Abstract
The excessive production of pro-inflammatory mediators, characteristic of obesity, leads to neuroinflammation. Zinc (Zn) and the branched-chain amino acids (BCAA) are supplements known for their immunomodulatory properties. Our goal was to evaluate if Zn or BCAA supplementation can affect long-term recognition memory and neuroinflammatory parameters of obese rats after a high-fat diet (HFD). Three-month-old Wistar rats were divided into six groups: Standard diet (SD) + vehicle; SD + Zn; SD + BCAA; High-fat diet (HFD) + vehicle; HFD + Zn; and HFD + BCAA. Diets were administrated for 19 weeks, Zn (1,2 mg/kg/day) or BCAA (750 mg/kg/day) supplementation was conducted in the last 4 weeks. Long-term recognition memory was evaluated by the novel object recognition test. IL-1β immunoreactivity in the cortex and hippocampus, and IL-6 levels in the cortex tissue were assessed. Astrogliosis were evaluated through GFAP + cell count and morphological analysis (Sholl Method). Zn supplementation improved object recognition memory in HFD-fed rats, which was not observed following BCAA supplementation. The levels of IL-6 in the cerebral cortex were higher after HFD, which was not diminished after neither supplementation. Obesity also led to increased IL-1β immunoreactivity in the cerebral cortex and hippocampus, which was reduced by Zn. BCAA supplementation also diminished IL-1β immunoreactivity, but only in the hippocampus. We also showed that astrocyte reactivity caused by HFD is area-dependent, being the cerebral cortex more susceptible to the diet. Even though BCAA and Zn can affect IL-1β immunoreactivity and astrocyte morphology, only Zn improved memory. Future studies are needed to clarify the pathways by which Zn improves cognition in obesity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural immune response of the central nervous system (CNS) is known as neuroinflammation. It is responsible for preserving the CNS environment by removing pathogenic organisms, cleaning cellular debris, and promoting tissue repair. However, sustained neuroinflammation has been extensively associated with neurodegenerative diseases, being correlated with the starting and progression of neurodegeneration (Neal and Richardson 2018; Kwon and Koh 2020).
In obesity, excessive body fat accumulation creates a low-level, chronic, and generalized inflammatory state known as meta-inflammation (Gregor and Hotamisligil 2011). Peripheral pro-inflammatory mediators, such as interleukin-1β (IL-1β) and tumor necrosis factor (TNF-α), can cross the blood–brain barrier (BBB) and reach the brain (Rhea et al. 2017). In the brain, glial cells, mainly microglia and astrocytes, respond by changing their morphology and becoming activated (Kwon and Koh 2020). Although microglia are the innate immune cell of the CNS, there is significant involvement of astrocytes in the neuroinflammation found in neurodegenerative disorders (Neal and Richardson 2018). Astrocytes are the most abundant glial cells in the central nervous system (CNS). They are entrusted with important tasks such as participating in neuronal synapses and maintaining the integrity of the BBB (Pekny and Pekna 2014; Sofroniew 2014; Rhea et al. 2017). When glial cells become chronically activated, a sustained increase of local pro-inflammatory elements triggers neuronal death. It may explain why obesity also have been considered a risk factor for the development of neurological diseases such as Alzheimer's disease (Samara et al. 2019). Obesity has become an epidemic and, according to the World Health Organization, in 2016 around 9% of the world population were obese (≥ 30 kg/m2) (WHO 2018).
Although obesity is a multifactorial disease, it is known that diet plays an important role in its increasing incidence worldwide (Malik et al. 2013). In that sense, animal models to mimic obesity are crucial to better understanding the mechanism of obesity-related neuroinflammation. High-fat diets (HFD) are high-energy diets, extensively used to mimic the pattern of human obesity with the development of characteristics such as weight gain and metabolic disorders (Beilharz et al. 2016, Thoen et al. 2018). Previous work by our research group using this diet model has already shown that the damage also extends to the central nervous system (de Andrade et al. 2017). Therefore, the use of diet-induced obesity models allows the search for treatment options capable of diminishing the deleterious neurological effects of the disease.
Among a range of commercial supplements that have known immuno-modulatory properties, we can highlight Zinc (Zn) and the branched-chain amino acids (BCAA). Zn is a mineral of nutritional importance. It is necessary as a component for more than 300 enzymes contributing to the functionality of over 2000 proteins in the human body, and its deficiency can cause a a range of acute and chronic detrimental effects, including growth retardation, infertility and immune dysfunction (Plum et al. 2010; Portbury and Adlard 2017). Obese and overweight individuals can present low serum Zn levels, leading to a low capacity to respond to oxidative damage and inflammation, aggravating symptoms already presented in obesity (Costarelli et al. 2010; Rios-Lugo et al. 2020). Also, Zn supplementation could ameliorate glucose homeostasis and lipid profile in humans (Skalny et al. 2021). In the brain, zinc is associated with cognition and memory regulation through N-methyl-D-aspartate receptors in processes such as long-term potentiation and long-term depression (Portbury and Adlard 2017). Also, zinc plays an important role in immune modulation, being capable of suppressing the allogeneic immune response at relatively low doses (Faber et al. 2004).
BCAA (valine, leucine and isoleucine) are essential amino acids. As an immune modulator, those amino acids can, for instance, provide important coenzymes that will support metabolic reprogramming of immune cells and support epigenetics modulation (Kelly and Pearce 2020). Evidence suggests that low BCAA dietary intake impairs the immune response and increases the vulnerability to certain diseases (Zhang et al. 2017). Unlike most amino acids, only a small fraction of BCAA is metabolized by the liver. About 10% of ingested BCAA crosses the blood–brain barrier and reaches the brain. In the brain, the BCAA will directly or indirectly participate in neurotransmitter synthesis and maintenance of nitrogen balance in the glutamate-glutamine cycle between astrocytes and neurons (De Simone et al. 2013). Other advantages of BCAA supplementation include controlling body weight, improving muscle protein synthesis, and maintaining glucose homeostasis (Bifari and Nisoli 2017).
Considering that obesity causes a chronic inflammatory state which can affect peripheral and CNS structures and that Zn and BCAA can improve metabolic and inflammatory parameters, the present study aimed to evaluate whether 4 weeks of Zn or BCAA supplementation can impact the recognition memory and neuroinflammatory parameters of obese rats.
Experimental procedures
Animals
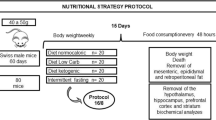
Male Wistar rats (n = 36) were provided by the Federal University of Health Sciences of Porto Alegre (UFCSPA) animal facility. To avoid hormonal fluctuation, only male rats were included. They were kept in plastic cages (2–3 rats per cage) under controlled temperature (22–24 °C) and light (12 h light/12 h dark cycle). Animals had free access to standard rat chow with a caloric content of 3.4 kcal/g (63% carbohydrates, 11% lipids, and 26% proteins; Nuvilab® CR-1 standard rat chow; NUVITAB, Brazil) and water until complete 12 weeks.
All procedures were approved by the Institutional Animal Care and Use Committee (UFCSPA, Brazil, protocol No. 359/16). All efforts were made to minimize animal suffering and reduce the number of animals used in the experiments, which were performed following the international laws for the care of laboratory animals, following the 3Rs guidelines.
Diet and supplementation
The diet and supplementations were performed as described in a previous study of our group (Thoen et al. 2018). Briefly, 12 weeks old male rats were randomly allocated in one of the 6 experimental groups (n = 6/group): Standard diet (SD) + vehicle; SD + Zn; SD + BCAA; High-fat diet (HFD) + vehicle; HFD + Zn; and HFD + BCAA. Diets were administrated for 19 weeks. As described above, the standard diet groups were fed with Nuvilab® CR-1 standard rat chow. Animals from the HFD groups were feed with Pragsoluções® high-fat diet, with a caloric content of 4.5 kcal/g (35.7% carbohydrates, 45.1% lipids, and 19.2% proteins, Pragsoluções Biociências, Brazil), and had free access to a solution containing 42 g/L of fructose and sucrose (55% fructose and 45% sucrose) (Synth, Brazil). Animals from all groups had access to water ad libitum.
The supplementation was administered by gavage. Zn and BCAA (1:1:1) were diluted in water at the doses of 1,2 mg/kg and 750 mg/kg, respectively. Animals received Zn or BCAA supplementation daily from the 15th week until the end of the experiment (4 weeks of supplementation). SD and HFD groups received an equivalent volume of water by gavage for the same period.
Recognition memory
The object recognition test was performed in the last week (19th week) of diet administration. The test is composed of 3 phases, divided into 3 days. First, rats were habituated for 10 min in an acrylic box (40 cm × 40 cm × 20 cm). Twenty-four hours after, the training session was conducted. In the training session animals were individually placed in the left rear quadrant of the box containing two identical objects (A and B) and allowed to explore for 5 min freely. Finally, twenty-four hours after the training session, one of the objects was replaced by a new object (C), and each rat was reintroduced into the box for 5 min to explore. Time exploring each object was recorded. Exploration was considered when the animals placed the nose towards the object at a distance less than 2 cm and/or when the animal touched the object with its nose (Ennaceur and Delacour 1988). Finally, the recognition index (RI) was calculated using the equation exhibited below.
Brain tissue samples
After 19 weeks of diet, the animals were fasted for 6 h and then euthanatized using 50 mg/kg of xylazine hydrochloride (Rompum®) and 100 mg/kg of ketamine hydrochloride (Ketalar®) for tissue collection. The brain was quickly removed, and the right and left hemispheres were separated. For histological analyses, the right hemisphere was placed in a fixative solution (zinc buffer solution). The cerebral cortex and the hippocampus were dissected from the left hemisphere and quickly frozen together with the cerebellum in liquid nitrogen, as previously described (de Moura et al. 2015), and stored at -80 °C for further analysis.
Immunohistochemistry
For immunohistochemistry, the brain's right hemisphere was fixed in a zinc buffer solution (pH 7.4) for 48 h at room temperature. After this period, tissues were dehydrated, embedded in paraffin, and sectioned (8 μm thick) using a microtome. Sections were treated with 3% H2O2 in 10% MeOH for 30 min, washed in PBS for 30 min, and incubated for 30 min in 3% goat serum (Millipore) in PBS containing 0.4% Triton X-100 (PBS-T). Then, sections were incubated overnight at 4 °C with a primary antibody (anti-GFAP, 1:750, Millipore, USA; anti-IL-1β (1:100, Santa Cruz Biotechnology, USA). Sections were then incubated with anti-mouse IgG peroxidase-conjugated secondary antibody (Sigma-Aldrich), diluted 1:500, for 90 min at room temperature. Immunoreaction was developed using a solution of 0.06% DAB (3,3′-diaminobenzidine tetrahydrochloride; Sigma-Aldrich) and 0.005% H2O2 in PBS. Sections were counterstained with hematoxylin, dehydrated, and covered with Entellan (Merck) and coverslips. A blinded researcher regarding the groups scored IL1-β immunorreactivity. The scoring system was adapted from a previous study (Clausen et al. 2020) considering four categories with basis on the number and the intensity of the immunoreactivity (IR): 0 = no immunoreactive cells; 1 = weak IR; 2 = moderate IR; 3 = intense IR. Three sections were scored per animal. Three to four animals per group were analyzed.
Astrocytes counting and morphological analyses
Digital images were acquired using a digital camera coupled to an Olympus BX-41 microscope using a 20 × objective lens. Four or five animals per group were analyzed. Three randomly selected fields were analyzed in the cortex in nonadjacent sections and two in each hippocampal region—CA1 and Dentate Gyrus. All the GFAP+ cells of the fields were counted. Results were expressed as the number of GFAP+ cells per field. The images were analyzed using the ImageJ software (Schneider et al. 2012).
Morphologic analysis was performed on 10–15 astrocytes of each animal. Three animals per group were analyzed. Images were analyzed using Image-Pro Plus software. For a general analysis of astrocytic ramifications, we used an adaptation of Sholl's concentric circle technique (Fig. 1) (Sholl 1953; Dall'Oglio et al. 2008). Shortly, the area around each astrocyte was divided into 4 quadrants, 2 laterals (i.e., right/left) and 2 central (i.e., top/bottom). Following, around 10 virtual circles with 2 μm intervals were drawn around each cell. All the processes extending directly from the soma were counted to quantify the number of primary ramifications. The longest primary process of the central and lateral quadrants was measured by tracing the process with an automated measurement tool; then, the results were summed to find the total length of the longest primary processes. Finally, the number of intersections of astrocytic processes with each virtual circle was quantified in all quadrants.
IL-6 dosage
Levels of inflammatory cytokine IL-6 were quantified in the cerebral cortex by ELISA (Invitrogen, USA) following the manufacturer’s instructions.
Zinc and BCAA determinations
Zinc content in the cerebellum was determined by flame atomic absorption spectrometry (FAAS) analysis using acid digestion. Nitric acid (65%, v:v) was added to samples, which were heated at 100 °C for 90 min. The determination of Zn was performed in a flame atomic absorption spectrometer (Shimadzu, model AA 7000F) equipped with a hollow cathode lamp and a deuterium lamp as a background corrector. The wavelength used was 213.9 nm. The current of the hollow cathode lamp was 8 mA for Zn, while the slit width of the monochromator was 0.7 nm. The mixture of gases was composed of air and acetylene. To carry out the analyzes, the analytical curve was performed with a standard Zn stock solution of 1000 mg/L (Merck) with a purity level of 99.9%.
BCAA content in the central nervous system was measured by UHPLC-ESI–MS/MS analysis. Briefly, cerebellum samples (300 mg) were homogenized with 1 mL of deionized water, frozen in liquid nitrogen, and kept under -80 °C until processing. Aliquots containing 10 μL of the obtained homogenates were added to 90 μL of an aqueous solution containing 0.1% formic acid and centrifuged at 9000 g for 10 min. The supernatants were collected to quantify the amino acids. The analytical system consisted of a Nexera UFLC system coupled to an LCMS-8040 triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). The electrospray parameters were set in the positive ion mode as follows: capillary voltage, 4500 V; desolvation line temperature, 250 °C; heating block temperature, 400 °C; drying gas, 18 l/min; and nebulizing gas, 2 l/min. Collision-induced dissociation was obtained with 230 kPa argon pressure. Analyses were carried out with multiple reaction monitoring (MRM) by using the following [M + H]+ fragmentations: m/z 117.9 → 72.1 for detection of valine; and m/z 131.9 → 86.1 for detection of isomers leucine and isoleucine. The calibration curves were constructed at the intervals of 2 to 14 ng for each amino acid. The chromatographic separation was achieved with a 250 × 4.6 mm i.d., 5 μm, Shim-pack ODS column (Shimadzu, Kyoto, Japan) isocratically eluted with an aqueous solution containing 10% of methanol and of 0.1% formic acid at a flow rate of 500 μL/min and 20 °C. For data evaluation, LabSolutions software (Shimadzu, Kyoto, Japan) was used for the data treatment.
Statistical analyses
The normality of data was analyzed by Kolmogorov–Smirnov test. Two-way ANOVA followed by Bonferroni post hoc test was used for most of the analysis. Treatment (vehicle, BCAA, or zinc) and diet (SD or HFD) were used as main effects for ANOVA. The data are expressed as mean ± SEM. GraphPad Prism 9.0 was used for the statistical analyses. The significance level was set at p < 0.05.
Results
In a previous study, we showed that HFD-fed rats had higher weight gain and visceral adiposity than the SD group, and Zn treatment could revert these results in obese rats. On the other hand, BCAA did not exert any effect on these measurements (Thoen et al. 2018). The present study evaluated the effect of Zn or BCAA supplementation associated with HFD on memory and neuroinflammatory markers.
Zinc and BCAA content were measured in the cerebellum to confirm that the supplementation reached the CNS (Table 1). The amount of Zn in the tissue of the Zn-treated rats was significantly higher when compared to rats that received the vehicle. Similar results were found for BCAA-treated rats, where isoleucine was significantly higher in the cerebellum of supplemented animals.
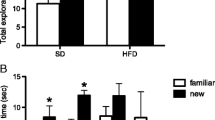
The object recognition test was performed to evaluate the long-term memory of rats (Fig. 2). There was no difference among the groups in the total exploration time independently of the diet and/or supplementation (interaction: F2,23 = 0.154, p = 0.858, Fig. 2A). Nonetheless, an interaction between diet and supplementation (Zn or BCAA) was found in the object recognition index (interaction: F2,24 = 6.165, p = 0.0069, Fig. 2B). Bonferroni post hoc evidenced that the HFD + BCAA group had reduced recognition index (RI) compared to the SD + BCAA group (p = 0.0060), and HFD + Zn rats showed a significant increase in the RI compared to the HFD + vehicle (p = 0.0247) and HFD + BCAA (p = 0.0091) groups. Therefore, Zn treatment can improve memory in obese rats. On the other hand, no positive effect was found after BCAA supplementation.
Zn supplementation improved object recognition memory in HFD-fed rats. A) There was no difference in the total exploration time (novel and familiar objects) among the experimental groups. B) Object recognition index was higher in the HFD + Zn group compared to HFD + vehicle and HFD + BCAA. Data are expressed as mean ± SEM. * (p < 0.05) when compared to HFD + vehicle and HFD + BCAA; ** (p < 0.01) when compared to SD + BCAA. SD, standard diet. HFD, high-fat diet. n = 5–6/group
To investigate the neuroinflammatory state in the brain following HFD, we assessed IL-6 protein levels in the cerebral cortex through an ELISA assay. We found higher levels of IL-6 in the cerebral cortex following the HFD (diet effect: F 1,27 = 5.348, p = 0.0286, Fig. 3), and neither Zn nor BCAA could reverse it (supplementation effect: F 2,27 = 1.302, p = 0.289). We also analyzed IL-1β immunoreactivity in the cerebral cortex and hippocampus (Fig. 4). In the cerebral cortex (Fig. 4A), there were a diet (F 1,84 = 41.54, p < 0.0001) and supplementation (F 2,84 = 7.464, p = 0.0010) effects. HFD + vehicle and HFD + BCAA IL-1β immunoreactivity were higher than HFD + Zn (p = 0.0025 and p = 0.0138, respectively). All HFD groups showed increased IL-1β immunoreactivity in the cerebral cortex in comparison to their SD-fed counterparts. Similarly, in the hippocampus, we also show a diet (F 1,69 = 8.265, p = 0.0054) and a supplementation (F 2,69 = 4.361, p = 0.0165) effects (Fig. 4B). The post hoc test showed that the HFD group had increased IL-1β immunoreactivity compared to all the other groups (HFD + vehicle vs. HFD + Zn: p = 0.0046; HFD + vehicle vs. HFD + BCAA: p = 0.0323; HFD + vehicle vs. SD + vehicle: p = 0.0015). Therefore, HFD consistently increased neuroinflammation.
IL-1β immunoreactivity in the cerebral cortex (upper) and hippocampus (lower). A) A diet and supplementation effects were found in the cerebral cortex, and Zn treatment reduced IL-1β immunostaining in obese rats. B) In the hippocampus, it was also showed diet and supplementation effects, but not only Zn but also BCAA decreased IL-1β immunoreactivity in HFD-fed animals. Data are expressed as mean ± SEM. * (p < 0.05) when compared to HFD + Zn and SD groups; # (p < 0.05) when compared to all groups. Scale bar = 50 µm. SD, standard diet. HFD, high-fat diet. n = 4–5/group
We also investigated the astrocytes morphology changes in response to HFD in the cerebral cortex and different regions of the hippocampus. Therefore, we performed the counting of astrocytes and morphological evaluation through Sholl circles. We found decreased GFAP positive cells in the cerebral cortex following HFD (diet effect, F1,77 = 16.73, p = 0.0001, Fig. 5A) with no Zn or BCAA supplementation effect (F2,77 = 0.3268, p = 0.72). Post-hoc analyses also demonstrated that the HFD + vehicle group had decreased GFAP positive cells than SD + vehicle (p = 0.0068). In the hippocampus, the dentate gyrus and CA1 areas were evaluated. Both dentate gyrus and CA1 showed a supplementation effect (dentate gyrus: F2,72 = 6.720, p = 0.0021, Fig. 5B; CA1: F2,75 = 4.447, p = 0.015, Fig. 5C). Divergent from what we found in the cerebral cortex, in the dentate gyrus, multiple comparisons demonstrated that both HFD + Zn (p = 0.0372) and HFD + BCAA (p = 0.0111) had reduced GFAP positive cells compared to HFD + vehicle. A similar pattern was found in CA1, where HFD + Zn (p = 0.0336) had reduced GFAP + cells compared to HFD + vehicle. Therefore, we were able to show that the effects of diet and treatments depend on the area of the brain, and both diet and treatment can result in changes in astrocytes number in a region-dependent way.
Immunohistochemistry for GFAP (number of cells per region). A) HFD reduced the number of GFAP positive cells in the cerebral cortex regardless of the Zn or BCAA supplementation. B) In the hippocampus, we found a supplementation effect, with a decrease in the number of GFAP positive cells in the dentate gyrus of obese rats that received Zn or BCAA treatment. C) In the CA1 area, a supplementation effect was also found, with a significant decrease in HFD + Zn compared to the HFD + vehicle group. Data are expressed as mean ± SEM. ** (p < 0,01) when compared SD and HFD with the same treatment. # (p < 0,05) when compared with HFD vehicle. Scale bar = 50 µm. SD, standard diet. HFD, high-fat diet. n = 4–5/group
Further, we investigated how diet or Zn and BCAA treatments could impact astrocyte morphology using Sholl circles analysis. The number of primary ramifications from the soma, total length, and the total number of intersections of astrocytic processes were measured. In the cerebral cortex, the HFD affected all morphological parameters evaluated. We found reduced number of ramifications (F1,169 = 18.39, p < 0.0001, Fig. 6A), total length (F1,167 = 18.17, p < 0.0001, Fig. 6B) and number of intersections (F1,167 = 47, p < 0.0001, Fig. 6C) following HFD. Post hoc tests showed that the number of primary ramifications in the HFD + BCAA group was lower than SD + BCAA (p = 0.0005). The total length of astrocytes in the HFD + Zn and HFD + BCAA groups were decreased compared to the SD + Zn group (p = 0.0207) and SD + BCAA (p = 0.0032), respectively. Similarly, the number of intersections of HFD + Zn and HFD + BCAA were lower than SD + Zn (p < 0.0001) and SD + BCAA (p < 0.0001) groups, respectively. In the dentate gyrus, there was an interaction in the number of intersections (Interaction: F2,180 = 3.357, p = 0.037, Fig. 6F). Post-hoc analysis showed that HFD + BCAA had an increased number of intersections than the SD + BCAA group (p = 0.0360). In the CA1 area, the number of primary ramifications (Fig. 6G) and the number of intersections (Fig. 6I) were not affected by diet and Zn and BCAA treatments. However, an interaction in the total length of the ramifications (Interaction: F2,237 = 10.37, p < 0.0001, Fig. 6H) was observed. Multiple comparisons showed that HFD + vehicle caused a decrease in the dimension of the ramifications compared to SD + vehicle (p = 0.0281). Also, HFD + BCAA had increased dimension of ramifications compared to HFD + vehicle (p = 0.0233) and SD + BCAA (p = 0.0055); and SD + BCAA group was decreased compared to SD + vehicle (p = 0.0057).
Sholl circles analysis of astrocytes. (A-C) Morphological analysis of the astrocytes in the cerebral cortex. HFD decreased astrocyte arborization, as showed by all parameters evaluated. (D-E) Morphological analysis of the astrocytes in the dentate gyrus of the hippocampus. HFD increased the number of process intersections, and only Zn supplementation could reverse it. (G-I) Morphological analysis of the astrocytes in the CA1 area of the hippocampus. HFD decreased the total length of the longest ramifications. Only BCAA supplementation could reverse it. Data are expressed as mean ± SEM. * (p < 0.05) ** (p < 0.01) *** (p < 0,001) when compared SD and HFD with the same treatment. # (p < 0.05) ## (p < 0.01) when compared with HFD vehicle. && (p < 0.01) when compared with SD vehicle. SD, standard diet. HFD, high-fat diet. n = 4–5/group
A summary of all morphological findings is shown in Table 2. These results corroborate the findings regarding cell number by demonstrating that the effects of HFD and treatments are dependent on the region analyzed.
Discussion
It is well established that obesity causes memory impairment (Cordner and Tamashiro 2015; Heyward et al. 2016; de Andrade et al. 2017). Multiple mechanisms are involved in obesity-related memory decline, and neuroinflammation is the most prominent (Leigh and Morris 2020). Related molecular features include deficits in synaptic plasticity, reduced hippocampal neurogenesis, epigenetic mechanisms, and alterations in the expression of memory-associated genes (Fischer et al. 2007; Heyward et al. 2016).
In the present study, we show that 4 weeks of Zn supplementation could improve the recognition memory of HFD-fed rats. It has been shown that Zn is fundamental for maintaining body homeostasis (Plum et al. 2010; Portbury and Adlard 2017). In obesity, there is an imbalance of Zn and a decrease in the expression of genes that regulate intracellular homeostasis of this mineral in the brain (Costarelli et al. 2010; Olesen et al. 2016). Cope et al. (2011) had demonstrated that 4 weeks of Zn supplementation improved spatial memory and learning after traumatic brain injury in rats (Cope et al. 2011). Moreover, one of our previous studies also demonstrated the cognitive benefits of Zn supplementation on Cafeteria Diet-fed rats (de Oliveira et al. 2021). The mechanism by which Zn affects memory is still poorly understood. However, intracellular Zn2+ signaling in the hippocampus is required for cognition and related to object recognition test performance, possibly acting through the long-term potentiation modulation (Tamano et al. 2015; Tamano et al. 2016). Therefore, Zn supplementation could be a promising strategy to ameliorate cognitive disorders found in obesity.
On the other hand, BCAA supplementation could not to improve recognition memory after HFD. In a model of Alzheimer's disease, Tournissac et al. (2018), also found no benefits on the object recognition test performance after supplementation with BCAA (Tournissac et al. 2018). BCAA reaches the CNS through an amino acid transporter in the BBB, which is also responsible for transporting other amino acids such as tryptophan (a precursor of serotonin) and threonine. Thus, the supplementation of BCAA can reduce the brain uptake of those amino acids (Coppola et al. 2013). A high level of threonine in the brain is related to better results in the object recognition test, and serotonin also affects behavior (Coppola et al. 2013; Scaini et al. 2014; Tournissac et al. 2018). Hence, this may be the reason why we did not find an improvement of the object recognition index after 4 weeks of BCAA supplementation.
Metabolic inflammation caused by obesity increases the peripheral and central levels of pro-inflammatory cytokines such as IL-6 and IL-1β (Ellulu et al. 2017; Rhea et al. 2017). Here we found higher levels of IL-1β immunolabeling in both the cerebral cortex and hippocampus and increased levels of IL-6 in the cerebral cortex following HFD. Hippocampus is highly susceptible to obesogenic diets. It is described that markers of inflammation are augmented in the hippocampus earlier than in the hypothalamus or perirhinal cortex (Beilharz et al. 2016). These signs of inflammation corroborates with the recognition memory impairment following HFD and are in line with the neuroinflammatory hypothesis of obesity-driven cognitive deficit (Guillemot-Legris and Muccioli 2017).
Zn supplementation improved the performance in the recognition object test and was shown to reduce levels of IL-1β in both the cerebral cortex and hippocampus. Although BCAA also reduced hippocampal levels of IL-1β, it did not sufficiently improve memory of obese rats. Furthermore, we have previously demonstrated that Zn treatment reverted metabolic dysfunction caused by HFD (Thoen et al. 2018). However, here we show that it was not sufficient to prevent the increase in cortical levels of IL-6. We hypothesize that Zn supplementation may be providing its beneficial effect on memory through a mechanism involving the brain-derived neurotrophic factor (BDNF) and metalloproteinase-9, both important modulators for learning and memory since Zn can activate this pathway, enhancing synaptic plasticity and, thus, neuronal functioning (Frazzini et al. 2018). On the other hand, BCAA administration did not alter the memory outcome and the obesity-related increase of IL-1β and IL-6 in the cerebral cortex. High doses of BCAA can alter the inflammatory profile in the rats’ cerebral cortex and hippocampus and mixed glial cell cultures (De Simone et al. 2013; Rosa et al. 2016). However, those changes seem insufficient to revert the detrimental effects of obesity.
Astrocyte activation is another feature of neuroinflammation caused by obesity (Buckman et al. 2013). Despite the changes in glial cells being more prominent in the hypothalamus in obesity, HFD may affect differently distinct brain areas (Lizarbe et al. 2018). Previous studies have found an altered number of GFAP positive cells in the cerebral cortex after an HFD (Tomassoni et al. 2013; de Andrade et al. 2017), which corroborates with our findings. This feature may be related to reactive astrocytes, which are widely associated with neurodegenerative disorders (Sofroniew 2014). In the present study, we showed that HFD did not change the number of GFAP+ cells in the hippocampus, whereas it caused a decrease in the number of astrocytes in the cerebral cortex. Curiously, both BCAA and Zn supplementation decreased the number of astrocytes in the hippocampus without interfering in the cortical number of these cells. It is well known that obesity leads to cerebral atrophy both in humans and animal models, which can be related to a disruption in cerebral vascular function and inflammation (Gómez-Apo et al. 2021). Nevertheless, the underlying mechanisms are not entirely understood (Gunstad et al. 2008; Raji et al. 2010; Nguyen et al. 2014). It is reasonable to suppose the effects of HFD administrated in combination with a high fructose and sucrose solution are more harmful than fed animals exclusively with an HFD. Thus, a process of cerebral atrophy promoted for that diet could explain the reduction observed in the number of cortical astrocytes.
When using Sholl’s method to analyze astrocytes, we found that HFD not only reduced the number of GFAP cells in the cerebral cortex, but it also reduced astrocytic arborization. Regarding the hippocampus, in the CA1 region, HFD reduced astrocytic arborization and it was reversed with BCAA supplementation. Gzielo et al. (2017), also found that obesity affects the morphology of the astrocytes in the CA1 area (Gzielo et al. 2017). Therefore, our findings indicate that following HFD, BCAA supplementation could avoid the decline in astrocyte arborization. On the other hand, HFD increased the number of processes intersections in the dentate gyrus, indicating a rise in astrocytes arborization. In this case, only Zn supplementation was able to reverse this effect. Thus, both treatments had some effect on reversing the HFD-elicited morphological changes in the hippocampus. In the cerebral cortex, all morphological parameters analyzed decreased after HFD, and none of the supplementations affected it. These results agree with the reduced number of astrocytes also described here, and it provides additional support to the hypothesis of cortical atrophy. Recent studies suggest that obesity-related astrocyte reactivity is area-dependent (Tsai et al. 2018), and our findings corroborate with it.
The results reported here should be considered in light of some limitations. Our study does not indicate the long-term effects of the treatments on neuroinflammation and memory. Also, besides being a fundamental tool, animal model studies should be carefully considered when translated to humans. The Zn and BCAA dosage were previously investigated based on their beneficial effects on metabolic parameters and oxidative stress of HFD-fed obese rats (Thoen et al. 2018). In humans, although Zn has been found to improve glycemic control (Cruz et al. 2017; Wang et al. 2019), decrease body weight (Abdollahi et al. 2020) and even to improve depressive symptoms in overweight/obese subjects(Solati et al. 2015; Yosaee et al. 2020). Even that Zn is commonly used as a supplement, to our knowledge, no study has assessed its cognitive effects. It is not clear in the literature the precise dosage of Zn to ensure safe and efficient treatment for cognitive decline related to human obesity. Therefore, new clinical studies are necessary to evaluate the effects of the BCAA or Zinc supplementations by dose and period in obese subjects and its long-term effects.
In conclusion, our study provides additional evidence on obesity-driven neuroinflammation, which is known to increase the risk of neurological diseases. Therefore, the search for treatments capable of minimizing or reversing the impact of obesity in the CNS is paramount. Here we show that Zn supplementation was able to ameliorate recognition memory decline related to obesity in rats. Also, the ability of BCAA and Zn supplementation to affect IL-1β immunoreactivity and astrocyte morphology was demonstrated. Future studies are needed to clarify the mechanisms by which Zn promotes memory improvement in obesity. However, we can presume that Zn supplementation may be a potential target for human therapeutic intervention.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abdollahi S, Toupchian O, Jayedi A, Meyre D, Tam V, Soltani S (2020) Zinc supplementation and body weight: a systematic review and dose-response meta-analysis of randomized controlled trials. Adv Nutr 11(2):398–411
Beilharz JE, Maniam J, Morris MJ (2016) Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res 306:1–7
Bifari F, Nisoli E (2017) Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol 174(11):1366–1377
Buckman LB, Thompson MM, Moreno HN, Ellacott KL (2013) Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol 521(6):1322–1333
Clausen BH, Wirenfeldt M, Høgedal SS, Frich LH, Nielsen HH, Schrøder HD, Østergaard K, Finsen B, Kristensen BW, Lambertsen KL (2020) Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol Commun 8(1):81
Cope EC, Morris DR, Scrimgeour AG, VanLandingham JW, Levenson CW (2011) Zinc supplementation provides behavioral resiliency in a rat model of traumatic brain injury. Physiol Behav 104(5):942–947
Coppola A, Wenner BR, Ilkayeva O, Stevens RD, Maggioni M, Slotkin TA, Levin ED, Newgard CB (2013) Branched-chain amino acids alter neurobehavioral function in rats. Am J Physiol Endocrinol Metab 304(4):E405-413
Cordner ZA, Tamashiro KL (2015) Effects of high-fat diet exposure on learning & memory. Physiol Behav 152(Pt B):363–371
Costarelli L, Muti E, Malavolta M, Cipriano C, Giacconi R, Tesei S, Piacenza F, Pierpaoli S, Gasparini N, Faloia E, Tirabassi G, Boscaro M, Polito A, Mauro B, Maiani F, Raguzzini A, Marcellini F, Giuli C, Papa R, Emanuelli M, Lattanzio F, Mocchegiani E (2010) Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J Nutr Biochem 21(5):432–437
Cruz KJ, Morais JB, de Oliveira AR, Severo JS, Marreiro DD (2017) The effect of zinc supplementation on insulin resistance in obese subjects: a systematic review. Biol Trace Elem Res 176(2):239–243
Dall’Oglio A, Gehlen G, Achaval M, Rasia-Filho AA (2008) Dendritic branching features of Golgi-impregnated neurons from the “ventral” medial amygdala subnuclei of adult male and female rats. Neurosci Lett 439(3):287–292
de Andrade AM, Fernandes MDC, de Fraga LS, Porawski M, Giovenardi M, Guedes RP (2017) Omega-3 fatty acids revert high-fat diet-induced neuroinflammation but not recognition memory impairment in rats. Metab Brain Dis 32(6):1871–1881
de Moura AC, Lazzari VM, Becker RO, Gil MS, Ruthschilling CA, Agnes G, Almeida S, da Veiga AB, Lucion AB, Giovenardi M (2015) Gene expression in the CNS of lactating rats with different patterns of maternal behavior. Neurosci Res 99:8–15
de Oliveira S, Feijó GDS, Neto J, Jantsch J, Braga MF, Castro L, Giovenardi M, Porawski M, Guedes RP (2021) Zinc supplementation decreases obesity-related neuroinflammation and improves metabolic function and memory in rats. Obesity (Silver Spring) 29(1):116–124
De Simone R, Vissicchio F, Mingarelli C, De Nuccio C, Visentin S, Ajmone-Cat MA, Minghetti L (2013) Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim Biophys Acta 1832(5):650–659
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y (2017) Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13(4):851–863
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31(1):47–59
Faber C, Gabriel P, Ibs KH, Rink L (2004) Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transplant 33(12):1241–1246
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature 447(7141):178–182
Frazzini V, Granzotto A, Bomba M, Massetti N, Castelli V, d’Aurora M, Punzi M, Iorio M, Mosca A, Delli Pizzi S, Gatta V, Cimini A, Sensi SL (2018) The pharmacological perturbation of brain zinc impairs BDNF-related signaling and the cognitive performances of young mice. Sci Rep 8(1):9768
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Guillemot-Legris O, Muccioli GG (2017) Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci 40(4):237–253
Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E (2008) Relationship between body mass index and brain volume in healthy adults. Int J Neurosci 118(11):1582–1593
Gzielo K, Kielbinski M, Ploszaj J, Janeczko K, Gazdzinski SP, Setkowicz Z (2017) Long-term consumption of high-fat diet in rats: effects on microglial and astrocytic morphology and neuronal nitric oxide synthase expression. Cell Mol Neurobiol 37(5):783–789
Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, Silva-Pereyra J (2021) Structural brain changes associated with overweight and obesity. J Obes 2021:6613385
Heyward FD, Gilliam D, Coleman MA, Gavin CF, Wang J, Kaas G, Trieu R, Lewis J, Moulden J, Sweatt JD (2016) Obesity weighs down memory through a mechanism involving the neuroepigenetic dysregulation of sirt1. J Neurosci 36(4):1324–1335
Kelly B, Pearce EL (2020) Amino assets: how amino acids support immunity. Cell Metab 32(2):154–175
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42
Leigh SJ, Morris MJ (2020) Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis 1866(6):165767
Lizarbe B, Soares AF, Larsson S, Duarte JMN (2018) Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front Neurosci 12:985
Malik VS, Willett WC, Hu FB (2013) Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 9(1):13–27
Neal M, Richardson JR (2018) Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta Mol Basis Dis 1864(2):432–443
Nguyen JC, Killcross AS, Jenkins TA (2014) Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci 8:375
Olesen RH, Hyde TM, Kleinman JE, Smidt K, Rungby J, Larsen A (2016) Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain. Transl Psychiatry 6(6):e838
Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94(4):1077–1098
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7(4):1342–1365
Portbury SD, Adlard PA (2017) Zinc signal in brain diseases. Int J Mol Sci 18(12):2506
Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM (2010) Brain structure and obesity. Hum Brain Mapp 31(3):353–364
Rhea EM, Salameh TS, Logsdon AF, Hanson AJ, Erickson MA, Banks WA (2017) Blood-brain barriers in obesity. Aaps j 19(4):921–930
Rios-Lugo MJ, Madrigal-Arellano C, Gaytán-Hernández D, Hernández-Mendoza H, Romero-Guzmán ET (2020) Association of serum zinc levels in overweight and obesity. Biol Trace Elem Res 198(1):51–57
Rosa L, Scaini G, Furlanetto CB, Galant LS, Vuolo F, Dall’Igna DM, Schuck PF, Ferreira GC, Dal-Pizzol F, Streck EL (2016) Administration of branched-chain amino acids alters the balance between pro-inflammatory and anti-inflammatory cytokines. Int J Dev Neurosci 48:24–30
Samara A, Murphy T, Strain J, Rutlin J, Sun P, Neyman O, Sreevalsan N, Shimony JS, Ances BM, Song SK, Hershey T, Eisenstein SA (2019) Neuroinflammation and white matter alterations in obesity assessed by diffusion basis spectrum imaging. Front Hum Neurosci 13:464
Scaini G, Jeremias GC, Furlanetto CB, Dominguini D, Comim CM, Quevedo J, Schuck PF, Ferreira GC, Streck EL (2014) Behavioral responses in rats submitted to chronic administration of branched-chain amino acids. JIMD Rep 13:159–167
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Skalny AV, Aschner M, Tinkov AA (2021) Zinc. Adv Food Nutr Res 96:251–310
Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7(2):a020420
Solati Z, Jazayeri S, Tehrani-Doost M, Mahmoodianfard S, Gohari MR (2015) Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: a double-blind, randomized, placebo-controlled trial. Nutr Neurosci 18(4):162–168
Tamano H, Koike Y, Nakada H, Shakushi Y, Takeda A (2016) Significance of synaptic Zn(2+) signaling in zincergic and non-zincergic synapses in the hippocampus in cognition. J Trace Elem Med Biol 38:93–98
Tamano H, Minamino T, Fujii H, Takada S, Nakamura M, Ando M, Takeda A (2015) Blockade of intracellular Zn2+ signaling in the dentate gyrus erases recognition memory via impairment of maintained LTP. Hippocampus 25(8):952–962
Thoen RU, Barther NN, Schemitt E, Bona S, Fernandes S, Coral G, Marroni NP, Tovo C, Guedes RP, Porawski M (2019) Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl Physiol Nutr Metab 44(6):580–586
Tomassoni D, Nwankwo IE, Gabrielli MG, Bhatt S, Muhammad AB, Lokhandwala MF, Tayebati SK, Amenta F (2013) Astrogliosis in the brain of obese Zucker rat: a model of metabolic syndrome. Neurosci Lett 543:136–141
Tournissac M, Vandal M, Tremblay C, Bourassa P, Vancassel S, Emond V, Gangloff A, Calon F (2018) Dietary intake of branched-chain amino acids in a mouse model of Alzheimer’s disease: Effects on survival, behavior, and neuropathology. Alzheimers Dement (NY) 4:677–687
Tsai SF, Wu HT, Chen PC, Chen YW, Yu M, Wang TF, Wu SY, Tzeng SF, Kuo YM (2018) High-fat diet suppresses the astrocytic process arborization and downregulates the glial glutamate transporters in the hippocampus of mice. Brain Res 1700:66–77
Wang X, Wu W, Zheng W, Fang X, Chen L, Rink L, Min J, Wang F (2019) Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 110(1):76–90
WHO, W. H. O (2018) Obesity and Overweight
Yosaee S, Soltani S, Esteghamati A, Motevalian SA, Tehrani-Doost M, Clark CCT, Jazayeri S (2020) Effects of zinc, vitamin D, and their co-supplementation on mood, serum cortisol, and brain-derived neurotrophic factor in patients with obesity and mild to moderate depressive symptoms: A phase II, 12-wk, 2 × 2 factorial design, double-blind, randomized, placebo-controlled trial. Nutrition 71:110601
Zhang S, Zeng X, Ren M, Mao X, Qiao S (2017) Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8:10
Funding
This study was supported by the Brazilian funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS); G.S. Feijó and J. Jantsch were recipients of FAPERGS and CNPq fellowships, respectivelly. L.L. Correia and S.Eller were recipients of UFCSPA fellowships.
Author information
Authors and Affiliations
Contributions
Renata Padilha Guedes and Marilene Porawski conceived the presented idea. Grace dos Santos Feijó, Jeferson Jantsch, Lidia Luz Correia, Sarah Eller, and Orlando Vieira Furtado Filho carried out the experiments. Grace dos Santos Feijó, Renata Padilha Guedes, Elizandra Braganhol and Márcia Giovenardi contributed to the interpretation of the results. Grace dos Santos Feijó and Jeferson Jantsch wrote the manuscript in consultation with Renata Padilha Guedes and Elizandra Braganhol. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were approved by the Institutional Animal Care and Use Committee (UFCSPA, Brazil, protocol No. 359/16).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feijó, G.d., Jantsch, J., Correia, L.L. et al. Neuroinflammatory responses following zinc or branched-chain amino acids supplementation in obese rats. Metab Brain Dis 37, 1875–1886 (2022). https://doi.org/10.1007/s11011-022-00996-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00996-5