon bearing pellet technology,” Disposal, Recycling and Recovery of Electric Furnace Exhaust Dust, Iron and Steel Society, AIME, 410, Commonwealth Drive, Warrendale, Pennsylvania 15086, USA (1987), pp. 115–20.
K. K. Sharma, S. Swaroop, and D. S Thakur, “Recycling of LD slag through sinter route on direct charging in blast furnace at Bhilai Steel Plant,” Proc. National Seminar on Pollution Control in Steel Industries (1993), pp. 72–9.
Y. D. Shi, J. Wang, and P. G. Tan, “Study on the Treatment of Mercury in Sea Water with Steel Slag,” J. Qingdao. Univ. Technol. (in Chinese), 32, No. 3, 80–3 (2011).
C. Oh, S. Rhee, M. Oh, J. Park, “Removal characteristics of As(III) and As(V) from acidic aqueous solution by steel making slag,” J. Hazard. Mater., 213–214, 147–155 (2012).
D. H. Kim, M. C. Shin, H. D. Choi, et al., “Removal mechanisms of copper using steel-making slag: adsorption and precipitation,” Desalination, 223, 283–289 (2008).
J. M. Duan, J. M. Lin, H. D. Fang, et al., “Adsorption characteristic of modified steel-making slag for simultaneous removal of phosphorus and ammonium nitrogen from aqueous solution,” Chin. J. Environ. Eng. (in Chinese), 6. No. 1, 201–4 (2012).
A. N. Shilton, I. Elmetri, A. Driz, et al., “Phosphorus removal by an ‘active’ slag filter-a decade of full scale experience,” Water Res., 40, 113–8 (2006).
J. Gao, S. Y. Liu, Y. J. Yang, et al., “Study on adsorptive removal of phenol by steel slag,” Chin. J. Environ. Eng. (in Chinese), No. 2(4), 323–6 (2010).
Y, Sun, M. S. Yao, J. P. Zhang, and G. Yang, “Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution,” Chem. Eng. J., 173, 437–445 (2011).
S. Eloneva, S. Tei, J. Salminen, et al., “Fixation of CO2 by carbonating calcium derived from blast furnace slag,” Energy, 33, 1461–7 (2008).
C. Kunzler, N. Alves, E. Pereira, et al., “CO2 storage with indirect carbonation using industrial waste,” Energy Procedia, 4,1010-7 (2011).
J. H. Feng, J. S. Wang, and S. H. Ke, “The basic study on desulfurisation of agglomeration gas by using converter steel sediment,” Hebei Polytech. Univ. (Nat. Sci. Ed.) (in Chinese), No. 1(32), 6–9 (2010).
X. L. Ding, Y. C. Guo, S. W. Tang, et al., “Experimental study on wet flue gas desulfurisation with scrap slag powder residue,” Environ Eng. (in Chinese), No. 3(27), 99–102 (2009).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chand, S., Paul, B. & Kumar, M. Sustainable Approaches for LD Slag Waste Management in Steel Industries: A Review. Metallurgist 60, 116–128 (2016). https://doi.org/10.1007/s11015-016-0261-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-016-0261-3