Abstract
The Linz–Donawitz (LD) slag is an industrial waste generated in Linz–Donawitz process of steel making. It is not gaining importance in construction industry because of its volume instability, and presence of excessive phosphorous and sulphur content. Suitable accelerated ageing and masking of harmful materials can improve the engineering properties of LD slag. The research shows that it is possible to use LD slag in sustainable construction applications including cementitious binders, aggregates in pavement and concretes, building products, soil improvement, etc. However, its use is presently limited to 25% of the total quantity generated and the rest is dumped as landfill. Thus, there is a strong need to review the available literature to explore the gainful utilization of LD slag in various applications. Aim of this paper is to highlight the potential of LD slag for a broad range of applications based on the published research over the past decade along with the recent research and developments. Physico-mechanical properties, microscopic characteristics, and mineralogical composition of LD slag observed in various studies are also reported. Efficacies of LD slag utilization in construction are also presented. This paper also discusses current challenges for its usage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India faces major environmental challenges due to waste generation and inadequate waste collection, transport, treatment, and disposal. Environmental and economic benefits can be achieved by sustainable utilization of solid wastes in construction. The challenges are extracting and transforming waste materials into useful product, which are currently in a great demand for their environmental friendly disposal and recycling. Other major challenge is rapid consumption of existing natural resources especially by construction industry. Sustainability in the construction industry is essential as it can save the environment by conserving natural resources, which are crucial for inclusive growth.
For centuries, huge amount of wastes was generated that need to be reutilized as resource materials. For instance, construction and demolition (C&D) waste is one of the predominant solid wastes. It is generated during the construction, renovation, and demolition of buildings or structures [1]. Similar types of inorganic wastes are mine tailings, granite slurry, limestone powder, cement kiln dust, etc.[2,3,4]. The organic wastes include paper production residue, kraft pulp production residue, wood sawdust, tea industry waste, rice husk, cotton waste, etc. [5,6,7,8]. Coal fly ash and steel slag are important industrial wastes [9,10,11]. Coal fly ash is one of the solid residues generated from coal-firing power stations and used in cement clinkers, concrete production, road embankment, waste stabilization/solidification, geopolymer concrete, etc. The incorporation of these resource materials is not only helping in waste management but also reduces handling cost of wastes. The production of steel slag around the world is increasing over the time. The integrated steel plants generate about 150–200 kg of waste materials per ton of steel production at different stages of processing and metallurgical operations [12]. Worldwide, steel production reaches 1691 million tons in the year 2017 [12]. India is world’s third largest steel producer with 101.4 million tons per year after China (831.7 million tons) and Japan (104.7 million tons).
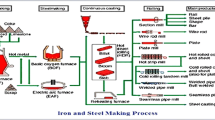
The production of steel mostly consumes iron ore, limestone, fuel, water, air, and power, and generates various kinds of slags [13]. These are categorised as Blast Furnace (BF) [14,15,16], Ladle Furnace (LF) [17,18,19,20], Electric Arc Furnace (EAF) [21, 22], and Linz–Donawitz (LD) [23, 24] slag according to steel making processes. These slags differ from each other in terms of mineralogical and chemical composition. Therefore, its utilization and possible applications may vary based on their physical, chemical, and mineralogical properties [25,26,27].
The BF slag mainly contains inorganic constituents [14, 15]. The presence of high silica (SiO2) and calcium oxide (CaO) along with glassy phase more than 90% and low iron content makes it useful in the production of cementitious binder along with the gypsum and clinker [17, 23, 24]. The benefits of using BF slag are well established [28,29,30]. It is widely used in production of Portland blast-furnace slag (PBFS) cement. It is also used in the preparation of ceramic glass, silica gel, ceramic tiles, and bricks [29, 31].
EAF slag is black in colour having high mechanical and abrasion resistance [21, 22]. It is stone like material, which is easy to crush. It contains low percentage of amorphous silica and high content of ferric oxides, which leads to low pozzolanic activities as compared to BF slag [29]. Studies are taken up to use EAF slag in the construction industry, in particular as an aggregate in road constructions. The main problems are durability and its environmental tolerance of concrete [21, 22].
LF slag is another industrial byproduct of steel industry which is produced during the second stage of basic refining process. Research shows that LF slag can be used in construction applications, soil improvement and masonry mortar production. Some studies [32, 33] refer LF slag as a low-quality material due to its fine grain size, adverse leaching potential, and expansive behaviours. The expansive characteristics of LF slag can be reduced by natural ageing or accelerated weathering to make it useful in construction [32, 33].
LD slag is generated from a Linz–Donawitz process. It is produced during the LD process as pig iron is processed into crude steel. The main components of slag are free lime, metallic and non-metallic iron, and calcium silicates, which make it highly basic in nature [23, 24]. Due to its specific characteristics, a part of LD slag is fed back into the iron and steel making process to increase efficiency of raw materials. The mineral phases present in different types of slags are summarized in Table 1.
This review seeks to find sustainable ways of LD slag utilization in manufacturing of newer cementitious binders and other building products. The chemical, physical, and mineralogical characterizations along with phases present in LD slag are presented for better understanding of as resource material. The associated issues with LD slag leading to its instability and other challenges are also discussed.
LD slag
In India, about 12 million tons of LD slag was generated in the year 2017 and expected to further increase with increase in production of steel [34, 35]. In the steel making process, hot metal is transferred from BF to LD converter where it undergoes oxidation process to remove carbon and other impurities (phosphorus & silicon) present in hot metal. Lime is added to the furnace and waste (slag) containing mainly calcium silicate and other impurities as oxides is skimmed out for open quenching, wherein it is cooled using water and air. The cooling rate of slag is slow which is responsible for its crystalline phase. Only 25% of total LD slag produced is used in various possible applications in India such as aggregates, soil conditioning agent, cementitious binders, building bricks, fertilizers, etc., whereas remaining 75% are disposed off in unplanned manner as landfills [36,37,38]. The BF slag is utilized nearly 50% [38, 39]. This is due to difference in composition and properties of these slags. BF slag is glassy (92–93%) and amorphous in nature, whereas LD slag contains only about 40–49% of amorphous phase [40,41,42]. Furthermore, the use of LD slag is limited due to the presence of lime, which absorbs moisture and carbon dioxide from air and form hydroxides and carbonates respectively as given in Eqs. 1–3:
This leads to volume expansion or swelling resulting to formation of cracks if used in concrete and building products. Similar types of reaction (Eqs. 4–6) occur with MgO in the presence of water and carbon dioxides:
The volume increases by about 91.7% and 119.6% of concrete due to the formation of calcium hydroxide and magnesium hydroxide, respectively [43]. These reactions are topo-chemical, in which these hydroxides evolve outward expansion, causing stress that leads to micro cracking [43]. To overcome these instability issues and gainful utilization of LD slag, different physical processes like accelerated ageing, magnetic separation, floatation, dual-phase separation, etc. are reported in previous studies [44, 45]. The main purpose of accelerated ageing is to remove free lime and magnesia present in LD slag. The ageing is also taken up under vigorous conditions of sunlight, heat, vibration, oxygen, etc. The accelerated and pressurized steam ageing methods also reduce ageing duration dramatically as compared to natural air ageing [44,45,46,47].
LD slag contains high percentage of phosphorus (1–7%) which reduces its chances of being recycled and reused. The presence of high P2O5 leads to corrosion of reinforced material in building structures. Several attempts are made for dephosphorization using chemical and biological methods [45, 48, 49]. The P2O5 is present as 3CaO.P2O5 which was reduced with carbon to metallic (Fe–P–C) and non-metallic phases (3(CaO.SiO2)) [45]. The overall reduction to non-metallic phases takes place according to the following equation:
The non-metallic phase can be used for production of Portland clinker. However, above conversion requires very high temperature of 1500 °C. Takeuchi et al. [50] have removed 60% of phosphorus from the slag using Fe–Si alloys as the reducing agent to P2. Phosphorus extraction behaviour from Fe–P–Csatd alloy at 1200 °C was also investigated by Morita et al. [51]. For the extraction of phosphorous from Fe–P–Csatd alloy, Na2CO3 is more effective as compared to K2CO3. Marhual et al. [52] removes phosphorous of LD slag with the help of phosphorus solubilizing microorganism (two Gram-negative bacteria belonging to genus Pseudomonas and two Gram-positive bacteria belonging to genus Bacillus). Phosphorous recovered from slag can be used for production of fertilizers.
LD slag has been used for the recovery metals such as Fe, Co, Cr, Ni, Cu, Al, Pb, Ta, Au, Zn, Nb, and Ag etc. by various physical, chemical, and biological techniques including grinding [53], crushing [53], leaching [54, 55], roasting [56, 57], magnetic separation [40], flotation, etc.[53]. Recovery of these metals and its utilization are important for saving metal resources and protecting the environment. Mirazimi et al. have used alkaline roasting-sulfuric acid leaching process for 96% recovery of vanadium from LD slag [56]. However, roasting requires high amount of energy for metallurgical process. Therefore, the same group in different studies uses direct acid leaching process for the recovery of 98% vanadium [55]. Menad et al. [40] suggested two flowsheets for treatment of LD slag to recover high-grade iron using magnet in metallurgical processes. Xiang et al. [57] carried out the mechanical activation study for the oxidation roasting. This mechanical activation pre-treatment destroyed the structure of vanadium slag and reduced oxidation duration. The vanadium from LD slag can be removed by means of three different species of microbial systems: acidithiobacillus thiooxidans (autotrophic bacteria), pseudomonas putida (heterotrophic bacteria), and aspergillus niger (fungi) [58].
Despite the fact that research and development on utilizing LD slag in various fields, disposal of LD slag by landfilling is a major concern of steel industries as it causes air, water, and soil pollution. The associated environmental problems are leaching of harmful metals into ground water and pollution of nearby water sources, lowering of moisture, chemical degradation, and lack of aesthetics. Therefore, the gainful utilization of LD slag as construction materials will not only help in sustainable utilization of waste but also help in preserving the valuable natural resources [59,60,61,62].
Physical properties
LD slag appears like a loose collection of subrounded-to-angular-shaped granules, as shown in Fig. 1a. The grain size distribution of ungrounded slag varies from 6 to 20 mm with uniformity coefficient (Cu = D60/D10) and coefficient of gradation (Ck = D302/D60D10) in the range of 7.55 and 0.67, respectively. The density of LD slag lies between 3300 and 3600 kg/m3, whereas natural aggregates density varies from 1500 to 1680 kg/m3 [63]. It is higher than those of the natural aggregates due to the high content of iron. Water absorption varies from 0.20 to 2.50%. The pH usually varies from 11.35 to 11.86. The Grindability Index is about 0.70–0.96. The resistance to impact is found to be as high as 10–26%. Crushing value is about 22–25%. The Los-Angeles abrasion value is 9–18%. It is hard and wear resistant due to high Fe content. The compressive strength is found to be more than 100 MPa which is close to granite stone [38, 64].
a View of LD slag and b SEM image of LD slag at ×5000 magnification [66]
Microscopic properties
Figure 1 shows image of LD slag and its FE-SEM image at 5000× magnification. The surface of LD slag is rough in texture and mainly formed of cubical and angular particles of various sizes. The presence of sand and silt size particles is also observed. Internal structures of slag consist of vesicular shaped particles which are not interconnected to each other. It is also observed by various authors [65, 66].
EDX of selected portion of LD slag is shown in Fig. 2. The major elements present in the LD slag are calcium, iron, and silicon, whereas phosphorous, sulphur, aluminium, and magnesium are present in appreciable amount.
EDX of LD slag [69]
Chemical and mineralogical composition
The chemical composition is mainly determined by X-ray fluorescence (XRF), inductively coupled plasma atomic emission spectroscopy (ICP-AES), CHNSO, and energy dispersive X-ray spectroscopy (EDS) by various studies [66,67,68]. The general chemical composition of Indian and International LD slag is summarized in Table 2. The chemical composition of steel slag varies significantly from source to source. It is clearly visible that chemical compositions are almost comparable with minor deviation. It mainly consists of inorganic constituents such CaO, SiO2, Al2O3, FeO, Fe2O3, MnO, and MgO, in which CaO content is high, varying from 42 to 50%. The different techniques used by various authors for the calculation of lime content (CaO) in LD slag are thermogravimetric analysis, Leduc test, and Bernard calcinatory analysis [23, 38, 66]. The free lime comes mainly from two sources: residual free lime from the raw material and precipitated lime from the molten slag. As reported, it is present in two forms: (1) lime nodules (size ranging from 20 to 100 μm) and (2) lime micro-inclusions (size ranging from 1 to 3 μm) [38, 66]. The silica content (SiO2) varies from 10 to 28%. Iron oxide content can be as high as up to 38% depending upon the efficiency of the furnace [68]. A high content of MgO can often be detected which comes from dolomite (used as flux) and refractory material, which also causes a soundness problem. A significant amount of P2O5 is also present in LD slag.
The XRD pattern of LD slag shows crystalline phase, as shown in Fig. 3 [69]. Similar patterns were also observed by various authors [38, 66, 68]. The XRD pattern is complex due to overlapping of peaks of many minerals. It is found that the intense peaks correspond to di-calcium silicate, di-calcium ferrite, and calcium hydroxide. Other phases present in LD slag from various studies are tri-calcium silicate, di-calcium aluminoferrite, MgO and free CaO. Among these, the reactive phases are di-calcium silicate, tri-calcium silicate, di-calcium aluminoferrite, and free CaO and MgO, while remaining phases are non-reactive (metals). Table 3 summarizes the major phases present in LD slag from various literatures.
Typical XRD pattern of LD slag [69]
Thermal properties
There is little investigation on thermal properties of LD slag. Ashrit et al. [70] conducted TGA/DTA test on LD slag fines (0–6 mm) in oxygen and nitrogen atmosphere up to a temperature of 850 °C. There are two peaks in the temperature range of 450–550 °C and 650–810 °C apart from a peak at 107 °C due to removal of moisture. Differential scanning calorimetry (DSC) shows the presence of two endothermic peaks at 103 °C and 488 °C, which are attributed to water evaporation and calcium hydroxide dehydration respectively [23]. At 804 °C, the DSC curve shows remarkable mass loss due to decomposition of one or more phases in the LD slag. For instance, β-C2S undergoes transition to α-C2S and wustite decomposes to magnetite.
Deleterious potential
Leaching of toxic elements from LD slag has adverse impact on soil, ground water, surface water, marine ecosystems, as well as human health. Therefore, scientists recommended the use of some tests as prerequisite to assess the impact of leaching and toxicity prior to its use in different fields [71,72,73]. A variety of short-term leaching procedures like United State Environmental Protection Agency (USEPA) [74], Strong Acid Digestion Test (SADT) [75], Toxicity Characteristic Leaching Procedure (TCLP) [74], Batch Leach Test (BLT) [76], Synthetic Precipitation Leaching Procedure (SPLP) [76], and American Society for Testing and Materials (ASTM) [77] shake test were carried out for evaluating heavy-metal leaching properties. Chand et al. [54] studied the short-term leaching behaviour of LD slag and found As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Se, V, and Zn as main leachates. The leaching properties of the different components vary with the pH of LD slag dumped sites. Aarabi-Karasgani et al. [78] studied the vanadium leaching and the effect of different parameters on kinetics by alkaline roasting-acid method. Short-term leaching studies are associated with some limitations such as assessing the environmental concerns which may arise due to leaching during long period. Therefore, long-term leaching studies are required for real impact of leaching on environment. Recently, Chand et al. [72] conducted a long-term leaching study for more than 1 year using long-term open column. The leachates were collected at interval of 3–5 days and showed variation in quantity and pattern of some important constituent elements such as Mn, Cu, Ni, Fe, Co, Cr, Zn, As, Cd, Pb, Se, and V. The leaching trend shows a fast leaching during initial period which become constant over a period of time.
Research and development trends
Various researchers [79,80,81] studied the applications and efficient utilization of LD slag. It is used in various applications of civil engineering such as cementitious binders, concrete, building products, etc. [82,83,84]. Other applications of LD slag include soil fertilization and conditioning, flux in metal recovery, treatment of wastewater with arsenic, CO2 absorption, and flue gas desulphurization. Possible uses of different phases of LD slag are represented in Table 4.
Pozzolanicity
Pozzolans, an amorphous siliceous or siliceous and aluminous material in finely divided form, reacts with calcium hydroxide in the presence of water to form cementitious hydrated products [85, 86]. The presence of C3S, C2S, and C4AF in LD slag is accountable for its pozzolanic properties. However, due to its crystalline nature, it does not show hydraulic and pozzolanic properties [86]. These properties can be enhanced by phase modification using alkalis and heat treatment [87]. The presence of water and alkalis (NaOH) accelerates the hydration of the slags. The pozzolanic reactivity occurs when silica reacts with calcium hydroxide as given below:
In addition, di- and tri-calcium silicate on exposure to CO2 can be converted to calcium silicate hydrates and calcium carbonates. The reactions of C2S and C3S with CO2 are given below:
Several studies on the use of LD slag in cementitious binders are discussed in subsequent section.
Cementitious binder
India is the second largest cement producing country with 420 MT production capacities in 2017, which is expected to touch 550 MT by 2020 [88]. Use of LD slag for cement manufacturing in India is minimal, whereas China is producing LD slag-based cement for last 20 years [89, 90]. Cement developed in China is a mixture of LD slag, blast-furnace slag, Portland cement clinker, gypsum, and admixture. The resulting cement has good durability and high resistance to sulphate and carbonate [61, 62].
The major mineral phases present in LD slag are di-calcium silicate (C2S), tri-calcium silicate (C3S), and tetracalcium aluminoferrite (C4AF), which are accountable for its cementitious properties [91, 92]. However, the amount of these components in LD slag is very low than in the cement, thus making their hydration rate very low. High FeO content in slag reduces the hydraulic character of the slag. Furthermore, during slow-cooling process, the more active β-C2S changes to less active γ-C2S and most of the C3S phase decomposes [93]. The alkalinity or basicity of the steel slag \(\left\{ {{{{\mathbf{CaO}}} \mathord{\left/ {\vphantom {{{\mathbf{CaO}}} {{\mathbf{SiO}}_{2} + {\mathbf{P}}_{2} {\mathbf{O}}_{5} }}} \right. \kern-\nulldelimiterspace} {{\mathbf{SiO}}_{2} + {\mathbf{P}}_{2} {\mathbf{O}}_{5} }}} \right\}\) is important factor which determines the hydraulic activity of the steel. For the use of steel slag as cementitious binder, the basicity of the slag should be > 1.8 [94]. The high CaO content in slag contributed towards high basicity. Based on these observations, the LD slag is not suitable for use as a cementitious binder in constructions. Therefore, for effective utilization of slag in construction, several prior modifications are needed. Free oxides present in the slag found its usefulness in activating other materials such as GGBFS (Ground Granulated Blast-Furnace Slag), fly ash, and other pozzolan [93].
The uses of slag in cementitious binders are studied by several researchers since 1980 [89, 90]. First time, Conjeaud et al. studied cementitious property of LD slag in 1981 [42]. Theoretical and semi-field trials were performed and observed that addition of 6–15% of alumina to slag in the oxygen furnace improves its cementitious property. The presence of high CaO content was observed beneficial due to strong alkaline substance. This accelerated the formation of Ca(OH)2 which acted as an activator and gave better mechanical strength properties. Cements made with a mixture of LD slag, blast furnace slag, clinkers, and gypsum were produced [61, 62]. This slag cement exhibited higher acid resistance and improved strength. The high initial porosity reduced after 28 days of curing. Experimental studies by Geiseler [95] found that the addition of slag as a clinker raw material permits a lower firing temperature and were energy efficient in clinker production. Reddy et al. [96] observed that the cooling rate has a profound effect on mineralogical and cementing properties of slag and did not show any cementitious properties on slow cooling.
Cementitious properties of cooled slag were improved by phase modifications using heat treatment. The early strength of slag can also be improved by carbonation reactions as given in Eqs. 8 and 9. The resulting carbonated slag was used as a binder to replace Portland cement [86]. Singh et al. [23] studied the possibility of using slag in place of iron ore in raw feed during clinker manufacture. The study shows that 2% slag can be used as raw mix component for correction of iron content in raw mix. Zhang et al. [97] studied the strength properties of Portland cement where LD slag was replaced by 30–60% in binder and found properties similar to Portland cement. Recently, Agrawal et al. [39] observed the viability of adding LD slag as partial replacement of granulated blast furnace slag (GBFS) in Portland slag cement (PSC). It was found that only 7.5% of LD slag by weight can be used. Presently, study on use of LD slag as a replacement of clinker was limited to a maximum level of 10% due to its chemical and phase composition [37,38,39]. It is highly crystalline in nature due to its higher CaO/SiO2 content. The crystalline phases in Portland cement are mostly hydraulic, but the ones present in LD slag are non-hydraulic and non-reactive [97,98,99]. As per Indian standard [IS: 12089] and ASTM C989, the glass content in slag and slag activity index should be ≥ 85% for its gainful utilization in cement [100, 101]. However, the glass content in LD slag is only 40–49% which restricts its use due to nonconformity to IS/ASTM. There are several other factors such as presence of P2O5, CaO, and FeO which restrict its usage. The presence of iron oxide in LD slag leads to the formation of tetracalcium aluminoferrite (C4AF) which affects the properties of cement adversely. Therefore, modifications in the treatment are required to generate binding properties.
The mechanical and chemical activation helps in the improvement of hydraulic behaviour [91, 92]. However, alkali activation of LD slag is a big challenge considering the fact that it is highly crystalline. Further, the presence of non-hydraulic phases such as C2S, bredigite, and merwinite restricts its activation [98, 99]. Duda et al. have studied the reactivity of crystalline LD slag by milling and blending it with BF slag and activating with NaOH [83]. The alkali activation of LD slag shows significant enhancement in cementitious properties such as improvement in fineness of the material and hydration reactions. The series of reaction for alkali activation of SiO2- and CaO-rich slags are given in Eqs. 10–12 [102, 103]:
The activation of SiO2- and CaO-rich slag involves the breakdown of Si–O–Si bonds and dissolution of Ca to form C–S–H-type reaction products with a low C/S ratio. Table 5 summarizes the recent research and development trends on the use of LD slag as a replacement in cementitious products.
Road pavements
Massive amount of non-renewable aggregate resources have been exploited for centuries in construction of road pavements such as road surfacing, and base and subbase layers. These resources are depleting at the faster rate due to continuous expansion of roads. The use of LD slag as aggregates displays several technical and environmental benefits as compared to natural aggregates making them potentially important road construction materials. It is well established that LD slag can be effectively utilized as partial replacement of coarse and fine aggregates in construction of road pavements. High binder adhesion as well as high frictional and abrasion resistance make it suitable for aggregates not only in surface layers of the pavement but also in unbound bases and sub-bases, especially in asphaltic surface layers [80, 104]. Stone mastic asphalt with LD slag is found exhibiting higher deformation resistance. It provides acceptable strength properties, suitable freeze/thaw durability, and exceptional fracture properties [104,105,106]. The performance and sound absorption studies of porous asphalt mixture with different proportion of steel slag were carried out by Shen et al. [107]. It was found that the mixtures with slag enhance skid resistance, rutting resistance, moisture susceptibility, and sound absorption. Asi et al. [108] found that replacement of both fine and coarse aggregate components by steel slag in hot mix asphalt cause lot of air voids, necessitating the use of high quantities of bitumen. The bonding behaviour of steel slag with bitumen was studied by Xie et al. [109] using a modified pull off test. The slag with higher CaO content has better affinity for bitumen, and strength decreases with increase in temperature and bitumen film thickness. The effect of LD slag as fine and coarse aggregate on compressive strength of pavements concrete is summarized in Table 6.
The initial gain in strength of concrete is attributed to high strength of slag. However, the presence of magnesia and free lime leads to expansion of slag in presence of moisture [66]. This in turn leads to expansion of concrete when LD slag is used as aggregate. At higher percentage this leads to dimensional instability and cracking of concrete leading to reduction in compressive strength [66].
Concrete construction
LD slag due to its high compressive, flexural and tensile strength can be processed to aggregates of high quality comparable with natural aggregates [110,111,112]. Most studies are showing that maximum strength is obtained up to 60% slag replacement as fine aggregate in concrete. These strengths go on decreasing marginally beyond this value due to porosity of slag and presence of free lime [112, 113]. At 60% replacement, the density of concrete is increased from 25 to 26 kN/m3 which makes is unsuitable for structural concrete due to increase in dead load. However, as shown in Fig. 5, Pajgade et al. [29] have shown the maximum strength at 75% replacement. This behaviour is attributed to the size, shape, and surface texture of steel slag aggregates, which offer a better adhesion between the particles and cement paste. Therefore, 45% replacement is considered as optimum, considering concrete density. The presence of free lime and phosphorous makes it unsuitable for replacement at higher percentages [83, 114]. Its optimum percentage of replacement is varying from 30 to 60%. Beyond 60% replacement, concrete starts exhibiting cracks and instability problem [65, 94]. The compressive strength of concrete is increased by 6% up to 50% LD slag replacements as both coarse and fine aggregates [79, 113]. However, it decreased by 7% to 10% at 100% replacement of fine aggregate [112, 113, 115]. This is attributed to the higher reactivity of free lime above 60% replacement as fine aggregates in comparison to coarse aggregates. IS: 383 [63] recommends the use of steel and iron slag as coarse aggregate up to 25% and 50% in plain concrete, respectively. The iron slag can be used up to 25% in reinforced concrete. Steel slag and iron slag as fine aggregate up to 25% and 50% in plain concrete, whereas iron slag up to 25% is permitted in reinforced concrete. The uses of steel slag aggregates are not permitted in reinforced concrete. The optimum percentage of replacement for use in various applications and related findings is summarized in Table 7. Figures 4, 5 show the effect of percentage replacement of LD slag as fine and coarse aggregate on compressive strength of concrete samples [116].
Bricks
Slags such as EAF slag, BF Slag, etc. are widely used in manufacturing of refractory lining and acid resistant bricks [5, 117, 118]. However, the use of LD slag in building bricks is limited. Singh et al. [23] manufactured LD slag bricks with different proportions of fly ash, LD slag, gypsum, quarry dust, lime, and CaCl2. These bricks are found to attain compressive strength of about 4.04–5.78 MPa at 7th day and about 10.33–12.82 MPa at 28th day. Additionally, the bricks exhibited a higher electrical conductivity and pH. Bricks with higher percentage of slag (greater than 40%) unable to sustain for a longer duration and showed more cracks. Burnt clay bricks with clay and 10% slag mixture exhibited better properties than other mixes. Compressive strength and firing shrinkage of these burnt clay bricks decreases with addition of slag [118]. The appropriate additions of LD slag (< 10%) in the burnt clay bricks help in reducing the firing temperature [118, 119]. Comparison of LD slag bricks with other type of bricks is given in Table 8.
Other applications
LD slag is used as a soil additive (liming agent) to improve the physicochemical properties of LD slag [120, 121]. It is found effective in soil neutralization [39,40,41]. The pH of soil is increased from 5.3 to 6.4 using 7500 kg of LD slag in 10,000 m2 land for the first year. In the second year, only 3000 kg is required to increase pH of soil by 41% [120]. Furthermore, the LD slag improves soil structure and reduce fungal infections. In South Nigeria, the pH and phosphorous content of acidic soil increases substantially on treatment with LD slag. The slag also increases calcium, potassium, and micronutrient up-take, and increases dry matter by plant [122]. In Sweden, the crop production increases with the use of LD slag as compared to limestone. LD slag also prevents the clubroot disease in Sugukina and effectively maintains the acidity of the soil [36, 37]. Tata steel, India is making efforts to use LD slag as a soil conditioner in tea gardens, paddy fields, etc. after grinding it to 45 μm sieve size. It is also used as fertilizers in agricultural applications [36, 37]. A process is developed in Japan to produce ecofriendly potassium silicate fertilizer from the slag [120]. The produced fertilizer is blackish grey in colour and comprises of vitric potassium silicate. It exhibits slower release effects than conventional quick acting chemical fertilizer such as potassium chloride, potassium sulphate, and urea [120]. The studies are carried out for production of fertilizer from LD slag, ammonium sulphate, and semi-calcined dolomite. The influence of these materials as new fertilizer on chemical composition of soil and grass was evaluated [123].
Current challenges
The review of literature and previous studies indicate that there is limited research on LD slag in comparison to blast furnace and other steel slag in production of cementitious binders and building products. The various limitations for use of LD slags are mainly due to the presence of CaO, MgO, P2O5, and FeO. It leads to unsoundness and instability. The instability and volume increases with increase in amount of free lime and/or periclase in the slag, and the resulting volume increase causes disintegration of the slag aggregates and loss of strength. Therefore, Volume instability is a vital aspect when LD slag is considered for use as a construction material. The LD slag can be used for unpaved roads as there is no restriction on volume stability. However, the volume instability must in within the permissible limit when it is used in bound and unbound layers of roads. The permissible limit of free lime content in bound layers is up to 4%, and for unbound layers, it is up to 7% [93]. The maximum volume change due to expansion should be less than 10%.
It was found that the slag expansion could be avoided using slag particle of size less than 13.2 mm in asphalt mix pavement layers. The bitumen coating of these slag particles will help in preventing hydration reaction and, therefore, limiting its expansion. The instability problem can be solved by appropriate ageing of the slag prior to its use in construction. In the ageing process, the slag is exposed to outdoor environmental conditions such as moisture from natural humidity or precipitation. The ageing process causes hydration of free lime and periclase. The ageing can be carried out for various time periods such as one to 3 months, 2–3 months, minimum 6 months, 9–12 months, and up to 2 years [89]. The required ageing time depends upon various factors including steel making process, amount of free lime/periclase, climatic conditions of the area, etc. After conditioning, it is important to confirm that the slag meets the specified requirements.
There are no few studies on stabilizing the free lime, MnO, and P2O5 that prevent the usage of LD slag aggregates at higher percentages [124–126]. The presence of phosphorus and sulphur make its suitability questionable for use in reinforced concrete structure. There are limited studies on infield improvement of LD slag for use in cement. Activation of LD slag to improve its suitability as cementitious binder is not studied. However, it is theorized that during activation with alkalis, the free lime may be consumed to form C–S–H gel and NaCO3. It is also believed that activation of slag may lead to the formation of alumino-silicates which may immobilize the MgO, hexavalent chromium and other heavy metals. Feasibility of use of LD slag with other industrial by products such as lime sludge, sugarcane bagasse ash, rice husk ash, etc. is not explored. Potential applications of LD slag in civil engineering area are possible with the help of hygrothermal treatment followed by mechanochemical activation. However, the same can be ascertained only after an intensive research on use of LD slag in construction after stabilization of unstable compounds of LD slag and activation of inactive crystalline components. Also, the durability of building products with LD slag shall be studied for long term as well as aggressive conditions.
Conclusions
The efforts were made towards utilization of LD slag. However, several durability issues have limited its applications in construction. Therefore, it is utmost important that research studies should focus on exploring the possibility of LD slag in cementitious binder. In addition, steel industry is now focusing on increasing the recycling of LD slags to conserve energy and natural resources and ultimately improve production with a target of “zero-waste” in future. The sustainable use of slags will also contribute to natural resource saving. To attain this, it is important to identify the wide areas for use of LD slag in construction. This paper gives an overview of characteristic of LD slag and the use in construction as under:
- 1.
LD slag is highly crystalline in nature due to slow-cooling conditions during processing and consists of high lime and low silica content.
- 2.
Use of LD slag is limited in brick production due to instability issue. The maximum possible percentage replacement of clay by LD slag is 60%.
- 3.
LD slag is being used as unbound aggregate for asphalt concrete pavement in many countries. This is also limited to 60% due to presence of phosphorous, free lime, and magnesia causing expansive behaviour.
- 4.
The volume instability, low hydraulic reactivity, and heavy-metal leaching also pose major problems when exposed to water.
- 5.
The use of steel slag as a cementing component should be given a priority from technical, economical, and environmental considerations. The use is limited to 10% as a replacement of clinker due to its crystalline nature which is responsible for its weak cementitious properties.
- 6.
These issues can be resolved by natural/accelerated ageing of LD slag. Therefore, a better understanding of the properties of LD slag is needed to improve its gainful utilization.
- 7.
Several drawbacks associated with application point of view still needed to be identified and resolved.
References
Behera M, Bhattacharyya SK, Minocha AK, Deoliya R, Maiti S (2014) Recycled aggregate from C&D waste & its use in concrete—a breakthrough towards sustainability in construction sector: a review. Constr Build Mater 68:501–516
Roy S, Adhikari GR, Gupta RN (2007) Use of gold mill tailings in making bricks: a feasibility study. Waste Manag Res 25:475–482
Menezes RR, Ferreira HS, Neves GA, Lira HDL, Ferreira HC (2005) Use of granite sawing wastes in the production of ceramic bricks and tiles. J Eur Ceram Soc 25:1149–1158
Ye G, Liu X, De Schutter G, Poppe AM, Taerwe L (2007) Influence of limestone powder used as filler in SCC on hydration and microstructure of cement pastes. Cement Concr Compos 29:94–102
Sutcu M, Akkurt S (2009) The use of recycled paper processing residue in making porous brick with reduced thermal conductivity. Ceram Int 35:2625–2631
Demir I, Baspinar MS, Orhan M (2005) Utilization of kraft pulp production residues in clay brick production. Build Environ 40:1533–1537
Demir I (2006) An investigation on the production of construction brick with processed waste tea. Build Environ 41:1274–1278
Rahman MA (1987) Properties of clay–sand–rice husk ash mixed bricks. Int J Cement Compos Lightweight Concr 9:105–108
Kayali O (2005) High performance bricks from fly ash World of coal ash (WOCA). Lexington, Center for Applied Energy Research, pp 1–13
Lin KL (2006) Feasibility study of using brick made from municipal solid waste incinerator fly ash slag. J Hazard Mater 137:1810–1816
Mahllawy MSE (2008) Characteristics of acid resisting bricks made from quarry residues and waste steel slag. Constr Build Mater 22:1887–1896
World Crude Steel Production–summary, world steel association (2017) https://www.worldsteel.org/media-centre/press-releases/2018/World-crude-steel-output-increases-by-5.3--in-2017.html, 24 Jan 2018
Tossavainen M, Engstrom F, Yang Q, Menad N, Larsson ML, Bjorkman B (2007) Characteristics of steel slag under different cooling conditions. Waste Manag 27:1335–1344
Haha MB, Lothenbach B, Saout GL, Winnefeld F (2011) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—part I: effect of MgO. Cem Concr Res 41:955–963
Yildirim IZ, Prezzi M (2011) Chemical, mineralogical, and morphological properties of steel slag. Adv Civ Eng 5:1–13
Kim JH, Lee HS (2017) Improvement of early strength of cement mortar containing granulated blast furnace slag using industrial byproducts. Materials 10:1050–1075
Rodriguez A, Manso JM, Aragon A, Gonzalez JJ (2009) Strength and workability of masonry mortars manufactured with ladle furnace slag. Resour Conserv Recycl 53:645–651
Setien J, Hernandez D, Gonzalez JJ (2009) Characterization of ladle furnace basic slag for use as a construction material. Constr Build Mater 23:1788–1794
Shi C (2002) Characteristics and cementitious properties of ladle slag fines from steel production. Cem Concr Res 32:459–462
Shi C, Hu S (2003) Cementitious properties of ladle slag fines under autoclave curing conditions. Cem Concr Res 33:1851–1856
Luxan MP, Sotolongo R, Dorrego F, Herrero E (2000) Characteristics of the slags produced in the fusion of scrap steel by electric arc furnace. Cem Concr Res 30:517–519
Proctor DM, Fehling KA, Shay EC, Wittenborn JL, Green JJ, Avent C, Bigham RD, Connolly M, Lee B, Shepker TO, Zak MA (2000) Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ Sci Technol 34:1576–1582
Singh R, Gorai AK, Segaran RG (2013) Characterization of LD slag of Bokaro steel plant and its feasibility study of manufacturing commercial ‘fly ash–LD slag’ bricks. Int J Environ Technol Manag 16:129–145
Ashrit S, Banerjee PK, Ghosh TK, Rayasam V, Nair UG (2015) Characterisation of LD slag fines by X-ray diffraction. Metall Res Technol 112:502–602
Das B, Prakash S, Reddy PSR, Misra VN (2007) An overview of utilization of slag and sludge from steel industries. Resour Conserv Recycl 50:40–57
Yi H, Xu G, Cheng H, Wang J, Wan Y, Chen H (2012) An overview of utilization of steel slag. Proc Environ Sci 16:791–801
Shi C (2004) Steel slag—its production, processing, characteristics, and cementitious properties. J Mater Civ Eng 16:230–236
Huang Y, Lin ZS (2010) Investigation on phosphogypsum–steel slag–granulated blast-furnace slag–limestone cement. Constr Build Mater 24:1296–1301
Pajgade PS, Thakur NB (2013) Utilisation of waste product of steel industry. Int J Eng Res Appl 3:2033–2041
Ozbay E, Erdemir M, Durmus HI (2016) Utilization and efficiency of ground granulated blast furnace slag on concrete properties—a review. Constr Build Mater 105:423–434
Sheshukov OY, Lobanov DA, Mikheenkov MA, Nekrasov IV, Egiazaryan DK (2017) The opportunity of silicate product manufacturing with simultaneous pig iron reduction from slag technogenic formations. AIP Conf Proc 1886:1–5
Manso JM, Losanez M, Polanco JA, Gonzalez JJ (2005) Ladle furnace slag in construction. J Mater Civ Eng 17:513–518
Radenovic A, Malina J, Sofilic T (2013) Characterization of ladle furnace slag from carbon steel production as a potential adsorbent. Adv Mater Sci Eng 2013:1–6
Annual Report 2017–2018, Ministry of steel, Government of India. https://steel.gov.in/annual-reports, 09 Feb 2018
Indian Minerals Yearbook (2015) Slag-iron and steel, 54th edition. Ministry of Mines. 16, 1–10
Pal J, Chaudhary PN, Goswami MC (2003) Utilisation of LD slag–An overview. J Metall Mater Sci 45:61–72
Chand S, Paul B, Kumar M (2016) Sustainable approaches for LD slag waste management in steel industries: a review. Metallurgist 60:116–128
Tiwari MK, Bajpai S, Dewangan UK (2016) Steel slag utilization—overview in Indian perspective. Int J Adv Res 4:2232–2246
Agarwal SK, Vanguri S, Chaturvedi SK, Kumar A, Reddy AS (2017) Performance evaluation of granulated BF slag -steel slag based Portland slag cement. 15th NCB International Seminar on Cement, Concrete and Building Materials. New Delhi, India. TS: VB (A413): 1–11
Menad N, Kanari N, Save M (2014) Recovery of high grade iron compounds from LD slag by enhanced magnetic separation techniques. Int J Miner Process 126:1–9
Chand S, Paul B, Kumar M (2016) A comparative study of physicochemical and mineralogical properties of LD slags from some selected steel plants in India. J Environ Sci Technol 9:75–87
Conjeaud M, George CM, Sorrentino FP (1981) A new steel slag for cement manufacture: mineralogy and hydraulicity. Cem Concr Res 11:85–102
Erlin B, Jana D (2003) Forces of hydration that can cause havoc in concrete. Concr Int 25:51–57
Gawwad HAEA, Khater HM, Mohamed SAE (2015) Impact of alkali concentration and metakaolin content on accelerated ageing of Egyptian slag. Am J Chem Eng 3:30–38
Shiomi S, Sano N, Matsushita Y (1977) Removal of phosphorus in BOF slag. Tetsu-to- Hagane 63:1520–1528 (in Japanese)
Sasaki T, Hamazaki T (2015) Development of steam-aging process for steel slag. Nippon Steel Sumitomo Metal Tech Rep 109:23–26
Mahieux PY, Aubert JE, Escadeillas G (2009) Utilization of weathered basic oxygen furnace slag in the production of hydraulic road binders. Constr Build Mater 23:742–747
Pradhan N, Das B, Acharya S, Kar RN, Shukla LB, Misra BN (2005) Removal of phosphorus from LD slag using a heterotrophic bacterium. Miner Metall Process 3:149–152
Panda R, Kar RN, Panda CR (2013) Dephosphorisation of LD slags by penicillium citrinum. Int Q J Environ Sci 3:247–250
Takeuchi S, Sano N, Matsushita Y (1980) Separate recovery of iron and phosphorus from BOF slags using Fe–Si alloys (in Japanese). Tetsu-to-Hagané 66:2050–2057
Morita K, Guo M, Oka N, Sano N (2002) Resurrection of the iron and phosphorus resource in steel-making slag. J Mater Cycles Waste Manag 4:93–101
Marhual NP, Pradhan N, Mohanta NC, Shukla LB, Misra BN (2011) Dephosphorisations of LD slag by phosphorus solubilising bacteria. Int Biodeterior Biodegrad 65:404–409
Shen H, Forssberg E (2003) An overview of recovery of metals from slags. Waste Manag 23:933–949
Chand S, Paul B, Kumar M (2017) Short-term leaching study of heavy metals from LD slags of important steel industries in Eastern India. J Mater Cycles Waste Manag 19:851–862
Mirazimi SMJ, Rashchi F, Saba M (2015) A new approach for direct leaching of vanadium from LD converter slag. Chem Eng Res Des 94:131–140
Mirazimi SMJ, Rashchi F, Saba M (2013) Vanadium removal from roasted LD converter slag: optimization of parameters by response surface methodology (RSM). Sep Purif Technol 116:175–183
Xiang J, Huang Q, Lv X, Bai C (2017) Mechanochemical effects on the roasting behavior of vanadium bearing LD converter slag in the air. Iron Steel Inst Japan Int 57:970–977
Mirazimi SMJ, Abbasalipour Z, Rashchi F (2015) Vanadium removal from LD converter slag using bacteria and fungi. J Environ Manag 153:144–151
Borges AC, Gadioli MCB, Junior LABP, Oliveira JR (2012) Mixture of granite waste and LD steel slag for use in cement production. Mater Sci Forum 727:1535–1540
Chandrasekhar SY (2016) An experimental study on mud concrete using soil as a fine aggregate and LD slag as coarse aggregate. Int J Res Eng Technol 5:264–268
Dongxue L, Xuequan W (1993) Technique methods for increasing early strength of steel slag cement. Jian Shu Build Mater 4:24
Dongxue L, Xinhua F, Xuequan W, Mingshu T (1997) Durability study of steel slag cement. Cem Concr Res 27:983–987
IS 383 (2016) Specification for coarse and fine aggregates from natural sources for concrete. Bureau of Indian Standards, New Delhi
Chand S, Paul B, Kumar M (2015) An overview of use of Linz–Donawitz (LD) steel slag in agriculture. Curr World Environ 10:975–984
Wang K, Qian C, Wang R (2016) The properties and mechanism of microbial mineralized steel slag bricks. Constr Build Mater 113:815–823
Waligora J, Bulteela D, Degrugilliers P, Damidot D, Potdevin JL, Measson M (2010) Chemical and mineralogical characterizations of LD converter steel slags: a multi-analytical techniques approach. Mater Charact 61:39–48
Pati PR, Satapathy A (2015) Development of wear resistant coatings using LD slag premixed with Al2O3. J Mater Cycles Waste Manag 17:135–143
Ashrit S, Banerjee PK, Chatti RV (2015) Characterization of gypsum synthesized from LD slag fines generated at a waste recycling plant of a steel plant. New J Chem 39:4128–4134
Singh SK, Rekha P, Surya M (2017) Utilization of LD slag in newer cementitious binder: A state of art report. CSIR-Central Building Research Institute Roorkee, India, pp 1–48
Ashrit S, Banerjee PK, Nair UG, Rayasam V (2017) Thermogravimetric analysis of LD slag waste fines in the range of 0–6 mm and establishing the correlation between free lime and weight loss of LD slag fines. Metall Res Technol 114:310
Tossavainen M, Forssberg E (1999) The potential leach ability from natural road construction materials. Sci Total Environ 239:31–47
Chand S, Chand SK, Paul B, Kumar M (2018) Long-term leaching assessment of constituent elements from Linz–Donawitz slag of major steel industries in India. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-2025-z
Makhija D, Rath RK, Chakravarty K, Patra AS, Mukherjee AK, Dubey AK (2016) Phosphorus partitioning and recovery of low-phosphorus iron-rich compounds through physical separation of Linz–Donawitz slag. Int J Miner Metal Mater 23:751–759
USEPA: SW-846 Test Method 1311 (1992) Toxicity characteristic leaching procedure. United States Environmental Protection Agency, USA
Arsenic AM, Beryllium AM (1996) Method 3050B acid digestion of sediments, sludges, and soils 1.0 scope and application
USEPA: SW-846 Test Method 1312 (1994) Synthetic precipitation leaching procedure. United States Environmental Protection Agency, USA
ASTM D4874–95 (2014) Standard test method for leaching solid material in a column apparatus. American Society for Testing and Materials, USA
Aarabi-Karasgani M, Rashchi F, Mostoufi N, Vahidi E (2010) Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 102:14–21
Chinnaraju K, Ramaleumar VR, Lineesh K, Nithya S, Sathish V (2013) Study on concrete using steel slag as coarse aggregate replacement and eco sand as fine aggregate replacement. Int J Res Eng Adv Technol 1:1–6
Gronniger J, Wistuba MP, Falchetto AC (2015) Reuse of Linz-Donawitz (LD) slag in asphalt mixtures for pavement application. Proceedings of the Interantional Conference on Industrial Wasted and Wastewater Treatment & Valorization, 1–17
Dominguez EA, Ullman R (1996) Ecological bricks made with clays and steel dust pollutants. Appl Clay Sci 11:237–249
Duda A (1987) Aspects of the sulphate resistance of steelwork slag cements. Cem Concr Res 17:373–384
Duda A (1989) Hydraulic reactions of LD steelwork slags. Cem Concr Res 19:793–801
Olonade KA, Kadiri MB, Aderemi PO (2015) Performance of steel slag as fine aggregate in structural concrete. Nigerian J Technol 34:452–458
Muhmood L, Vitta S, Venkateswaran D (2009) Cementitious and pozzolanic behavior of electric arc furnace steel slags. Cem Concr Res 39:102–109
Mahoutian M, Shao Y, Mucci A, Fournier B (2015) Carbonation and hydration behavior of EAF and BOF steel slag binders. Mater Struct 48:3075–3085
Salman M, Cizer O, Pontikes Y, Vandewalle L, Blanpain B, Balen KV (2014) Effect of curing temperatures on the alkali activation of crystalline continuous casting stainless steel slag. Constr Build Mater 71:308–316
Indian Cement Industry Analysis, https://www.ibef.org/industry/cement-presentation. April 2018
Tufekci M, Demirbas A, Genc H (1997) Evaluation of steel furnace slags as cement additives. Cem Concr Res 27:1713–1717
Tsakiridis PE, Papadimitriou GD, Tsivilis S, Koroneos C (2008) Utilization of steel slag for Portland cement clinker production. J Hazard Mater 152:805–811
Kriskova L, Pontikes Y, Zhang F, Cizer O, Jones PT, Van Balen K, Blanpain B (2014) Influence of mechanical and chemical activation on the hydraulic properties of gamma dicalcium silicate. Cem Concr Res 55:59–68
Kriskova L, Pontikes Y, Cizer O, Mertens G, Veulemans W, Geysen D, Jones PT, Vandewalle L, Van Balen K, Blanpain B (2012) Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cem Concr Res 42:778–788
Kambole C, Paige-Green P, Kupolati WK, Ndambuki JM, Adeboje AO (2017) Basic oxygen furnace slag for road pavements: a review of material characteristics and performance for effective utilisation in southern Africa. Constr Build Mater 148:618–631
Taylor HFW (1997) Cement chemistry, 2nd edn. Telford Publishing, London
Geiseler J (1996) Use of steel works slag in Europe. Waste Manag 16:59–63
Reddy AS, Pradhan RK, Chandra S (2006) Utilization of basic oxygen furnace (BOF) slag in the production of a hydraulic cements binder. Int J Miner Process 79:98–105
Zhang T, Yu Q, Wei J, Li J, Zhang P (2011) Preparation of high performance blended cements and reclamation of iron concentrate from basic oxygen furnace steel slag. Resour Conserv Recycl 56:48–55
Salman M, Cizer O, Pontikes Y, Snellings R, Dijkman J, Sels B, Vandewalle L, Blanpain B, Balen KV (2015) Alkali activation of AOD stainless steel slag under steam curing conditions. J Am Ceram Soc 98:3062–3074
Salman M, Cizer O, Pontikes Y, Snellings R, Vandewalle L, Blanpain B, Balen KV (2015) Cementitious binders from activated stainless steel refining slag and the effect of alkali solutions. J Hazard Mater 286:211–219
IS 12089 (1987) Specification for granulated slag for the manufacture of Portland slag cement. Bureau of Indian Standards, New Delhi
ASTM: C989, C989M (2017) Standard specification for slag cement for use in concrete and mortars. American Society for Testing and Materials, USA
Krivenko P (1994) Progress in alkaline cements. Proceedings of the 1st International. Conference, Alkaline cements and Concretes. (Kiev, Ukraine), 11–129
Glukhovsky V (1994) Ancient, modern and future concretes. First International. Conference. Alkaline Cements and Concretes, (Kiev, Ukraine), 1, 1–8
Motz H, Geiseler J (2001) Products of steel slags—an opportunity to save natural resources. Waste Manag 21:285–293
Kumar DS, Sah R, Prasad G, Prasad SMR, Yadav D, Gupta S, Chaturvedi SK (2020) Use of granulated steel slag in manufacture of cement. JSW Steel Ltd, and NCCB, India, (www.ncbindia.com/pdf_seminar/050-FP.pdf)
Wu S, Xue Y, Ye Q, Chen Y (2007) Utilization of steel slag as aggregates for stone mastic asphalt (SMA) mixtures. Build Environ 42:2580–2585
Shen DH, Wu C-M, Du J-C (2009) Laboratory investigation of basic oxygen furnace slag for substitution of aggregate in porous asphalt mixture. Constr Build Mater 23:453–461
Asi IM, Qasrawi HY, Shalabi FI (2007) Use of steel slag aggregate in asphalt concrete mixes. Can J Civ Eng 34:902–911
Xie J, Chen Z, Pang L, Wu S (2014) Implementation of modified pull-off test by UTM to investigate bonding characteristics of bitumen and basic oxygen furnace slag (BOF). Constr Build Mater 57:61–68
Pang B, Zhou Z, Xu H (2015) Utilization of carbonated and granulated steel slag aggregate in concrete. Constr Build Mater 84:454–467
Kotresh KM, Kebede YB, Behre S, Gethaun M, Honnappanavar ML (2016) Study focus on concrete replacing LD slag as fine aggregate. Int J Adv Eng Manag Sci 2117–2121
N N, Maheshchandra KV (2014) A study on flexural behaviour of reinforced concrete beams by replacement of Linz–Donawitz (LD) slag as fine aggregate. Int J Civ Struct Eng Res 2:89–96
Suri N, Babu YA (2016) Experimental investigations on partial replacement of steel slag as coarse aggregates and eco sand as fine aggregate. Int J Civ Eng Technol 7:322–328
Brand AS, Roesler JR (2015) Steel furnace slag aggregate expansion and hardened concrete properties. Cem Concr Compos 60:1–9
Sezer GI, Gulderen M (2015) Usage of steel slag in concrete as fine and/or coarse aggregate Indian. J Eng Mater Sci 22:339–344
Bodor M, Santos RM, Cristea G, Salman M, Cizer O, Iacobescu RI, Chiang WY, Balen KV, Vlad M, Gerven TV (2016) Laboratory investigation of carbonated BOF slag used as partial replacement of natural aggregate in cement mortars. Cement Concr Compos 65:55–66
Emery JJ (1982) Slag utilization in pavement construction. Extending Aggregate Resources. ASTM Special Technical Publication 774, ASTM, Washington, DC
Shih PH, Wu ZZ, Chiang HL (2004) Characteristics of bricks made from waste steel slag. Waste Manag 24:1043–1047
Shakir AA, Naganathan SK, Mustapha KNB (2013) Development of bricks from waste material: a review paper. Aust J Basic Appl Sci 7:812–818
Pinto M, Rodrigvez M, Besga G (1995) Effects of Linz–Donawitz (LD) slag as soil properties and pasture production in the Basque country (Northern Spain). N Z J Agric Res 38:143–155
A guide for the use of steel slag in agriculture and for reclamation of acidic lands, https://www.nationalslag.org/sites/nationalslag/files/ag_guide909.pdf, 09 Feb 2018
Poh HY, Ghataora GS, Ghazireh N (2006) Soil stabilization using basic oxygen steel slag fines. J Mater Civ Eng 18:229–240
Lopez Gomez FA, Aldecoa R, Fernandez Prieto MA, Rodrigues JM, Simoes C (1999) Preparation of NPK fertilisers from ferrous-metallurgy. Eur Comm 1
Kumar DS (2015) JSW steel’s granulated LD slag for cement production
Kumar DS, Sah R, Prasad G, Prasad SMR, Yadav D, Gupta S, Chaturvedi SK (2020) Use of granulated steel slag in manufacture of cement. JSW Steel Ltd, and NCCB, India, (www.ncbindia.com/pdf_seminar/050-FP.pdf)
Kumar P, Kumar DS, Marutiram K, Prasad SMR (2017) Pilot-scale steam aging of steel slags. Waste Manag Res pp 1–8
Xuequan W, Hong Z, Xinkai H, Husen L (1999) Study on steel slag and fly ash composite Portland cement. Cem Concr Res 29:1103–1106
Xue Y, Wu S, Hou H, Zha J (2006) Experimental investigation of basic oxygen furnace slag used as aggregate in asphalt mixture. J Hazard Mater B 138:261–268
Murphy JN, Meadowcroft TR, Barr PV (1997) Enhancement of the cementitious properties of steelmaking slag. Can Metall Q 36:315–331
Monshi A, Asgarani MK (1999) Producing Portland cement from iron and steel slags and limestone. Cem Concr Res 29:1373–1377
Li YS (1999) The use of waste basic oxygen furnace slag and hydrogen peroxide to degrade 4-chlorophenol. Waste Manag 19:495–502
Belhadj E, Diliberto C, Lecomte A (2012) Characterization and activation of basic oxygen furnace slag. Cem Concr Compos 34:34–40
Chen Z, Wu S, Xiao Y, Zeng W, Yi M, Wan J (2016) Effect of hydration and silicone resin on basic oxygen furnace slag and its asphalt mixture. J Clean Prod 112:392–400
Yildirim IZ, Prezzi M (2009) Use of steel slag in subgrade applications, in: joint transportation research program: Final Report—FHWA/IN/JTRP-2009/32, SPR-3129, Indiana Department of Transportation—Office of Research and Development, West Lafayette, USA, 2009, 1–274
Lun Y, Zhou M, Cai X, Xu F (2008) Methods for improving volume stability of steel slag as fine aggregate. J Wuhan Univ Technol 23:737–742
Miraoui M, Zentar R, Abriak N-E (2012) Road material basis in dredged sediment and basic oxygen asphalt mixture, furnace steel slag. Constr Build Mater 30:309–319
Björkman B, Eriksson J, Nedar L, Samuelsson C (1996) Waste reduction through process optimization and development. JOM 48:45–49
Wang Q, Yan P, Han S (2011) The influence of steel slag on the hydration of cement during the hydration process of complex binder. Sci China Technol Sci 54:388–394
Alanyali H, Col M, Yilmaz M, Karagoz S (2009) Concrete produced by steel-making slag (basic oxygen furnace) addition in Portland cement. Int J Appl Ceram Technol 6:736–748
Reddy AS, Pradhan RK, Chandra S (2006) Utilization of Basic Oxygen Furnace (BOF) slag in the production of a hydraulic cement binder. Int J Miner Process 79:98–105
Vlcek J, Tomkova V, Ovcacikova H, Ovcacik F, Topinkova M, Matejka V (2013) Slags from steel production: properties and their utilization. Metalurgija 52:329–333
Altun IA, Yılmaz I (2002) Study on steel furnace slags with high MgO as additive in Portland cement. Cem Concr Res 32:1247–1249
Ahmedzadea P, Sengoz B (2009) Evaluation of steel slag coarse aggregate in hot mix asphalt concrete. J Hazard Mater 165:300–305
Devi VS, Gnanavel BK (2014) Properties of concrete manufactured using steel slag. 12th Global congress on manufacturing and management (GCMM). Proc Eng 97:95–104
IS 1077 (1992) Common burnt clay building bricks specifications. Bureau of Indian Standards, New Delhi, India
IS 12894 (2002) Pulverized fuel ash-lime bricks-specification. Bureau of Indian Standards, New Delhi, India
Acknowledgements
The paper forms part of research and development project at CSIR-Central Building Research Institute, Roorkee under financial assistance from Ministry of Steel, Government of India (Grant No. 11(24)/GBS/2017-TD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S.K., Rekha, P. & Surya, M. Utilization of Linz–Donawitz slag from steel industry for waste minimization. J Mater Cycles Waste Manag 22, 611–627 (2020). https://doi.org/10.1007/s10163-020-00981-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-00981-z