Results are presented for a study of medium-carbon structural steels for the effect of microalloying with nitrogen, vanadium, and other nitride-forming elements on the microstructure and processes of nitride formation after hardening with tempering and rolling. The level of mechanical properties is compared after different treatment. It is shown that a lower bainite structure forms with high dislocation density after rolling and cooling steel to 550°C. Within ferrite plates there is precipitation of finely dispersed densely located vanadium carbonitride. Strength as a result of good dispersion of the fine lower bainite structure, high dislocation density, and dispersion strengthening with precipitation of nanosize carbonitride phase, provides a high level of mechanical properties after rolling and cooling to 550°C, comparable with the level of properties after hardening and tempering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The task of preparing structural steels exhibiting high strength and fracture toughness as a result of alloying is always important. In particular, recent research [1–3] has shown that a good set of unique properties may be achieved as a result of microalloying with nitrogen and nitride-forming elements. Significant austenite grain refinement, strengthening (by 30–50%) after heat treatment, and an increase in resistance to brittle and fatigue failure are achieved. It has been established [4, 5] that hot-rolled microalloyed steels after “controlled” forming with achievement of a lower bainite structure have properties equivalent to heat treated steels. Exclusion of heat treatment operations both from the point of view of obtaining properties, and from the point of view of ecology and economics, is very important, for example for engine (connecting rod, crankshaft, etc.) components, and also for such articles high-strength bolts, in order to avoid formation of quenching cracks, improving fatigue properties. In this case a delay necessary at the bainite transformation temperature, a type of tempering, and therefore sometimes this treatment is called “bainite heat treatment.” With a bainitic structure there is an increase in the difference between ultimate strength (failure stress) and breaking strength, being a measure of bolt ductility [6], even with high strength values (up to 1400 MPa).
Data provided in publications concerning this problem are very limited, and therefore it is interesting to study features of structure formation and microalloyed steel nitride formation after rolling compared with a quenched and tempered condition.
Medium-carbon steels with basic alloying with chromium type 30Kh2, containing nitrogen, titanium and other nitride formers, were studied. Melting was carried out in a 200-kg induction furnace. Metal (Table 1) was poured into ingots weighing 10 kg, which after homogenizing were forged into strip with a cross section of 30 × 45 mm2. Then strip was heated for rolling to 1200°C and rolled at a rate of 0.04 sec−1 for one pass in a universal mill with a degree of deformation of 35% and a temperature for the end of rolling of 950°C. After rolling specimens were air cooled with blowing at a rate of 20°C/sec to a temperature of the order of 550°C (bainite transformation temperature) and stacked, and control billets were water cooled. Some specimens were tempered at 550°C.
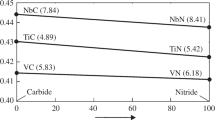
It is well known that the bainite transformation temperature is reduced by almost all alloying elements, it has been detected empirically that it decreases linearly in relation to steel composition [7]
Bainite transformation temperature for the test steels was 530–550°C, and therefore accelerated cooling of billets from the temperature for the end of rolling was carried out to 530–550°C followed by holding at this temperature by stacking billets, i.e., “bainite heat treatment”.
The set of studies used included light, transmission, and scanning electron microscopy, x-ray structural phase analysis, local x-ray spectral, chemical analyses of an anodic deposit, and mechanical tests.
Analysis of data obtained shows that the properties of microalloyed steels cooled after rolling to 550°C and quenched and tempered at 550°C, are comparable (Table 2). Values of steel 30Kh2AFB properties are somewhat lower than for steel 30Kh2AF. Austenite grain size is 3 μm, and ferrite grain size is 1 μm.
According to electron microscope study data within the structure of air cooled specimens there are crystals, i.e., laths of lower bainite of different orientation, within which cementite particles are seen. Cementite particles within bainite are aligned in regular rows at an angle 58–65° to ferrite crystals (Fig. 1), within which they are located. In contrast to this within laths of tempered martensite there are carbide precipitates of two to three orientations.
Alongside cementite precipitates in areas of foil where the contrast due to dislocations is suppressed, particles are revealed with a size of about 10 nm with a diffraction pattern corresponding to vanadium carbonitride.
x-Ray studies of anodic deposits, chemically deposited from specimens, according to relative intensity of interference lines and crystal lattice spacing showed the following.
A set of interference lines is present in diffraction patterns for quenched steel 30Kh2AF, typical for a cubic phase with a lattice spacing a = 0.41419, corresponding to V(C0.4C0.6). The form of interference lines indicates that the precipitates are partly undissolved phases at 950°C. The size of V(C0.4C0.6) phase determined from the width of interference lines is 14 nm, and lines are also detected corresponding to TiN. With accelerated cooling (AC) in air from the rolling temperature to 550°C a set of interference lines is observed for steel 30Kh2AF typical for cementite, which may indicate formation of bainite. In addition, new interference lines are detected, typical for V(C0.2C0.8). The width of interference lines obtained for these particles is greater than that for V(C0.4N0.6) particles. Calculation of particle average size from the integral width of lines showed that particle size is 8–10 nm. It should be noted that judging from deposit chemical analysis a small amount of chromium is dissolved within cementite present in hot-rolled specimens.
A fragment of a diffraction pattern is presented in Fig. 2 from a carbide precipitate, separated from steel 30Kh2AFB after deformation and subsequent quenching. In the diffraction pattern for specimens of this steel after rolling with t e.r = 950°C, water quenched, a set of interference lines is present typical for cubic phase with crystal lattice spacing a = 0.4435 nm and corresponding to niobium carbide of the composition Nb(C0.5N0.5) with an average particle size of 18 nm.
Accelerated cooling to 550°C after rolling also leads to precipitation of a phase with lattice spacing a = 0.4271, corresponding to Nb0.25V0.75(C, N) with a particle size of 12 nm. The width of interference lines, obtained from Nb0.25V0.75(C, N) particles is greater than the width of lines recorded from NbC0.5N0.5 particles, which is connected strong dispersion of phases. Analysis shows that the first particles are soluble phase on heating for rolling, and the second phase precipitates within austenite during rolling. After AC from the temperature for the end of rolling to 550°C, the same as in steel 30Kh2AF, V(C0.2N0.8) vanadium carbonitride is detected, but without bainitic cementite.
In both steels after hardening and tempering a set of interference lines of small width, typical for Me 7C3 type carbide containing a larger amount of chromium. In electron microscope pictures needle-like “transparent” Me 7C3 carbide precipitates are seen. In pictures of foil specimens, water quenched from 550°C, the amount of carbonitride particles is significantly greater than in the hot-rolled condition.
It is well known that lower bainite provides steel strengthening due to a combination of such strengthening mechanisms as:
-
1)
presence of fine ferrite (strengthening due to fine-grained austenite);
-
2)
high dislocation density, being a result of shear arising during bainite transformation; and
-
3)
strength of ferrite matrix, including solid solution strengthening by alloying elements.
As mechanical tests showed, yield strength reaches 1180 MPa. On the basis of Pickering’s opinion it may be suggested that the contribution of the three main strengthening mechanisms mentioned is estimated as follows [8]:
-
1)
fine ferrite platelets with a size of 1 μm “contribute” ≈260 MPa [8];
-
2)
strengthening due to dislocation substructure of bainite (with a dislocation density of 6·1014 cm−2) “gives” 400 MPa [9]; and
-
3)
the strength of matrix, including solid solution strengthening by alloying elements “contributes” of the order of 200 MPa [8].
It has been shown [3] that relative high V(C, N) solubility and low VN solubility compared with VC makes microalloying with vanadium in the presence of nitrogen more preferable for providing reliable and readily controllable dispersion strengthening. Precisely dispensed addition of nitrogen facilitates control of dispersion hardening, density of nanosize nitride precipitation, and finally, strengthening due to vanadium nitride. Ultrafine densely located precipitates have a marked effect on rolled product final strength by two routes:
-
1)
dispersion hardening of steel increases strength;
-
2)
nanosize precipitates slow and even prevent weakening of a dislocation structure of bainitic ferrite and thus retain the primary bainite strength.
The contribution of dispersion strengthening due densely located nanosize carbonitride particles, evaluated according to the Orowan mechanism, comprises roughly 320 MPa [9]. Strengthening as a result of precipitation of nanosize densely located vanadium carbonitride phases with presence of a high-dislocation substructure is very strong, and presence of these phases prevents weakening during exposure, which significantly increases steel yield strength.
Conclusion. A feature of nitride formation in steels, microalloyed with nitrogen, vanadium, and niobium, is presence of niobium carbonitride of the composition NbC0.5N0.5 soluble during rolling and precipitation of complex carbonitride Nb0.25V0.75(C, N) within austenite. In the test steels, cooled after rolling, within bainitic ferrite there is precipitation of nanosize densely located V(C0.2N0.8) vanadium carbonitride. After cooling from rolling heating steel with a lower bainite structure with a high dislocation density, strengthened by nanosize densely located carbonitride, has good set of properties, identical with those of the same steels quenched and tempered.
References
M. I. Gol’dshtein, A. V. Grin’, E. E. Blyum, and A. M. Panfilova, Structural Steel Strengthening with Nitruides, Metallurgiya, Moscow (1970).
A. M. Panfilova, “New generation of structural high-strength steels with vanadium,” Proc. Seminar Use of Vanadium in Steel, Moscow, 2002, Izd. UrO RAN, Ekaterinburg (2002), pp. 89–109.
R. Lagneborg, T. Siwecki, S. Zajac, and B. Hutchinson, “The role of vanadium in microalloyed steels,” Scand. J. Metallurgy 28, No. 5, 186–241 (1999).
A. A. Smirnov, L. M. Panfilova, and B. Z. Belen’kii, “Problems of expanding production of vanadium-containing steels within Russia,” Stal, No. 6, 108–115 (2005).
M. Korchinskii, “Advanced metal structural materials and new role of steel microalloying,” Stal, No. 6, 124–130 (2005).
V. Shmidt et al., “Alloyed steels,” Coll. Statistical Strength and Mechanical Failure, [Russian translation], Metallurgiya, Moscow (1986), pp. 511–544.
E. Gudremont, Special Steels [Russian translation], Metallurgiya, Moscow (1966), Vol. 1.
M. I. Goldshtein et al., High-Strength Alloy Metal Physics, Metallurgiya, Moscow (1986).
R. Lagneborg, T. Siwecki, S. Zajac, and B. Hutchinson, The Role of Vanadium in Microalloyed Steels, Swerea KIMAB, Sweden (2014).
This work was carried out with financial support UrO RAN directed fundamental research projects (grant No. 13-3-029 UZTM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 10, pp. 77–80, October, 2014.
Rights and permissions
About this article
Cite this article
Panfilova, L.M., Smirnov, L.A. Structural Features of Structural Steels Microalloyed with Nitrogen and Vanadium. Metallurgist 58, 916–920 (2015). https://doi.org/10.1007/s11015-015-0017-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-015-0017-5