Abstract
Gestational diabetes mellitus (GD) is a form of insulin resistance triggered during gestation, which affects approximately 10% of pregnant women. Although previously considered a transient condition with few long-term consequences, growing evidence suggest that GD may be linked to permanent metabolic and neurologic changes in the offspring. Currently available GD models fail to recapitulate the full spectrum of this disease, thus providing limited information about the true burden of this condition. Here, we describe a new mouse model of GD, based on the administration of an insulin receptor antagonist (S961, 30 nmol/kg s.c. daily) during pregnancy. Pregnant mice developed increased fasting glycemia and glucose intolerance in the absence of maternal obesity, with a return to normoglycemia shortly after parturition. Moreover, we showed that the adult offspring of GD dams presented pronounced metabolic and cognitive dysfunction when exposed to short-term high-fat diet (HFD). Our data demonstrate that S961 administration to pregnant mice comprises a valuable approach to study the complex pathophysiology of GD, as well as strategies focused on prevention and treatment of both the mother and the offspring. Our findings suggest that the offspring of GD mothers are more susceptible to metabolic and cognitive impairments when exposed to high-fat diet later in life, thus indicating that approaches to prevent and treat these late effects should be pursued.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive research has been conducted to unravel the bases of type 1 and type 2 diabetes, but few studies have assessed the physiopathological mechanisms and long-term consequences on gestational diabetes (GD) [1]. Risk factors for GD, a form of insulin resistance triggered during pregnancy in previously normoglycemic women, include family history of overweight and obesity, nonwhite race, and advanced maternal age [2, 3]. The prevalence of GD is increasing over the years and it is currently estimated to affect up to 10% of all pregnancies in the USA [4]. The consequences of GD to infants include macrosomia, neonatal hypoglycemia, hypocalcemia, and respiratory distress syndrome [5]. For many years, the transient nature of GD has led researchers and physicians to underestimate its long-term consequences. However, evidences for late metabolic and behavioral consequences are emerging, and late effects of intra-uterine exposure to high levels of glucose and insulin as well as a pro-inflammatory profile in this critical period of development are only beginning to be understood.
Infants born from diabetic mothers show increased risk of becoming obese and developing type 2 diabetes later in life [6]. More recent and concerning evidences, however, point to detrimental neurological effects of GD in their offspring. An increased risk of developing schizophrenia in children from GD mothers has been suggested [7], and other neuropsychiatric disorders might also be associated [8]. Several putative mechanisms, common to other cognitive dysfunctions, including iron deficiency, increased oxidative stress, and lipid peroxidation as well as inflammation and maternal immune activation, have been proposed [7, 9, 10]. Previous studies described that transient intra-uterine exposure to these stressors could program fetal brain and peripheral cells to respond differently when exposed to a second hit later in life, increasing the susceptibility to develop metabolic and behavioral alterations [7, 11, 12].
Animal models of GD are mandatory to studying its long-term consequences and molecular mechanisms. However, the current models of GD have several limitations [13]. Strategies described include surgical, pharmacological, nutritional, and genetic approaches. Pancreatectomy removes both endocrine and exocrine tissues, and results in changes in not necessarily related to diabetes, such as inflammation [14]. Diabetogenic drugs like streptozotocin induce widespread destruction of β-cells and have been demonstrated to have toxic effects on the embryos [15, 16]. Both surgical and chemical inductions result in a permanent state of diabetes and severe elevation of blood glucose whereas GD is characterized by mild glucose intolerance. Diet interventions like high-fat diet reproduce GD phenotype but induce increased adiposity [17], a condition associated but not necessary for GD development, as many women manifest the disease despite being lean [18]. Genetic models including mutants for the leptin receptor [19, 20], mice deficient for prolactin or insulin receptor [21, 22], or mice deficient for the hepatocyte nuclear factor 4α (HNF-4α) [23] provide important insight into pathways that may influence disease development. However, these single-gene models are in contrast to the disease in humans, which present a phenotype resulting from complex interactions between environmental and polygenic factors. Although these models have been proven useful for the assessment of the consequences of hyperglycemia on mother and offspring, they fail to truly mimic human GD, as most of them fail to recapitulate key aspects of this condition, such as maternal peripheral tissue insulin resistance and the return to euglycemia soon after labor [13, 24]. The development of animal models that more closely recapitulate its key aspects will undoubtedly help to identify the long-lasting consequences of GD to brain and behavior of the offspring and guide the development of prevention strategies.
In this study, we developed a new mouse model of GD to gain further insight into how hyperglycemia and glucose intolerance during pregnancy affect metabolism and cognition in the offspring. To this end, we used the insulin receptor antagonist S961, which has been shown to induce features of type 2 diabetes in rodents including hyperglycemia, hyperinsulinemia, and glucose intolerance [25,26,27]. When administered daily during pregnancy, starting on gestational day 7, S961 increased fasting glycemia and glucose intolerance in pregnant dams, in the absence of obesity or changes in chow intake. Moreover, animals recovered normoglycemia shortly after parturition. This model resembles key aspects of GD that have not been previously recapitulated in other models. In addition, we found that both male and female offspring from diabetic dams are more susceptible to the deleterious metabolic effects of short-term high-fat diet (HFD), which also triggered cognitive impairment. In conclusion, we showed that the administration of S961 to pregnant mice comprises an interesting alternative approach to study the complex pathophysiology of GD. Using this model, we showed that the offspring of GD mothers are more susceptible to late metabolic and cognitive effects induced by diet, thus indicating that approaches to prevent and treat these late effects should be pursued.
Methods
Animals
Swiss mice were obtained from our own breeding facilities at the Federal University of Rio de Janeiro (UFRJ), Brazil. All procedures performed in the present study were approved by the Ethics in Research Committee of the UFRJ (protocol 045/16), followed the Brazilian legislation, and were in accordance with the National Institutes of Health and ARRIVE guidelines. Animals from our own breeding facilities were housed 5 per cage with free access to food and water, under a 12-h light/dark cycle, with controlled room temperature (22 ± 2 °C). Twelve-week-old female and male Swiss mice were mated in a 2:1 ratio, respectively, for 48 h, during the female estrus phase. The estrus cycle was always identified between 3 and 5 pm. Successful mating was confirmed by the formation of a vaginal plug and daily measures of body weight on the following 7 days were used to confirm pregnancy. After mating, females were individually housed in cages until they gave birth. Body weight, chow, and water consumption were evaluated once a week during the gestational period and every 5 days after birth of the offspring.
Treatment
S961 (30 nmol/kg/day; a kind gift from Dr. Lauge Schäffer, Novo Nordisk) or an equal volume of vehicle (saline) was injected daily at the same time of the day (5:00 pm) subcutaneously from the seventh day of pregnancy until the birth of the offspring (Fig. 1a). The dose of S961 and the administration schedule were chosen based on previous work [25, 28] and preliminary pilot studies.
S961 induces transient glucose intolerance in pregnant mice. a Swiss mice were mated and pregnant females were treated daily with 30 nmol/kg of S961 or equal volume of saline subcutaneously (s.c.), from gestational day (GD) 7 until the day before labor. b–d Fasting glucose (b; n = 5 saline, 6 S961), glucose tolerance test curves (c; n = 5 saline, 6 S961), and corresponding area under the curve (AUC) (d; n = 5 saline, 6 S961) of data shown in C. e Body weight gain during pregnancy in S961- and saline-treated dams (n = 11 saline, 13 S961). f Fasting blood glucose of S961- and saline-treated mice, measured 1 day after parturition (n = 5 saline, 6 S961). Food (g; n = 6 saline, 7 S961) and water intake (h; n = 6 saline, 7 S961) measured during S961 or saline treatment. In b: *p = 0.0004; in d: *p = 0.0032; in h: *p = 0.009; Student’s t test. White bars, saline; black bars, S961
Fasting Glucose Determination and Glucose Tolerance Test
Glucose tolerance test (GTT) was performed on the seventh day of treatment with S961 or saline in all dams and also in their offspring when they reached 90 days of age. Pregnant mice were fasted for 5 h while non-pregnant female and male mice (offspring of GD dams or control) were fasted for 12 h before glucose measurements began. Blood samples were collected from a tail incision for baseline values (fasting blood glucose). Then, mice received an i.p. injection of glucose solution (2 g/kg) and blood glucose measurements were performed after 15, 30, 45, 60, 90, and 120 min using a One-Touch® Ultra® Glucose Meter and strips (Johnson & Johnson). For i.p. glucose administration, animals were gently restrained and injection was performed between inner thigh and genitals with an inclination of approximately 35°, in order to ensure that no organs were punctured. Mice showing no changes in blood glucose levels at any time point after glucose injection were excluded from the study. The number of dams with no changes in glucose levels did not exceed 5% of animals, which is in agreement with previous studies from our group [29].
High-Fat Diet Administration
All litters were normalized to eight animals per dam: 4 males and 4 females whenever possible. At weaning, animals were housed in groups of no more than five in each cage and were fed with regular chow until they were 60 days old. Adult offspring of S961- and saline-treated groups were subdivided to receive normal diet (ND) or high-fat diet (HFD) ad libitum from the 60th to the 90th day of life (saline + ND, S961 + ND, saline + HFD, S961 + HFD, respectively). The HFD (Prag Solutions—Jaú, São Paulo, Brazil) contained 45% of energy derived from fat, 36% from carbohydrates, and 19% from protein. Body weight was evaluated every 5 days during this period. GTT was performed at the end of the HFD administration, as described above. Maternal behavior, as well as physical and reflex development of the pups, was evaluated between post-natal days 2 and 8, as described below. Other behavioral sessions were carried out between post-natal days 90 and 100.
Evaluation of Physical Development
Pups from each dam were observed daily between post-natal days 2 and 8, to evaluate if intra-uterine exposure to S961 could cause a delay or hastening in the appearance of physical developmental hallmarks. Variables analyzed were as follows: beginning of fur growth, pinna detachment, incisor eruption, and eye opening [30, 31].
Evaluation of Reflex Development
Pups from four S961- and four saline-treated dams were evaluated in terms of reflex development and neuromuscular maturation on the negative geotaxis and posture straightening reflex tests, performed between post-natal days 2 and 8 [32]. Negative geotaxis is an orientation movement that reflects vestibular and/or proprioceptive function. For evaluation of this reflex, pups were individually placed on a 45° inclined surface facing downwards. Latency to turn 180° (facing up) was measured. For the evaluation of posture straightening reflex, pups were individually placed laying on their backs on a flat surface and the time to turn and stand on the four limbs was recorded.
Evaluation of Maternal Behavior
Maternal behavior from S961- and saline-treated dams (n = 5/group) were evaluated between post-natal days 2 and 8. The number of events licking or feeding pups were assessed in four 1-h-long observation sessions performed each day at the following hours of the day: 8:00 AM, 1:00 PM, 4:00 PM, and 7:00 PM [33, 34]. Data are expressed as average number of events per day.
Open Field Test
For the open field test, animals were individually placed in the center of an arena made of wood measuring 30 × 30 × 45 cm. All sessions were video recorded and the total distance traveled and time in the center of the arena were evaluated during a 5-min-long session using the ANY-maze software (Stoelting, Wood Dale, IL). The arena was thoroughly cleaned with 20% ethanol in between trials to eliminate olfactory cues [35].
Novel Object Recognition Test
The test was carried out in the same arena used for the open field test. The training consisted in a 5-min session during which animals were placed at the center of the arena in the presence of two identical objects. The amount of time spent exploring each object was recorded. Sniffing and touching the object were considered as exploratory behavior. The arena was thoroughly cleaned with 20% ethanol in between trials to eliminate olfactory cues. Two hours after the training, animals were again placed in the arena for the test session, in which one of the objects was replaced by a new one, and the amount of time spent exploring familiar and novel objects was measured. All sessions were video recorded and evaluated using the ANY-maze software, and the results were expressed as percentage of time exploring each object [35].
Statistical Analysis
All data are expressed as means ± standard error mean (SEM). Significant differences were assessed by one sample Student’s t test, or one-way ANOVA followed by Tukey’s post hoc, as indicated in figure legends, using the GraphPad Prism 6.0 Software. A value of p < 0.05 was considered statistically significant.
Results
Treatment of Pregnant Mice with S961 Recapitulates Several Aspects of Gestational Diabetes
In order to develop a rodent model that mimics different aspects of GD, pregnant Swiss mice were treated daily with s.c. injections of the insulin receptor antagonist, S961 (30 nmol/kg) from gestational day 7 until the day before parturition (Fig. 1a). Pregnant females treated with S961 showed increased fasting blood glucose (Fig. 1b) and became glucose-intolerant (Fig. 1c, d), reproducing the main features of gestational diabetes in humans. Most animal models designed to recapitulate insulin resistance are associated to obesity and ingestion of fat-enriched diets [13], but it is known that over 25% of all cases of GD occur in non-obese women [36]. We found that S961-treated females did not develop obesity, as their body weight gain was comparable with that seen saline-treated dams throughout the pregnancy (Fig. 1e). Moreover, as shown for over 70% of patients [37], and rarely resembled by animal models of the disease, blood glucose levels of S961-treated females returned to normality shortly after the birth of the offspring (Fig. 1f). In addition, no changes in daily food intake were observed (Fig. 1g), but a significant increase in water consumption during the period of drug administration was observed (Fig. 1h), in agreement with the high blood glucose levels seen in S961-treated dams.
S961 Does Not Affect Maternal Behavior or Neonatal Development of the Offspring
Extensive evidence has shown that decreased maternal care during the neonatal period has persistent effects on adult behavior [38, 39]. To investigate whether S961 administration during pregnancy in mice interfered with maternal care, the behavior of dams was evaluated between post-natal days 2 and 8, at different moments of the day (see Methods). No changes in licking (Fig. 2a) or feeding (Fig. 2b) behaviors were seen between S961- and Saline-treated dams, thus ruling out the possibility that impaired maternal behavioral during early neonatal period could account for changes in behavior of the offspring later in life.
S961 given to pregnant mice does not affect maternal behavior. Pregnant females were treated daily with 30 nmol/kg of S961 or equal volume of saline subcutaneously (s.c.), from gestational day 7 until the day before labor. After birth of pups, number of events licking pups (a) and number of events feeding pups (b) were evaluated between post-natal days (PND) 2 and 8, as a measure of maternal behavior. n = 5 saline- and 6 S961-treated dams
We further investigated whether S961 treatment interfered with normal physical and reflex development of pups. Physical development of pups exposed to S961 was comparable with the control group (Table 1). Moreover, pups from S961-treated dams showed normal performance in the posture straightening reflex and negative geotaxis tasks (Table 2), indicating that the administration of the insulin antagonist to the pregnant dams has no effect on normal physical or neurological development of the offspring.
Offspring from Diabetic Dams Show Increased Susceptibility to High-Fat Diet–Induced Metabolic and Cognitive Impairments
It is known that the offspring of diabetic mothers are at increased risk of developing metabolic disorders throughout life [40, 41]. In order to evaluate whether mice born from S961-treated dams were more susceptible to metabolic dysfunction, adult mice (post-natal day 60) were kept in normal diet (ND) or received high-fat diet (HFD) for 30 days (Fig. 3a). HFD induced increase in fasting glycemia both in male and female mice born from saline-treated dams, when compared with mice fed with ND (Fig. 3b, e). Of interest, the impact of HFD on blood glucose levels was markedly higher in female mice born from S961-treated dams compared with those born from Saline-treated dams (Fig. 3e). Male (Fig. 3c, d) and female (Fig. 3f, g) offspring of GD dams showed worse performance than the offspring born from saline-treated dams, in a GTT performed after 30 days of HFD administration.
Adult offspring of diabetic dams show exacerbated glucose intolerance when submitted to 30 days of high-fat diet. a The offspring from control or diabetic dams were weaned at post-natal day 21 (P21) and animals received normal diet (ND) or high-fat diet (HFD) for 30 days starting at post-natal 60. b, e Fasting blood glucose measured in male (b; n = 7 Sal + ND, 7 Sal + HFD, 8 S961 + ND, 6 S961 + HFD) and female (e; n = 5 Sal + ND, 8 Sal + HFD, 11 S961 + ND, 10 S961 + HFD) mice at P90. c, d, f, g Glucose tolerance test curves in male (c; n = 7 Sal + ND, 7 Sal + HFD, 8 S961 + ND, 6 S961 + HFD) and female (f; n = 5 Sal + ND, 8 Sal + HFD, 11 S961 + ND, 10 S961 + HFD) mice at P90, and corresponding area under the curve (AUC) (d, g). In b: *p = 0.0153; **p = 0.0002, one-way ANOVA followed by Tukey. In d: *p = 0.0086; **p = 0.0453; ***p = 0.0005, one-way ANOVA followed by Tukey. In e: *p < 0.0001; **p < 0.0001; ***p < 0.0001, one-way ANOVA followed by Tukey. In g: *p = 0.0273; **p = 0.0003; ***p = 0.0006; #p = 0.0532, one-way ANOVA followed by Tukey. White/black bars, Sal + ND; black bars, Sal + HFD; white/gray bars, S961 + ND; gray bars, S961 + HFD
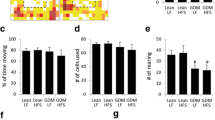
Maternal diabetes is a known risk factor for perinatal complications, but long-term consequences on offspring cognition remain poorly understood. Thus, we assessed the impact of intra-uterine hyperglycemia on cognitive function of adult offspring subjected to normal diet or HFD for 30 days (Fig. 4a). When evaluated in our open field paradigm, mice showed no differences in locomotion (Fig. 4b, c) or anxiety-like behavior (Fig. 4d, e) regardless of gender, diet or maternal S961 treatment. All experimental groups showed similar exploratory behavior during training session of NOR (Fig. 4e, f). HFD-treated mice born from normoglycemic dams (Sal + HFD) had no effect on cognitive function (Fig. 4g, h), when evaluated in the novel object recognition (NOR) task. Conversely, both male (Fig. 4g) and female (Fig. 4h) mice born from S961-treated dams and exposed to HFD were not capable of differentiating familiar from novel objects in the NOR test session, indicating that the offspring from GD dams is more susceptible to cognitive impairment in adulthood.
Adult mice born from diabetic dams show cognitive impairment when submitted to 30 days of high-fat diet. a Adult offspring born from control or diabetic dams received normal diet (ND) or high-fat diet (HFD) for 30 days starting at post-natal 60 (P60). At post-natal day 90 (P90), animals were submitted to the open field and novel object recognition (NOR) tasks. b–c Distance traveled by male (b) and female mice (c) in the open field task. d–e Time spent by male (d) and female (e) mice in the center of the open field arena at P90. White/black bars, Sal + ND; black bars, Sal + HFD; white/gray bars, S961 + ND; gray bars, S961 + HFD. f–g The percentage of time spent by male (f) and female (g) mice exploring the two identical objects used in NOR training session. h–i The percentage of time spent by male (h) and female (i) mice exploring familiar (Fam.) and novel objects in NOR test session. White bars, fam. object; black bars, novel object. n = 7 Sal + ND, 8 Sal + HFD, 11 S961 + ND, 10 S961 + HFD for experiments in male mice. n = 5 Sal + ND, 5 Sal + HFD, 6 S961 + ND, 6 S961 + HFD for experiments in female mice. In h: *p = 0.0294 for Sal + ND, *p = 0.0396 for Sal + HFD, *p = 0.0104 for S961 + ND, one sample Student’s t test. In i: *p = 0.0213 for Sal + ND, *p = 0.0147 for S961 + ND, one sample Student’s t test
Discussion
Gestational diabetes (GD) is a transient and multifactorial condition, making it challenging to experimentally recapitulate. Currently available GD animal models rely on surgical, chemical, nutritional, or genetic approaches. Surgical and chemical models consist on the removal or drug-induced permanent damage to the pancreas, causing a drastic and irreversible reduction in insulin secretion [13, 24]. These models are useful tools for studying the effects of severe hyperglycemia in the offspring, but fail in recapitulating key aspects of GD, such as the transient nature of the disease. Moreover, it is known that although 3–5% of GD patients remain diabetic, most women regain euglycemia shortly after parturition [42], highlighting that chemical and surgical models are not the best models to evaluate the late consequences of GD since hyperglycemia remains throughout lactation. Here, we describe a mouse model associated to moderately increased fasting blood glucose levels and glucose intolerance during late stages of pregnancy in mice, that mimicked the transitory condition of GD, since fasting blood glucose returned to control levels at the end of pregnancy.
Since obesity is a well-known risk factor for GD, several studies perform nutritional manipulations to induce β-cell dysfunction and diabetes in pregnant mice and rats [43,44,45]. However, the increasing evidence suggest that GD incidence is rising especially among non-obese women, and in certain populations, obesity is the main driving cause of diabetes in only 15% of cases [18]. Here, we describe a mouse model of hyperglycemia and glucose intolerance during pregnancy, with no induction of maternal obesity. Although we did not directly assess whether our model was associated to peripheral insulin resistance, previous studies have found that S961 administration to mice leads to hyperinsulinemia and marked insulin resistance [26, 27]. These findings suggest that our model is useful to study the long-term consequences of moderate and transient glucose intolerance during late stages of gestation on the health of females and their offspring, since it closely resembles the time course of GD seen in humans. Our findings also suggest that S961 has no direct effect on pups since there were no changes in physical and reflex development at any stage after birth. However, in our study, we did not directly address whether S961 crosses the placental barrier and reaches the fetuses. S961 is a peptide containing 43 amino acid residues and therefore is not likely to freely cross the brain and placental barriers. It is known that maternal insulin cannot cross the placenta, indicating that this tissue lacks insulin transporters [46], thus ruling out the possibility that these transporters recognize S961 and carry it across the placenta. It is unlikely that S961 is transported unspecifically by other transporters present in the placenta, since it binds with high selectivity to insulin receptors [28]. However, future studies should experimentally address whether the placental barrier is really impermeable to S961, especially under hyperglycemic conditions.
Early studies suggested a higher incidence of diabetes in adults born to mothers with GD [47,48,49], but until recently, the disease was considered a transient condition associated with no major consequence to the mother or child. Longitudinal studies following this population are still rare and with poor conclusions regarding the long-term effects of GD on the offspring, which possibly underestimated the late consequences of this metabolic misbalance [50]. More recent and concerning experimental evidence point to detrimental effects of GD on the development, metabolism, and behavior of the offspring [51,52,53]. Our data showed that, while pups born from GD dams are normoglycemic when become adults, they are more susceptible to becoming glucose-intolerant when exposed to a short period of high-fat diet. These findings are in agreement with previous clinical and epidemiological data and further strengthen the idea that exposure to high glucose and insulin levels during critical embryonic stages may have persistent effects on metabolic profile of the offspring.
The developing brain is extremely sensitive to endogenous and exogenous signals. GD involves fetal exposure to high levels of pro-inflammatory mediators during a critical period of brain development [54, 55] and could have latent effects and program brain’s response to aversive stimuli later in life. Clinical studies have reported delayed neurocognitive development in babies born from GD mothers compared with those born from patients who remained euglycemic throughout pregnancy [56,57,58]. This delayed cognitive development appears to be reversible, since older children regained normal memory function [58]. In agreement with these clinical findings, we found that adult mice born from GD dams show normal cognitive performance in the object recognition paradigm, although we did not evaluate cognitive abilities of these mice during infancy. Long-term treatment of mice with high-fat diet has been associated to cognitive impairment [59, 60], impaired insulin signaling in memory-related brain regions [60, 61], and increased brain generation of amyloid-β peptides, the main neurotoxins in Alzheimer’s disease [61, 62]. Here, we report that mice born from GD dams show increased susceptibility to develop cognitive impairment in the object recognition paradigm when exposed to a short-term period of high-fat diet. Altogether, our results suggest that although individuals might develop normal neurocognitive capacities in adulthood, intra-uterine exposure to the diabetic environment programs brain’s response to aversive stimuli later in life. Disrupted maternal care early in life has been shown to interfere with the proper development of the progeny, resulting in neurobiological and behavioral abnormalities in adulthood [63]. One could argue that the susceptibility to behavioral alterations found in our study could be related to variations in maternal care related to DG. However, we observed no alteration on maternal care parameters shortly after birth, thus excluding an unspecific effect of the perinatal manipulation on late-life behavior alterations found in the offspring.
Studies performed in other animal models have described persistent molecular changes in the brains of GD offspring. It is known that brain inflammation and microglial activation are associated to cognitive impairment in several conditions [64,65,66,67], and previous studies have showed that increased hippocampal production of TNF-α can lead to impaired neuronal insulin signaling and cognitive impairment in mice [68, 69]. Increased brain levels of pro-inflammatory markers [55, 70] and persistent microglial activation were previously shown in brains of mice born from GD dams [71]. In addition, deregulation in the expression of both IGF-1 and insulin receptors was described in the hippocampus of GD offspring [72], and another study reported insulin signaling resistance in other brain regions of GD offspring, mainly the hypothalamus [70]. Therefore, whether insulin signaling is disrupted in memory-related brain regions of the GD offspring and if contributes to the increased susceptibility to cognitive impairment in our study should be subject of future investigations. One study also found that levels of endoplasmic reticulum stress markers were higher in the brains of adolescent mice born from GD dams, suggesting that an unfolded protein response is triggered in the brains of these animals [70]. Leptin resistance and reduced neural hypothalamic projections in adult mice born from hyperglycemic dams were also reported [73]. Future studies should be performed to elucidate the molecular mechanisms involved in the behavioral programing induced by GD in the offspring, thus contributing to describe molecular targets for intervention.
In conclusion, our findings provide evidence that treatment of female pregnant mice with the insulin receptor antagonist S961 comprises an interesting model of GD, recapitulating key aspects of this condition which have not been mimicked by other animal models. This might be an interesting tool to study the mechanisms underlying GD and to test alternative treatments to prevent the late consequences associated to this disease. Our findings also suggest that the offspring of GD mothers are more susceptible to metabolic and cognitive impairments when exposed to high-fat diet later in life, thus indicating that approaches to prevent and treat these late effects should be pursued.
Abbreviations
- GD:
-
Gestational diabetes
- GTT:
-
Glucose tolerance test
- HFD:
-
High-fat diet
- i.p.:
-
Intraperitoneal
- ND:
-
Normal diet
- NOR:
-
Novel object recognition
References
Plows JF, Stanley JL, Baker PN, Reynolds C, Vickers M (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 19. https://doi.org/10.3390/ijms19113342
Chen P, Wang S, Ji J, Ge A, Chen C, Zhu Y, Xie N, Wang Y (2015) Risk factors and management of gestational diabetes. Cell Biochem Biophys 71:689–694. https://doi.org/10.1007/s12013-014-0248-2
Zhang C, Rawal S, Chong YS (2016) Risk factors for gestational diabetes: is prevention possible? Diabetologia 59:1385–1390. https://doi.org/10.1007/s00125-016-3979-3
DeSisto CL, Kim SY, Sharma AJ (2014) Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 11:E104. https://doi.org/10.5888/pcd11.130415
Mitanchez D, Burguet A, Simeoni U (2014) Infants born to mothers with gestational diabetes mellitus: mild neonatal effects, a long-term threat to Global Health. J Pediatr 164:445–450. https://doi.org/10.1016/j.jpeds.2013.10.076
Dabelea D (2007) The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 30(Suppl 2):S169–S174. https://doi.org/10.2337/dc07-s211
Van Lieshout RJ, Voruganti LP (2008) Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. J Psychiatry Neurosci 33:395–404
Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, Landau D, Sheiner E (2016) Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol 215:380.e1–380.e7. https://doi.org/10.1016/J.AJOG.2016.03.030
Chandna AR, Kuhlmann N, Bryce CA, Greba Q, Campanucci VA, Howland JG (2015) Chronic maternal hyperglycemia induced during mid-pregnancy in rats increases RAGE expression, augments hippocampal excitability, and alters behavior of the offspring. Neuroscience 303:241–260. https://doi.org/10.1016/J.NEUROSCIENCE.2015.06.063
Georgieff MK (2008) The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans 36:1267–1271. https://doi.org/10.1042/BST0361267
Bilbo SD, Schwarz JM (2012) The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33:267–286. https://doi.org/10.1016/j.yfrne.2012.08.006
Bilbo SD, Tsang V (2010) Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24:2104–2115. https://doi.org/10.1096/fj.09-144014
Pasek RC, Gannon M (2013) Advancements and challenges in generating accurate animal models of gestational diabetes mellitus. Am J Physiol Endocrinol Metab 305:E1327–E1338. https://doi.org/10.1152/ajpendo.00425.2013
Morisset J, Morisset S, Lauzon K, Côté S, Lainé J, Bourassa J, Lessard M, Echavé V (2000) Pancreatic inflammation, apoptosis, and growth: sequential events after partial pancreatectomy in pigs. Pancreas 21:321–324
Serradas P, Giroix MH Portha B evaluation of the pancreatic B-cell function in the rat after prenatal exposure to streptozotocin or N-nitrosomethylurea. Diabete Metab 15:30–37
Gutierrez JC, Bahamonde J, Prater MR, Yefi CP, Holladay SD (2010) Production of a type 2 maternal diabetes rodent model using the combination of high-fat diet and moderate dose of streptozocin. Endocr Res 35:59–70. https://doi.org/10.3109/07435801003641939
Dahlhoff M, Pfister S, Blutke A, Rozman J, Klingenspor M, Deutsch MJ, Rathkolb B, Fink B et al (2014) Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim Biophys Acta 1842:304–317. https://doi.org/10.1016/j.bbadis.2013.11.021
Kim SY, England L, Sappenfield W et al (2012) Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004-2007. Prev Chronic Dis 9:E88
Kaufmann RC, Amankwah KS, Dunaway G, Maroun L, Arbuthnot J, Roddick JW Jr (1981) An animal model of gestational diabetes. Am J Obstet Gynecol 141:479–482
Yamashita H, Shao J, Qiao L, Pagliassotti M, Friedman JE (2003) Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice. Pediatr Res 53:411–418. https://doi.org/10.1203/01.PDR.0000049667.58071.7D
Huang C, Snider F, Cross JC (2009) Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150:1618–1626. https://doi.org/10.1210/en.2008-1003
Kahraman S, Dirice E, De Jesus DF et al (2014) Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring. Am J Physiol Endocrinol Metab 307:E906–E918. https://doi.org/10.1152/ajpendo.00210.2014
Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G et al (2007) Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev 21:756–769. https://doi.org/10.1101/gad.1535507
de Sousa RAL, Torres YS, Figueiredo CP et al (2018) Consequences of gestational diabetes to the brain and behavior of the offspring. An Acad Bras Cienc 90:2279–2291. https://doi.org/10.1590/0001-3765201720170264
Vikram A, Jena G (2010) S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem Biophys Res Commun 398:260–265. https://doi.org/10.1016/j.bbrc.2010.06.070
Cieniewicz AM, Kirchner T, Hinke SA, Nanjunda R, D’Aquino K, Boayke K, Cooper PR, Perkinson R et al (2017) Novel monoclonal antibody is an allosteric insulin receptor antagonist that induces insulin resistance. Diabetes 66:206–217. https://doi.org/10.2337/db16-0633
Kleiner S, Gomez D, Megra B, Na E, Bhavsar R, Cavino K, Xin Y, Rojas J et al (2018) Mice harboring the human SLC30A8 R138X loss-of-function mutation have increased insulin secretory capacity. Proc Natl Acad Sci U S A 115:E7642–E7649. https://doi.org/10.1073/pnas.1721418115
Schäffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J (2008) A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 376:380–383. https://doi.org/10.1016/J.BBRC.2008.08.151
Clarke JR, Lyra E, Silva NM, Figueiredo CP et al (2015) Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol Med 7:190–210. https://doi.org/10.15252/emmm.201404183
Colomina MT, Albina ML, Domingo JL, Corbella J (1997) Influence of maternal stress on the effects of prenatal exposure to methylmercury and arsenic on postnatal development and behavior in mice: a preliminary evaluation. Physiol Behav 61:455–459
Santillán ME, Vincenti LM, Martini AC, Fiol de Cuneo M, Ruiz RD, Mangeaud A, Stutz G (2010) Developmental and neurobehavioral effects of perinatal exposure to diets with different omega-6:omega-3 ratios in mice. Nutrition 26:423–431. https://doi.org/10.1016/j.nut.2009.06.005
Zbinden G (1981) Experimental methods in behavioral teratology. Arch Toxikologie 48:69–88. https://doi.org/10.1007/BF00310480
De Castro VLSS, Destefani CR, Diniz C, Poli P (2007) Evaluation of neurodevelopmental effects on rats exposed prenatally to sulfentrazone. Neurotoxicology 28:1249–1259. https://doi.org/10.1016/j.neuro.2007.06.001
Reis AR, de Azevedo MS, de Souza MA, Lutz ML, Alves MB, Izquierdo I, Cammarota M, Silveira PP et al (2014) Neonatal handling alters the structure of maternal behavior and affects mother-pup bonding. Behav Brain Res 265:216–228. https://doi.org/10.1016/j.bbr.2014.02.036
Fortuna JTS, Gralle M, Beckman D, Neves FS, Diniz LP, Frost PS, Barros-Aragão F, Santos LE et al (2017) Brain infusion of α-synuclein oligomers induces motor and non-motor Parkinson’s disease-like symptoms in mice. Behav Brain Res 333:150–160. https://doi.org/10.1016/j.bbr.2017.06.047
Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P (2010) Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health 100:1047–1052. https://doi.org/10.2105/AJPH.2009.172890
Carson MP, Frank MI, Keely E (2013) Original research: postpartum testing rates among women with a history of gestational diabetes--systematic review. Prim Care Diabetes 7:177–186. https://doi.org/10.1016/j.pcd.2013.04.007
Francis DD, Champagne FA, Liu D, Meaney MJ (1999) Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci 896:66–84
Franks B, Champagne FA, Curley JP (2015) Postnatal maternal care predicts divergent weaning strategies and the development of social behavior. Dev Psychobiol 57:809–817. https://doi.org/10.1002/dev.21326
Burlina S, Dalfrà MG, Lapolla A (2017) Short- and long-term consequences for offspring exposed to maternal diabetes: a review. J Matern Fetal Neonatal Med 32:1–8. https://doi.org/10.1080/14767058.2017.1387893
Wicklow BA, Sellers EAC, Sharma AK, Kroeker K, Nickel NC, Philips-Beck W, Shen GX (2018) Association of gestational diabetes and type 2 diabetes exposure in utero with the development of type 2 diabetes in first nations and non-first nations offspring. JAMA Pediatr 172:724–731. https://doi.org/10.1001/jamapediatrics.2018.1201
Gilmartin ABH, Ural SH, Repke JT (2008) Gestational diabetes mellitus. Rev Obstet Gynecol 1:129–134
Holemans K, Caluwaerts S, Poston L, Van Assche FA (2004) Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol 190:858–865. https://doi.org/10.1016/j.ajog.2003.09.025
Liang C, DeCourcy K, Prater MR (2010) High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism 59:943–950. https://doi.org/10.1016/j.metabol.2009.10.015
Gauguier D, Bihoreau MT, Ktorza A, Berthault MF, Picon L (1990) Inheritance of diabetes mellitus as consequence of gestational hyperglycemia in rats. Diabetes 39:734–739
Ruiz-Palacios M, Ruiz-Alcaraz AJ, Sanchez-Campillo M, Larqué E (2017) Role of insulin in placental transport of nutrients in gestational diabetes mellitus. Ann Nutr Metab 70:16–25. https://doi.org/10.1159/000455904
Dörner G, Mohnike A (1976) Further evidence for a predominantly maternal transmission of maturity-onset type diabetes. Endokrinologie 1:121–124. https://doi.org/10.1093/cercor/bhw375
Silverman BL, Metzger BE, Cho NH, Loeb CA (1995) Impaired glucose tolerance in adolescent offspring of diabetic mothers. Diabetes Care 18:611–617
Poston L, Health F (2010) Best Practice & Research Clinical Endocrinology & Metabolism Developmental programming and diabetes – the human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab 24:541–552. https://doi.org/10.1016/j.beem.2010.05.007
Poston L (2011) Intergenerational transmission of insulin resistance and type 2 diabetes. Prog Biophys Mol Biol 106:315–322. https://doi.org/10.1016/j.pbiomolbio.2010.11.011
Daraki V, Roumeliotaki T, Koutra K, Georgiou V, Kampouri M, Kyriklaki A, Vafeiadi M, Papavasiliou S et al (2017) Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: the Rhea mother-child cohort, Crete, Greece. Eur Child Adolesc Psychiatry 26:1–12. https://doi.org/10.1007/s00787-016-0934-2
Yessoufou A, Moutairou K (2011) Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory.”. Exp Diabetes Res 2011:1–12. https://doi.org/10.1155/2011/218598
Garcia-vargas L, Addison SS (2012) Gestational diabetes and the offspring: implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal Med 65212:134–142. https://doi.org/10.1159/000337734
Li H-P, Chen X, Li M-Q (2013) Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol 6:650–659
Tang X, Qin Q, Xie X, He P (2015) Protective effect of sRAGE on fetal development in pregnant rats with gestational diabetes mellitus. Cell Biochem Biophys 71:549–556. https://doi.org/10.1007/s12013-014-0233-9
DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA (2005) Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol 47:525–531
Torres-Espinola FJ, Berglund SK, García-Valdés LM, Segura MT, Jerez A, Campos D, Moreno-Torres R, Rueda R et al (2015) Maternal obesity, overweight and gestational diabetes affect the offspring neurodevelopment at 6 and 18 months of age--a follow up from the PREOBE cohort. PLoS One 10:e0133010. https://doi.org/10.1371/journal.pone.0133010
Riggins T, Bauer PJ, Georgieff MK, Nelson CA (2010) Declarative memory performance in infants of diabetic mothers. Adv Child Dev Behav 38:73–110
Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC et al (2010) Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 18:463–469. https://doi.org/10.1038/oby.2009.273
Sims-Robinson C, Bakeman A, Bruno E et al (2016) Dietary reversal ameliorates short- and long-term memory deficits induced by high-fat diet early in life. PLoS One 11:e0163883. https://doi.org/10.1371/journal.pone.0163883
Kothari V, Luo Y, Tornabene T, O’Neill AM, Greene MW, Geetha T, Babu JR (2017) High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta 1863:499–508. https://doi.org/10.1016/j.bbadis.2016.10.006
Walker JM, Dixit S, Saulsberry AC, May JM, Harrison FE (2017) Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol Dis 100:87–98. https://doi.org/10.1016/j.nbd.2017.01.004
Van der Kooij MA, Grosse J, Zanoletti O et al (2015) The effects of stress during early postnatal periods on behavior and hippocampal neuroplasticity markers in adult male mice. Neuroscience 311:508–518. https://doi.org/10.1016/j.neuroscience.2015.10.058
Medeiros R, Figueiredo CP, Pandolfo P, Duarte FS, Prediger RDS, Passos GF, Calixto JB (2010) The role of TNF-α signaling pathway on COX-2 upregulation and cognitive decline induced by β-amyloid peptide. Behav Brain Res 209:165–173. https://doi.org/10.1016/j.bbr.2010.01.040
Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, Kolpakov MA, Bashkirova YV et al (2019) Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol 56:1883–1896. https://doi.org/10.1007/s12035-018-1195-5
Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S et al (2017) Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation 14(222):222. https://doi.org/10.1186/s12974-017-0998-z
Cabrera-Pastor A, Hernandez-Rabaza V, Taoro-Gonzalez L, Balzano T, Llansola M, Felipo V (2016) In vivo administration of extracellular cGMP normalizes TNF-α and membrane expression of AMPA receptors in hippocampus and spatial reference memory but not IL-1β, NMDA receptors in membrane and working memory in hyperammonemic rats. Brain Behav Immun 57:360–370. https://doi.org/10.1016/j.bbi.2016.05.011
Neves FS, Marques PT, Barros-Aragão F et al (2018) Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol Neurobiol 55:435–444. https://doi.org/10.1007/s12035-016-0307-3
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest 122:1339–1353. https://doi.org/10.1172/JCI57256
Melo AM, Benatti RO, Ignacio-Souza LM, Okino C, Torsoni AS, Milanski M, Velloso LA, Torsoni MA (2014) Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metabolism 63:682–692. https://doi.org/10.1016/j.metabol.2014.02.002
Vuong B, Odero G, Rozbacher S, Stevenson M, Kereliuk SM, Pereira TJ, Dolinsky VW, Kauppinen TM (2017) Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J Neuroinflammation 14(80):80. https://doi.org/10.1186/s12974-017-0859-9
Hami J, Sadr-Nabavi A, Sankian M, Balali-Mood M, Haghir H (2013) The effects of maternal diabetes on expression of insulin-like growth factor-1 and insulin receptors in male developing rat hippocampus. Brain Struct Funct 218:73–84. https://doi.org/10.1007/s00429-011-0377-y
Steculorum SM, Bouret SG (2011) Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 152:4171–4179. https://doi.org/10.1210/en.2011-1279
Acknowledgments
We thank Melissa Florence, Jadilma Ferreira, and Ana Claudia Rangel.
Funding Sources
This work was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (E.V.L., C.P.F., G.F.P., J.R.C.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (C.P.F., J.R.C.), Institutos Nacionais de Pesquisa - Inovação em Medicamentos e Identificação de Novos Alvos Terapêuticos (C.P.F., G.F.P.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (R.A.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Sousa, R.A., de Lima, E.V., da Silva, T.P. et al. Late Cognitive Consequences of Gestational Diabetes to the Offspring, in a New Mouse Model. Mol Neurobiol 56, 7754–7764 (2019). https://doi.org/10.1007/s12035-019-1624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1624-0