Abstract
Gestational diabetes (GD) is the glucose intolerance that occurs during pregnancy. Mothers who develop diabetes during gestation are at increased risk of developing type 2 diabetes mellitus (T2DM) later in life, and the risk of adverse fetal and neonatal outcomes are also increased as a function of maternal hyperglycemia. Infants who are exposed to fetal hyperglycemia show an increased risk of becoming obese and developing T2DM later in life. Due to the need of new research on this field, and the difficulty of performing studies in human brain, studies using experimental models are necessary to suggest possible ways to avoid or inhibit offspring brain damage or harmful metabolic alterations. Here, it was made a review about the characteristics of the main animal models of GD, and what are the consequences to the brain and behavior of the offspring. In many experimental models, either by pharmacological induction, diet manipulation, or in the use of transgenic animals, glycemic conditions are severe. S961, a selective insulin receptor antagonist, revealed an increased fasting blood glucose level and glucose intolerance during mid-gestation, which returned to basal levels postpartum in mice. GD contributes to offspring neuroinflammation, influences neuronal distribution in central nervous system (CNS), and apoptosis during embryogenesis, which in turn may contribute to changes in behavior and memory in adult life and aging. The usage of animal models to study GD allows to examine extensively the characteristics of this condition, the molecular mechanisms involved and the consequences to the brain and behavior of the offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes (GD) is defined by the American Diabetes Association (ADA) as a form of glucose intolerance with onset or first recognition during pregnancy (ADA 2014). GD is the most important metabolic condition during pregnancy, and occurs in more than 15% of all pregnancies in the United States of America (Desisto et al. 2014). For many years, GD was considered to be a transient condition, ruling out investigation of whether it was related to long-lasting consequences to the mother or the fetus (Agha-Jaffar et al. 2016). It is known that mothers who develop diabetes during gestation are at increased risk of developing type 2 diabetes mellitus (T2DM) later in life, and the risk of adverse fetal and neonatal outcomes are also increased as a function of maternal hyperglycemia (Sharpe et al. 2005).
The immediate consequences of GD to infants include neonatal hypoglycemia, hypocalcemia, respiratory distress syndrome at birth, but the most common morbidity is macrosomia occurring in 30% of infants (Frías et al. 2007). Moreover, infants who are exposed to fetal hyperglycemia show an increased risk of becoming obese and developing T2DM later in life (ADA 2014). GD is related to several detrimental effects to the offspring, including an increased risk of congenital malformations and inflammation that affect several organs and tissues (Mills et al. 1979), including the central nervous system (CNS) (Golalipour et al. 2012).
Due to the need of new research on this field, and the difficulty of performing studies in brains of children and adult subjects, studies using experimental models are necessary to indicate possible ways to avoid or inhibit offspring brain damage or harmful metabolic alterations (De Sousa et al. 2020f). The aim of this study was to review some of the characteristics of the main animal models of GD and what are the consequences to the brain and behavior of the offspring.
Characteristics of the main animal models of GD
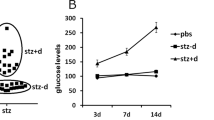
Since the first experimental models of GD were developed (Carlson and Drennan 1911; Markowitz and Soskin 1927; Cuthbert et al. 1936) many alternative models have been created (Yang et al. 2016; Vuong et al. 2017) with the purpose of studying this condition deeply. Most of them use rodents (Yao et al. 2015), but there are also models of GD induction developed in dogs (Fall et al. 2010), pigs (Kobayashi et al. 2004), sheep (Bergman et al. 1971) and primates (McCurdy et al. 2009). Among the main genetic models of GD, there is a classic model to study diabetes and obesity called db/+ female mice, which had the leptin receptors deleted characterized by the inability to suppress the feeding behavior (Chen et al. 1996). Diet manipulation making usage of high-fat diet (HFD) is also a common way to produce diabetes or GD (McCurdy et al. 2009). In addition, there are a variety of chemical compounds used to produce diabetes either through β-cell death or failure such as streptozotocin (STZ), which after entering the beta (β) cells of the pancreas carried by a specific glucose transporter, GLUT2, takes them to failure by the damage caused to deoxyribonucleic acid (DNA) leading to cellular necrosis and apoptosis by alkylation (Murata et al. 1999). The usage of many other chemicals to induce GD is very common, such as aloxan, which is also taken to β cells by GLUT2 and favors apoptosis by promoting oxidative stress (Szkudelski 2001), and methylation (Yang et al. 2016) to produce GD, but diabetes and obesity are persistent characteristics to the dams in these models.
Intra-uterine glycemic conditions in animal models of GD
Animal models of GD currently available involve severe and irreversible hyperglycemia and hyperinsulinemia, making them distant from the effects of GD in humans (Markowitz and Soskin 1927; Bergman et al. 1971; Szkudelski 2001; McCurdy et al. 2009; Yang et al. 2016). In many GD experimental models, either by pharmacological induction (Chandna et al. 2015), diet manipulation (Vuong et al. 2017), or transgenic animals (Yang et al. 2016), glycemic conditions are severe. However, there is a substance that has been used in rodents to induce T2DM that is S961 (Schäffer et al. 2008), and recently our group developed the first GD model using S961 in Swiss mice.
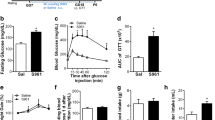
S961 is a single chain biosynthetic peptide of 43 amino acids (Vikram and Jena 2010) that acts as a selective and high affinity insulin receptor antagonist, and its activity has been shown both in vitro and in vivo (Schäffer et al. 2008; Vikram and Jena 2010). S961 induces hyperinsulinemia, insulin-resistance and depletion of energy stores in rats (Vikram and Jena 2010), and mice (De Sousa et al. 2019). Nevertheless, the effects of S961 usage to induce GD in experimental models to evaluate the offspring behavior and cognition has not been entirely documented yet. There is a recent study from our research group that was able to develop a new model of GD using S961 and revealed an increased fasting blood glucose level and glucose intolerance during mid-gestation, which returned to basal levels postpartum (De Sousa et al. 2019). The authors also showed, in this same study, that the dams did not develop obesity during the gestational period. These results happened because S961 is not acting directly in β pancreatic cells, and for this reason is not causing severe hyperglycemia that remains postpartum (Fig. 1.).
It is important to emphasize that S961 has a dose dependent effect (Yi et al. 2013). S961 exhibited partial agonistic effects at low doses, in the 1–10 nM range, where it could be seen many different effects, such as S961 significantly increased cell proliferation L6-hIR cells, which are used in assays to stimulate insulin receptors and AKT phosphorylation, and in MCF-7 cells, a breast cancer cell line (Knudsen et al. 2012). It was also reported that at 2.5 nmol/week or 5 nmol/week no disruption of insulin signalling that leads to insulin resistance can be seen in vitro and in vivo, while concentrations ranging between 10 nmol/week and 20 nmol/week led to insulin resistance, glucose intolerance, and hyperglycemia in both conditions (Yi et al. 2013). At higher concentrations S661 totally inhibits the action of the hormone insulin, both in cellular assays and in vivo experiments in rats (Schäffer et al. 2008), and mice (Yi et al. 2013; De Sousa et al. 2019). In conclusion, S961 presents high affinity and selectivity for the insulin receptor and can be used in concentrations over 10 Nm for at least 1 week to trigger insulin resistance and hyperglycemia in both in vivo and in vitro experiments (Schäffer et al. 2008).The first study to use S961 to develop a new model of GD was published in 2019 by our group, where we used an intraperitoneal injection at the concentration of 17 nmol/week during 2 weeks (De Sousa et al. 2019). The authors successfully developed a new GD model mimicking many different features of GD. However, new studies testing different doses and approaches to the usage of S961 in animal models of GD are necessary.
Despite all this, during implantation, organogenesis and fetal development, the induction of GD can cause changes in the neonate that affects the adult life of the offspring (Fu et al. 2006; Liao et al. 2004). These microenvironmental changes during GD within the placenta contribute to fetus developmental abnormalities (Chandna et al. 2015). A recent study using different types of animal models of GD showed that a Glo1-methylglyoxal pathway is always perturbed in this condition contributing to the dysregulation of neural precursors in the developing murine cortex, which lead to long-lasting alterations in adult neurons of the offspring (Yang et al. 2016). Higher hyperglycemia was kept in the animals who were born from a GD pregnancy and changes in behavior were also related to the offspring with higher glycemic levels.
The importance of the evaluation of maternal behavior in animal models of GD
The environment in which the neonate lives early in life is determined by the mother who is responsible for maintaining the survival of the fetus. The first source of comfort, cleanliness and nourishment is the mother who determines the development of physiological systems that will modulate the neonate’s behavior, being determinant for the development of the brain architecture after birth. Modifications in the mother-infant relationship different behaviors and responses to stress can affect offspring (Huot et al. 2004). Thus, the interaction between mother and offspring is essential for the behavioral development and somatic growth (Reis et al. 2014; Moussaoui et al. 2016).
Changes in maternal behavior have been associated to affect the hypothalamic pituitary adrenal (HPA) axis in both sexes, male and female pups, and intestinal barrier function in male offspring (Moussaoui et al. 2016). Interestingly, positive outcomes in the offspring born from dams who exercised before and during pregnancy resulted in the promotion of physical activity in adult offspring (Eclarinal et al. 2016).
The impact of changes of maternal behavior on pups affects brain development and may contribute to disease in adulthood or during aging (Reis et al. 2014). Animals born from diabetic mothers are more prone to develop obesity and T2DM (Silverman et al. 1995; Buchanan et al. 2012). A life based on a sedentary lifestyle or exposure to HFD can lead to the development of obesity (Sousa et al. 2019), and glucose intolerance (De Sousa et al. 2020a, c). However, offspring born from diabetic mothers who are exposed HFD can develop obesity earlier (El Hajj et al. 2014; De Sousa et al. 2019).
The consequences of GD to the brain and behavior of the offspring
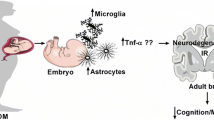
GD contributes to offspring neuroinflammation and influences neuronal distribution in CNS (Yang et al. 2016; De Sousa et al. 2017), and apoptosis during embryogenesis(Liu et al. 2015), which in turn may contribute to changes in behavior (Chandna et al. 2015) and memory (Yang et al. 2016; de Sousa 2018a) in adult life and during aging. Learning and memory deficits have been associated to inflammation and changes in insulin signaling in the brain which have been well documented in neurodegenerative disorders and different conditions, such as Alzheimer’s disease (Improta-Caria et al. 2020), and sepsis (Neves et al. 2016). Evidences suggest that inhibition or mal functioning of the phosphatidylinositol 3 kinase (PI3K) pathway in the CNS can have a negative effect on memory and cognition (de Sousa 2018b; De Sousa et al. 2020d), and can be caused due to a greater activation of glycogen synthase kinase 3 (GSK3) (Kaluski et al. 2017; De Sousa et al. 2020b).There are two isoforms of GSK3, alpha (GSK3α) and beta (GSK3β), with the latter being involved in energy metabolism and neuronal apoptosis (Plyte et al. 1992). However, the changes in memory and cognition in the offspring of GD, and the molecular mechanisms are poorly understood with a few studies in literature addressing the theme [15,17,18].
A study published by Vuong et al. (2017) provided evidence that maternal obesity associated to GD in rats influences neuroinflammation and microglial activation in the newborn offspring (Vuong et al. 2017). GD was induced in female Sprague-Dawley rats using a high-fat and sucrose diet for 6 weeks prior mating, throughout gestation and lactation, while lean control dams were fed a low-fat diet. Offspring presented impaired recognition memory in the object recognition test, associated to increased levels of pro-inflammatory cytokines like interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α), and also showed a reduced synaptophysin expression in different hippocampus areas, such as CA1 and dentate gyrus, which is related to neurogenesis. Finally, the microglia morphological transformation observed in the GD offspring persisted into young adulthood.
Another recent study investigated the development of inflammation in the CNS of the offspring through changes in the expression of genes responsible for regulating apoptosis in the hippocampus of neonate Wistar rats born to dams who were induced to diabetes with a single intraperitoneal injection of STZ (Chandna et al. 2015). The study evaluated male offspring at P0, P7, and P14 and revealed that maternal hyperglycemia may cause disturbances in the expression of Bcl-2 and Bax genes, two extremely important genes in apoptosis regulation. The authors suggest that these disturbances may be related to the anomalies in cognition and behavior observed in offspring born to diabetic mothers. Basically, hyperglycemia during pregnancy would contribute to a maternal pro-inflammatory state that can influence the brain development in the offspring affecting cognition as a result of disturbances in apoptosis regulation (Fig. 2.).
The molecular mechanisms that involve beneficial or negative adaptations in the brain mediated by glucose-related genes are partially understood. It has been showed that cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), cAMP-element binding protein (CREB), and brain-derived neuro factor (BDNF) are some of the genes involved with neuroprotection and memory preservation in mice (Wrann et al. 2013; Lourenco et al. 2019), while excessive expression of glial fibrillary acid protein (GFAP) and ionized calcium-binding adapter molecule 1 (IBA-1) are involved with neuroinflammation, cognitive decline, memory loss and changes in behavior (De Sousa et al. 2020e). Future studies are necessary in order to better understand the changes nn these genes and their relation to changes in cellular and morphological mechanisms in the brain of the dams and offspring in GD, and others conditions and pathologies.
Conclusions
The usage of animal models to study GD allows to examine extensively the characteristics of this condition, the molecular mechanisms involved, and the consequences to the brain and behavior of the offspring. A full understanding of the causes and consequences of GD in humans remains controversial, and the development of GD animal models that facilitate translation from basic research to clinical practice would be extremely useful, and may even impact public health policies.
References
Agha-Jaffar R, Oliver N, Johnston D, Robinson S (2016) Gestational diabetes mellitus: does an effective prevention strategy exist? Nat Rev Endocrinol 12:533–546. https://doi.org/10.1038/nrendo.2016.88
American Diabetes Association (ADA) (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. https://doi.org/10.2337/dc14-S081
Bergman EN, Havel RJ, Wolfe BM, Bohmer T (1971) Quantitative studies of the metabolism of chylomicron triglycerides and cholesterol by liver and extrahepatic tissues of sheep and dogs. J Clin Invest 50:1831–1839. https://doi.org/10.1172/JCI106674
Buchanan TA, Xiang AH, Page KA (2012) Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 8:639–649. https://doi.org/10.1038/nrendo.2012.96
Carlson A, Drennan F (1911) The control of pancreatic diabetes in pregnancy by the passage of the internal secretion of the pancreas of the fetus to the blood of the mother. Am J Physiol 28:391–395. https://doi.org/10.1017/S0029665113001286
Chandna AR, Kuhlmann N, Bryce CA et al (2015) Chronic maternal hyperglycemia induced during mid-pregnancy in rats increases RAGE expression, augments hippocampal excitability, and alters behavior of the offspring. Neuroscience 303:241–260. https://doi.org/10.1016/j.neuroscience.2015.06.063
Chen H, Charlat O, Tartaglia LA et al (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495. https://doi.org/10.1016/S0092-8674(00)81294-5
Cuthbert FP, Ivy AC, Isaacs BL, Gray J (1936) The relation of pregnancy and lactation to extirpation diabetes in the dog. Am Physiol Soc 115:480–496
De Sousa RAL, Torres YS, Figueiredo CPCP et al (2017) Consequences of gestational diabetes to the brain and behavior of the offspring. An Acad Bras Cienc 90:2279–2291. https://doi.org/10.1590/0001-3765201720170264
de Sousa RAL (2018a) Gestational diabetes is associated to the development of brain insulin resistance in the offspring. Int J Diabetes Dev Ctries 39:408–416. https://doi.org/10.1007/s13410-018-0618-1
de Sousa RAL (2018b) Brief report of the effects of the aerobic , resistance , and high-intensity interval training in type 2 diabetes mellitus individuals Diabetes mellitus. Int J Diabetes Dev Ctries 38:138–145. https://doi.org/10.1007/s13410-017-0582-1
De Sousa RAL, de Lima EV, da Silva TP et al (2019) Late cognitive consequences of gestational diabetes to the offspring, in a new mouse model. Mol Neurobiol 56:1–11. https://doi.org/10.1007/s12035-019-1624-0
De Sousa RAL, Azevedo LM, Improta-Caria A et al (2020a) Type 2 diabetes individuals improve C-reactive protein levels after high-intensity weight lift training. Sci Sport:1–7. https://doi.org/10.1016/j.scispo.2020.05.008
De Sousa RAL, Caria ACI, De Jesus Silva FM et al (2020b) High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol Behav 223:1–7. https://doi.org/10.1016/j.physbeh.2020.112998
De Sousa RAL, Hagenbeck KF, Arsa G, Pardono E (2020c) Moderate / high resistance exercise is better to reduce blood glucose and blood pressure in middle-aged diabetic subjects. Rev Bras Educ Física e Esporte 34:165–175
De Sousa RAL, Harmer AR, Freitas DA et al (2020d) An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05693-z
De Sousa RAL, Peixoto MFD, Leite HR et al (2020e) Neurological consequences of exercise during prenatal Zika virus exposure to mice pups Ricardo. Int J Neurosci. https://doi.org/10.1080/00207454.2020.1860970
De Sousa RAL, Rodrigues CM, Mendes BF et al (2020f) Physical exercise protocols in animal models of Alzheimer ’ s disease : a systematic review. Metab Brain Dis:1–11. https://doi.org/10.1007/s11011-020-00633-z
Desisto CL, Kim SY, Sharma AJ (2014) Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System ( PRAMS ), 2007–2010. Prev Chronic Dis 11:1–9
Eclarinal JD, Zhu S, Baker MS et al (2016) Maternal exercise during pregnancy promotes physical activity in adult offspring. FASEB J 30:2541–2548. https://doi.org/10.1096/fj.201500018R
El Hajj N, Schneider E, Lehnen H, Haaf T (2014) Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 148:R111–R120. https://doi.org/10.1530/REP-14-0334
Fall T, Hedhammar A, Wallberg A et al (2010) Diabetes mellitus in elkhounds is associated with Diestrus and pregnancy. J Vet Int Med 65:1322–1328
Frías JL, Frías JP, Frías PA, Frías MLM (2007) Infrequently studied congenital anomalies as clues to the diagnosis of maternal diabetes mellitus. Am J Med Genet Part A 2909:2904–2909. https://doi.org/10.1002/ajmg.a
Fu J, Tay SSW, Ling EA, Dheen ST (2006) High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia 49:1027–1038. https://doi.org/10.1007/s00125-006-0153-3
Golalipour MJ, Kafshgiri SK, Ghafari S (2012) Gestational diabetes induced neuronal loss in CA1 and CA3 subfields of rat hippocampus in early postnatal life. Folia Morphol (Warsz) 71:71–77
Huot R, Gonzalez M, Ladd C et al (2004) Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology 29:279–289. https://doi.org/10.1016/S0306-4530(03)00028-3
Improta-Caria AC, Nonaka CKV, Cavalcante BRR et al (2020) Modulation of microRNAs as a potential molecular mechanism involved in the beneficial actions of physical exercise in Alzheimer disease. Int J Mol Sci 21:1–35. https://doi.org/10.3390/ijms21144977
Kaluski S, Portillo M, Besnard A et al (2017) Neuroprotective functions for the histone report neuroprotective functions for the histone deacetylase SIRT6. CellReports 18:3052–3062. https://doi.org/10.1016/j.celrep.2017.03.008
Knudsen L, Hansen BF, Jensen P et al (2012) Agonism and antagonism at the insulin receptor. PLoS One 7. https://doi.org/10.1371/journal.pone.0051972
Kobayashi K, Kobayashi N, Okitsu T et al (2004) Development of a porcine model of type 1 diabetes by total pancreatectomy and establishment of a glucose tolerance evaluation method. Artif Organs 28:1035–1042. https://doi.org/10.1111/j.1525-1594.2004.00002.x
Liao DM, Ng YK, Tay SSW et al (2004) Altered gene expression with abnormal patterning of the telencephalon in embryos of diabetic Albino Swiss mice. Diabetologia 47:523–531. https://doi.org/10.1007/s00125-004-1351-5
Liu S, Guo Y, Yuan Q et al (2015) Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J Pineal Res:508–517. https://doi.org/10.1111/jpi.12282
Lourenco MV, Frozza RL, de Freitas GB et al (2019) Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med:165–175. https://doi.org/10.1038/s41591-018-0275-4
Markowitz J, Soskin S (1927) Pancreatic diabetes and pregnancy. Am J Phys 11:553–558
McCurdy CE, Bishop JM, Williams SM et al (2009) Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335. https://doi.org/10.1172/JCI32661
Mills JL, Baker L, Goldman AS (1979) Malformations in infants of diabetic mothers occur before the seventh gestational week implications for treatment. Diabetes 28:292–293
Moussaoui N, Larauche M, Biraud M et al (2016) Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS One:1–14. https://doi.org/10.1371/journal.pone.0155037
Murata M, Takahashi A, Saito I, Kawanishi S (1999) Site-specific DNA methylation and apoptosis: induction by diabetogenic streptozotocin. Biochem Pharmacol 57:881–887. https://doi.org/10.1016/S0006-2952(98)00370-0
Neves FS, Marques PT, Aragão FB et al (2016) Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol Neurobiol:1–10. https://doi.org/10.1007/s12035-016-0307-3
Plyte SE, Hughes K, Nikolakaki E et al (1992) Glycogen synthase kinase-3 : functions in oncogenesis and development. Biochim Biophys Acta 1114:147–162
Reis AR, de Azevedo MS, de Souza MA et al (2014) Neonatal handling alters the structure of maternal behavior and affects mother-pup bonding. Behav Brain Res 265:216–228. https://doi.org/10.1016/j.bbr.2014.02.036
Schäffer L, Brand CL, Hansen BF et al (2008) A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 376:380–383. https://doi.org/10.1016/j.bbrc.2008.08.151
Sharpe PB, Chan A, Haan EA, Hiller JE (2005) Maternal diabetes and congenital anomalies in South Australia 1986–2000: a population-based cohort study. Birth Defects Res A Clin Mol Teratol 73:605–611. https://doi.org/10.1002/bdra.20172
Silverman BL, Metzger BE, Cho NH, Loeb CA (1995) Impaired glucose tolerance in adolescent offspring of diabetic mothers. Diabetes Care 18:611–617
Sousa RAL, Freitas DA, Leite HR (2019) Cross-talk between obesity and central nervous system: role in cognitive function. Interv Obes Diabetes 3:7–9. https://doi.org/10.31031/IOD.2019.03.000551
Szkudelski T (2001) The mechanism of Alloxan and Streptozotocin action in B cells of the rat pancreas. Physiol Res 50:536–546
Vikram A, Jena G (2010) S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem Biophys Res Commun 398:260–265. https://doi.org/10.1016/j.bbrc.2010.06.070
Vuong B, Odero G, Rozbacher S et al (2017) Exposure to gestational diabetes mellitus induces neuroinflammation , derangement of hippocampal neurons , and cognitive changes in rat offspring. J Neuroinflammation:1–13. https://doi.org/10.1186/s12974-017-0859-9
Wrann CD, White JP, Salogiannnis J et al (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Yang G, Cancino GI, Zahr SK et al (2016) A glo1-methylglyoxal pathway that is perturbed in maternal diabetes regulates embryonic and adult neural stem cell pools in murine offspring. Cell Rep 17:1022–1036. https://doi.org/10.1016/j.celrep.2016.09.067
Yao L, Wan J, Li H et al (2015) Resveratrol relieves gestational diabetes mellitus in mice through activating AMPK. Reprod Biol Endocrinol 13:118. https://doi.org/10.1186/s12958-015-0114-0
Yi P, Park JS, Melton DA (2013) Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 153:747–758. https://doi.org/10.1016/j.cell.2013.04.008
Funding
RALS received fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil - Finance Code 001.
Author information
Authors and Affiliations
Contributions
RALS wrote the manuscript; performed the literature research; analyzed and critically discussed the data. The author read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
The author declares no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Sousa, R.A.L. Animal models of gestational diabetes: characteristics and consequences to the brain and behavior of the offspring. Metab Brain Dis 36, 199–204 (2021). https://doi.org/10.1007/s11011-020-00661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00661-9