Abstract
The main objective of the present study is to investigate potential effects of PCA in OBX induced depressive-like behavior in rat model. PCA was administered at a dose of 100 mg/kg and 200 mg/kg, by per oral in OBX and sham operated rats. Behavioral (ambulatory and rearing activity and immobility time), neurochemical [serotonin (5-HT), dopamine (DA), norepinephrine (NE) and brain derived neurotrophic factor (BDNF) expression], biochemical (MDA formation, IL-6, TNF-α and antioxidants) changes in hippocampus and cerebral cortex along with serum corticosterone were investigated. Experimental findings reveals that OBX subjected rats showed alteration in behaviors like, increase in immobility time, ambulatory and rearing behaviors significantly, reduced BDNF level, 5-HT, DA,NE and antioxidant parameters along with increased serum corticosterone, MDA formation, IL-6, and TNF-α in hippocampus and cerebral cortex compared to sham operated rats. Administration of PCA significantly attenuated behavioral and neurobiochemical alterations, thus, its antidepressant-like activity is largely mediated through modulation of neurotransmitter, endocrine and immunologic systems, mainly by improvements of BDNF, 5-HT, DA, NE, reduced MDA, IL-6, and TNF-α in hippocampus and cerebral cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olfactory bulbectomized (OBX) induced depression is largely employed rodent method for screening of drugs in depressive mood behavior (Song and Leonard 2005). OBX rats exhibits behavioral and neurobiochemical alterations, mainly, explorative changes which are mainly seen in agitated depression (Song and Leonard 2005). Brain neurogenesis is mainly routed via modulation of brain-derived neurotrophic factor (BDNF), a member of nerve growth factor. The BDNF expression and its level found to be altered during OBX, while antidepressant therapy improve such level in various brain regions of rodents (Duman 2004; Shimizu et al. 2003; Antunes et al. 2016). Further, OBX subjected rats elicited higher proinflammtory cytokines level mainly tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) the brain areas (Antunes et al. 2016) suggesting that these cytokines participate in the development of depressive state. OBX model of depression exhibited significant impairment of behavioral changes like increased immobility time, exploratory activity, biochemical alterations mainly, attenuation of monoamines, BDNF, TNF-α and IL-6 along with impaired endogenous defense system (Thakare et al. 2017a, b). The antidepressants like activity of these drugs probably mediated via neuroprotection mechanism produce by attenuation of neuroinflammatory effects.
Protocatechuic acid (PCA) induced neuroprotection in stress animals by improving endogenous antioxidant enzymatic activity (Shi et al. 2006) and antidepressant activity was by enhancing brain monoamines (Kim et al. 2014). PCA ameliorates neurocognitive dysfunction induced by chronic intermittent hypoxia; increased 5-HT in the brain tissues of rats along with decreased MAO-B activity (Kim et al. 2014). In our earlier findings, we observed that PCA prevented the elevation of malondialdehyde (MDA) formation, and improved endogenous antioxidant system in cerebral ischemic rats (Muley et al. 20122013). In addition, administration of PCA attenuates lipid peroxidation and subsequently restored the antioxidant enzymes (Zhang et al. 2015). We recently documented antidepressant potential of PCA in acute restraint stress by attenuation of MDA formation and improved antioxidant defense system, thus control oxidative stress (Thakare et al. 2016). However, effects of PCA in OBX induced depressive-like behavior are not available in literature. Hence, the work presented in this communication attempts to demonstrate the antidepressant potential of PCA in OBX induced depression, and to explore associated mechanism(s) of action.
Materials and methods
Animals
Wistar rats of either sex weighing 200–250 g, 80–100 days old were procured from Institute of Biosciences, India. Rats were housed separately in groups of 8 per cage (polycarbonate cage size: 29 cm × 22 cm × 14 cm) under standard laboratory conditions with alternating light and dark cycle of 12 h each. The animals had free access to food and water unless specified. Experimental protocol was approved (SIPS/IAEC/2014–15/01) by the Institutional Animal Ethics Committee and experiment is complying with the guidelines in accordance of National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Drugs and chemicals
Test drug PCA and standard fluoxetine, Enzyme-linked immunosorbent assay (ELISA) kits for estimation of brain derived neurotrophic factor, corticosterone and cytokines were obtained from various repute vendors. The other chemicals utilized in the research studies were obtained from local vendors.
Olfactory bulbectomy (OBX)
Olfactory bulbectomy (OBX) surgery was carried out as per the method described by Van Reizen and Leonard (1990) and also employed at our lab earlier (Thakare et al. 2017a, b). Briefly, anaesthetized rats (Anaesthesia consist of ketamine and xylazine at the ratio of 10:1). Anesthetized rats were incised as 1-cm on midline of rat head by steriotaxically (Inco, Ambala, India) and OBX done with use of coordinates, from bregma, AP +6 mm, ML ±1 and DV 2 mm. Olfactory bulbs were ablated by use of suction and haemostatic sponge was introduced into bulb cavity in order to prevent the excessive hemorrhage, and finally incision was sutured. In Sham animals, same process was performed except olfactory bulbs removal. The model was corroborated by study of behavioral changes like, hyperactivity, duration of immobility time. The OBX/Sham rats were housed singly in cages for 15 days for rehabilitation. Pictogram of the study is shown in Fig. 1.
Schematic presentation of the study is shown in Fig.1
Experimental design
Two weeks of surgical rehabilitation, animals were grouped as per following approach-
-
Sham control group: Vehicle (Carboxy Methyl Cellulose (CMC), 10 ml/kg, per oral (po)
-
Sham standard group: Fluoxetine 20 mg/kg, po
-
Sham drug treated group 1 and 2- treated with PCA 100 and 200 mg/kg,po respectively
-
OBX control group: Vehicle, 10 ml/kg, po
-
OBX standard group: Fluoxetine 20 mg/kg, po
-
OBX drug treated group 1 and 2- treated with PCA 100 and 200 mg/kg, po respectively
Selection of PCA doses
The doses of PCA were chosen from previous documented findings of our lab (Muley et al. 2012, 2013; Thakare et al. 2016, 2017a, b). In the present studies we have chosen 100 and 200 mg/kg by oral route in order to study the dose dependent effects on various depressive behaviors in OBx rats.
In addition, As a safety measure we did not observed any significant unwanted behavioral changes with the employed doses of PCA viz. excitement and/convulsion with and itching, skin rashes, tremors etc. which was significantly observed with tricyclic antidepressants and fluoxetine respectively (Rang et al. 2007).
PCA and fluoxetine was suspended in CMC (1%, (w/v) and maximum volume was administered as 10 ml/kg by oral route. PCA treatment was given once a daily at 9.00 AM for 14 days post surgical rehabilitation period.
Behavioral studies
Forced swimming test (FST)
The duration of immobility time in FST was determined as per the procedure documented earlier by Porsolt et al. (1977).
Open-field test (OFT)
Ambulatory behaviors were recorded in the OFT as per the method previously employed by Rodrigues et al. (1996). The number of section crossed and rearing frequency were determined as ambulatory behavior in 6 min time period. The equipment was cleaned with a solution of 10% ethanol between tests in order to hide animal clues.
Biochemical studies
Post behavioral investigation, blood was withdrawn from retro-orbital plexus under light isoflurane anaesthesia at 8.30 am and serum was separated and used in corticosterone (CORT) measurement. Animals were sacrificed, brain samples were quickly isolated, and hippocampus and cerebral cortex were separated and homogenized in 0.1 M PBS (pH 7.4) and utilized for measurement of malondialdehyde (MDA) an index of lipid peroxidation process. The residual brain homogenate further centrifuged (10,000×g) at 4 °C for 15 min, resulting supernatant was employed for measurement of various neurobiochemical.
Estimation of malondialdehyde (MDA) formation
The MDA formation was estimated in homogenates of hippocampus and cerebral cortex as per the method of Ohkawa et al. (1979).
Antioxidant studies
The catalase (CAT) activity and reduced glutathione (GSH) was measured as per the procedure described by of Aebi (1984) and Ellman (1959) respectively.
Neurochemicals estimation
The monoamines, serotonin (5-HT), norepinephrine (NE) and dopamine (DA) were estimated in the tissue homogenates as per the method of Kent Shellenberger and Gordon (1971).
Determination of BDNF, and cytokines
BDNF, IL-6 and TNF-α levels were investigated by ELISA techniques by the methodology mentioned in the manufacturer’s kit.
Determination of serum CORT
Serum CORT was measured by ELISA techniques by the methodology mentioned in the manufacturer’s kit.
Statistical analysis
Data was expressed as the mean ± SEM. Statistical data was analyzed by two-way ANOVA followed by Bonferroni post hoc test. Probability value p < 0.05 was considered to be statistically significant.
Results
Effects of PCA or fluoxetine on immobility time
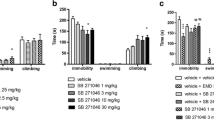
Experimental data suggested that rats underwent OBX elicit significantly (p < 0.0001) higher immobility time compared to sham control vehicle group. Treatment with PCA and fluoxetine attenuated the immobility time (p < 0.0001) when compared to vehicle treated OBX group (Fig. 2).
Effects of PCA or fluoxetine on open field behaviors
In open file test, we found increased exploratory behaviors, mainly ambulation and rearing in OBX group as compared to sham control group. Administration of PCA and significantly (p < 0.0001) attenuated these behavior when compared to vehicle treated OBX rats (Fig. 3a and b).
Effects of PCA or fluoxetine on monoamines level
In monoamines studies, we found significant reduction (p < 0.0001) of 5-HT, DA and NE level were observed in OBX operated group as compared to sham operated group. PCA and fluoxetine treatments significantly (p < 0.0001) improved levels of these monoamines in hippocampus (Figs. 4a, 5a and 6a). Similarly, in cerebral cortex too, we observed that, OBX rats exhibits significant decrements in levels of 5-HT, DA and NE compared to sham control. Treatment with PCA and fluoxetine significantly (p < 0.0001) prevented decline in these monoamines compared to OBX group (Figs. 4b, 5b and 6b).
Effects of PCA or fluoxetine on BDNF level
The experimental data reveals that OBX rats showed significant p = 0.0036) reduction in BDNF level in hippocampus compared to sham operated group (Fig. 7a). Administration of PCA significantly prevented the decline in BDNF contents in hippocampus of compared to OBX rats. Likewise, in cerebral cortex, treatment with PCA and fluoxetine prevented decrement in BDNF level due to OBX (Fig. 7b).
Effects of PCA or fluoxetine on cytokines
There was significant increase (p < 0.0001) cytokines TNF-α and IL-6 in hippocampai of OBX subjected rats compared to sham group (Figs. 8a, 9a). PCA administration significantly attenuated the both cytokines levels compared to vehicle treated OBX rats. For cerebral cortex, PCA treatment significantly (p < 0.0001) prevented the elevation of TNF-α and IL-6 levels compared to OBX operated rats (Figs. 8b, 9b).
Effects of PCA or fluoxetine on serum CORT level
OBX subjected rats elicited significant (p < 0.0001) elevation of serum CORT level compared to sham operated group. Administration of PCA at both doses significantly prevented after serum CORT elevation compared to OBX subjected rats. (Fig. 10).
Effects of PCA or fluoxetine on MDA formation
OBX rats exhibited increased hippocampal (Fig. 11a) and cerebral cortex (Fig. 11b) MDA formation compared to sham control group which was subsequently attenuated with the PCA treatments.
Effects of PCA or fluoxetine on CAT and GSH
OBX subjected rats showed significant decrement in both CAT activity and GSH contents in hippocampus compared to sham operated group (Figs. 12a, 13a). Treatment with PCA significantly improved antioxidants CAT and GSH compared to vehicle treated OBX group. Similarly, GSH content in cerebral cortex (Figs. 12b, 13b) was reversed with PCA treatment compared to OBX treated rats.
Discussion
In the present study we proposed that, PCA could able to attenuate OBX induced depressive-like behaviors by improving behavioral and neurobiochemical alternations. Ablation of olfactory bulb in rats elicits increased hyperactivity, loss of pleasure which manifested to depressive state (Song and Leonard 2005; Thakare et al. 2017a, b). Our experimental findings revealed that, OBX rats elicited increase immobility time which was subsequently attenuated with PCA administration. Further, we have measured exploratory activity, ambulatory and rearing behavior in OBX rats to identify the effects of PCA on depressive state that might augment such behaviors. We observed that, rats subjected to OBX exhibited increase ambulation and rearing behavior, suggested agitated depressive which was subsequently prevented with administration PCA at doses dependent manner.
Further, in order to understand involvements of monoamines in antidepressant like activity of PCA, we measured monoamines level 5-HT, NE and DA in hippocampus and cerebral cortex of OBX and sham control rats (Mao et al. 2011). It is apparent that deficiency of monoamines in the brain is largely participated in induction of depressive-like disorders (Elhwuegi 2004; Nutt 2008). The depressive symptoms like mood alterations, anhedonia, pessimism and feeling of worthlessness are connected by deficiency of brain monoamines (Elhwuegi 2004; Nutt 2008). Similarly, in our earlier OBX model, we found that OBX rats showed lower monoamines levels in hippocampus and cerebral cortex (Thakare et al. 2017a, b). Our present data indicated that decrease 5-HT, DA and NE level in hippocampus and cerebral cortex in OBX group while PCA and fluoxetine, noticeably improved these monoamines. Our present findings are in agreements with the findings of Kim et al. (2012), who demonstrated that PCA isolated from Gardenia jasminoides showed significant antidepressant activity by inhibiting MAO-A and MAO-B enzymes. As MAO-A is involved in the metabolism of serotonin, noradrenaline and to lesser extent dopamine, whereas, MAO-B metabolize the dopamine (Youdim and Weinstock 2004). Thus, we believed the improvements in monoamines levels with PCA in hippocampus and cerebral cortex in OBX rats is might be due to that ability of PCA to inhibit the MAO-A and MAO-B enzymes. However, we did not investigate the inhibitory effects of PCA on these enzymes in the present studies.
The documented literature reveals that, there is substantial correlation between depression and neuronal degeneration or damage at brain regions (Fuchs et al. 2004; Manji and Duman 2001). The chief action of BDNF is stimulation of development and subsequently formation of neurons and synapses mainly in the hippocampus, cortex (Fuchs et al. 2004). Documented reports revealed that depressed patients showed lower BDNF level (Matrisciano et al. 2009; Fernandes et al. 2011). In addition, it was noted that reduce BDNF content in hippocampus and prefrontal cortex of mice in chronic stress induce depressive behavior (Mao et al. 2014; Shen et al. 2016; Thakare et al. 2017a, b) and subsequent antidepressant treatment reversed the BDNF contents. In our findings too, OBX rats showed decrement in BDNF which is presumably due to increased free radicals formations due to oxidative stress and finally that induce neuronal damage. Thus, it is possible that prevention of BDNF decrement with PCA is largely through its scavenging or/neutralizing of generated free radicals, which are known to cause neuronal damage and subsequently attenuate depressive like behavior in OBX rats. Our findings are in agreement with Numakawa et al. (2011), where in they demonstrated that BDNF can significantly prevent neuronal damage caused due to oxidative stress, as found in neurodegenerative diseases. Furthermore, their findings showed that PCA administration to significantly increase the level of BDNF expression during a period of chronic intermittent hypoxia, in the hippocampus and prefrontal cortex.
Inflammatory cytokines, the immune system and neuroendocrinological components are largely participated in the development of mood related behavior (Schiepers et al. 2005). Tang et al. (2016) demonstrated that elevated TNF-α and IL-6 implicated in the pathophysiology of depression; intracerebroventricular injection of TNF-α known to exhibited depressive-like state in FST in mice (Kaster et al. 2012). Furthermore, it was found that the alteration in hippocampal neurogenesis was interrelated to the behavioral performances, as evidenced as relationship of depressive state and the dysfunctional neurogenesis. Hence, it might possible that OBX causes neuroinflammation due to increased production of pro-inflammatory cytokines, TNF-α and IL-6 in hippocampus are thought be culprit in neurogenesis process and subsequently induction of depressive like behavior. Administrations of PCA induce reduction in IL-6 and TNF-α in hippocampus and cerebral cortex. The ability of PCA due to its anti-inflammatory potential attenuates the neuroinflammation and thus subsequently prevented the elevation of IL-6 and TNF-α in hippocampus and cerebral cortex due to OBX.
It was observed that dysfunction of hypothalamic-pituitary-adrenal axis (HPA) results into increased CORT level and related alterations were also noticed post OBX in rodents (Cairncross et al. 1977; Pariante and Lightman 2008; Thakare et al. 2017a, b). Elevation of CORT level induce significant behavioral and neurochemical alterations that results in the development of depressive state were documented previously (Mao et al. 2010; Thakare et al. 2016, 2017a, b) and subsequently reduction of serum CORT suggested to induce antidepressant like effects. Likewise, we also observed that, OBX rats showed elevated serum CORT level pointing out impairment in the HPA axis. Our findings also substantiated with the documented studies of Jindal et al. (2015a, b) and Rinwa and Kumar (2014), wherein they demonstrated increased serum CORT in OBX animals which were attenuated with rolipram and Panax quinquefolium respectively. In the present studies, we observed that administration of PCA attenuated the elevated serum CORT level in dose dependent manner indicating normalization HPA axis and consequently antidepressant activity.
In order to understand the participation/involvement of oxidative stress in mood alterations in OBX; we measured MDA formation, CAT and GSH as endogenous antioxidants markers. We earlier reviewed that significant correlation between oxidative stress and depressive disorder, as corroborated by free radicals generation due to peroxidation process (Thakare and Patel 2015). Hence, oxidative stress is considered as main cause in the development of depressive behavior and consequently improvement of antioxidant paradigms. PCA exhibited significant neuroprotection through attenuation of the elevated MDA formation in the brain of ischemic rats (Muley et al. 2012, 2013). In present study, OBX rats elicited lipid peroxidation by higher MDA level which was consequently prevented significantly with PCA treatment thus protects hippocampal and cerebral cortex damage.
Reduced glutathione (GSH) is an important a non-enzymatic antioxidant component in biological tissue, that modulate oxidative stress via scavenging formed free radicals in biological reactions. The decrement in GSH contents are known to participate in the various neuropathological changes (Lovell et al. 1998) including mood related behavior. To validate such action, GSH treatment showed antidepressant potential in experimentally induced depressive like behavior (Rosa et al. 2013). The SOD and CAT activity involved in scavenging free radicals, thus, oxidative stress might results into disturbances in their activities. We in the present study showed that significant declined in CAT activity and GSH contents in hippocampus and cerebral cortex, PCA and fluoxetine treatment for 21 days significantly prevented alterations of these antioxidants. In addition, removal of olfactory bulb causes impaired endogenous antioxidants, CAT and GSH in the brain of OBX rats (Jindal et al. 2015a, b). The impaired antioxidants, CAT and GSH in OBX groups were found to return almost to normal with the PCA or fluoxetine treatments.
Therefore, we presumed herewith, scavenging and or neutralization free radicals mainly accomplished by phenolic group of PCA, it is possible that PCA due to its antioxidant nature participate in the antidepressant activity by scavenging free radicals which are subsequently abrogate oxidative stress and thereby improved the BDNF and antioxidant biomarkers and, attenuate neuroinflammation (reduced IL-6, and TNF-α). Our present data further substantiate with our earlier findings that PCA promote the improvement antioxidant marker levels/activities in ARS and OBX models of depression (Thakare et al. 2016, 2017a, b) which is subsequently responsible for antidepressant like activity.
As safety measure, PCA at 200 mg/kg did not induce any significant unwanted behavioral changes viz. excitement and/convulsion with and itching, skin rashes, tremors etc. and thus found to be safe.
Conclusion
In conclusion, our data indicate that PCA mainly at 200 mg/kg exhibited antidepressant activity in OBX model by attenuation of oxidative stress through prevention of MDA formation, restoration of antioxidants, and augmentation of neurotransmitter, neurotrophic factor, and attenuation of proinflammatory cytokines IL-6, and TNF-α in hippocampus and cerebral cortex. However, further experiments are needed to warrant the antidepressant potential of PCA in mood disorders.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Antunes MS, Jesse CR, Ruff JR, de Oliveira Espinosa D, Gomes NS, Altvater EET, Donato F, Giacomeli R, Boeira SP (2016) Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur J Pharmacol 789:411–420
Cairncross KD, Wren A, Cox B, Schnieden H (1977) Effects of olfactory bulbectomy and domicile on stress-induced corticosterone release in the rat. Physiol Behav 19:485–487
Duman RS (2004) Role of neurotrophic factors in the etiology and treatment of mood disorders. NeuroMolecular Med 5:11–25
Elhwuegi AS (2004) Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 28:435–451
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–72
Fernandes BS, Gama CS, Ceresér KM, Yatham LN, Fries GR, Colpo G et al (2011) Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res 45:995–1004
Fuchs E, Czéh B, Kole MH et al (2004) Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol 14:S481–S490
Jindal A, Mahesh R, Bhatt S (2015a) Type 4 phosphodiesterase enzyme inhibitor, rolipram rescues behavioral deficits in olfactory bulbectomy models of depression: involvement of hypothalamic–pituitary–adrenal axis, cAMP signaling aspects and antioxidant defense system. Pharmacol Biochem Behav 132:20–32
Jindal A, Mahesh R, Bhatt S (2015b) Etazolate, a phosphodiesterase-4 enzyme inhibitor produces antidepressant-like effects by blocking the behavioral, biochemical, neurobiological deficits and histological abnormalities in hippocampus region caused by olfactory bulbectomy. Psychopharmacology (Berlin) 232:623–637
Kaster MP, Gadotti VM, Calixto JB (2012) Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacol 62:419–426
Kim JH, Kim GH, Hwang KH (2012) Monoamine oxidase and dopamine b-hydroxylase inhibitors from the fruits of Gardenia jasminoides. BiomolTher 20:214–219
Kim YS, Seo HW, Lee MH, Kim DK, Jeon H, Cha DS (2014) Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans. Archives of Pharmacal Research 37(2):245–252
Lovell MA, Xie C, Markesbery WR (1998) Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology 51(6):1562–1566
Manji HK, Duman RS (2001) Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull l35:5–49
Mao Q, Xian YF, Ip SP et al (2010) Long-term treatment with peony glycosides reverses chronic unpredictable mild stress-induced depressive-like behavior via increasing expression of neurotrophins in rat brain. Behav Brain Res 210:171–177
Mao QQ, Xian YF, Ip SP, Che CT (2011) Involvement of serotonergic system in the antidepressant-like effect of piperine. Prog Neuropsychopharmacol Biol Psychiatry 35:1144–1147
Mao QQ, Huang Z, Zhong X et al (2014) Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res 26:140–145
Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, Ruberto A, Tatarelli R, Nicoletti F, Girardi P, Shelton RC (2009) Changes in BDNF serum levels in patients with major depression disorder MDD after 6months treatment with sertraline escitalopram or venlafaxine. J Psychiatr Res 43:247–254
Muley MM, Thakare VN, Patil RR, Kshirsagar AD, Naik SR (2012) Silymarin improves the behavioural, biochemical and histoarchitecture alterations in focal ischemic rats: comparative evaluation with piracetam and protocatachuic acid. Pharmacol Biochem Behav 102:286–293
Muley MM, Thakare VN, Patil RR, Bafna PA, Naik SR (2013) Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci 93:51–57
Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H (2011) Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration. Journal of Toxicology 2011:1–12
Nutt DJ (2008) Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry 69:4–7
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Rang HP, Dale MM, Ritter JM, Flower RJ (2007) Selective serotonin reuptake inhibitors. Pharmacology, pp 566. Churchill Livingstone, Elsevier Publication
Rinwa P, Kumar A (2014) Panax quinquefolium involves nitric oxide pathway in olfactory bulbectomy rat model. Physiol Behav 129:142–151
Rodrigues ALS, Rocha JBT, Mello CF, Souza DO (1996) Effect of perinatal Lead exposure on rat behaviour in open-field and two-Wky avoidance tasks. Basic Clin Pharmacol Toxicol 79:150–156
Rosa JM, Dafre AL, Rodrigues AL (2013) Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behavioural Brain Research 253:165–172
Schiepers OJ, Wichers MC, Maes M (2005) Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29:201–217
Shellenberger MK, Gordon JH (1971) A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem 39:356–372
Shen J, Ma LG, Hu CY, Pei YY, Jin SL, Fang XY, Li YC (2016) Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci Lett 614:77–82
Shi GF, An LJ, Jiang B, Guan SI, Bao YM (2006) Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci Lett 403:206–210
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada SI, Iyo M (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54:70–75
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647
Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC (2016) Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav 161:166–173
Thakare VN, Patel BM (2015) Potential targets for the development of novel antidepressants: future perspectives. CNS Neurol Disord Drug Targets 14:270–281
Thakare VN, Dhakane VD, Patel BM (2016) Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol Rep 68:1020–1027
Thakare VN, Dhakane VD, Patel BM (2017a) Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab Brain Dis 32:401–413
Thakare VN, Aswar MK, Kulkarni YP, Patil RR, Patel BM (2017b) Silymarin ameliorates experimentally induced depressive like behavior in rats: involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiol Behav 179:401–410
Van Reizen H, Leonard BE (1990) Effects of psychotropic drugs on the behavior and neurochemistry of olfactory bulbectomised rats. Pharmacol Ther 47:21–34
Youdim MB, Weinstock M (2004) Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation. Neurotoxicology 25:243–250
Zhang H, Li G, Szeto S, Chong C, Quan Q et al (2015) Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson's disease. Free Radic Biol Med 84:331–333
Acknowledgements
Authors are grateful to Prof. M. N. Navale, Founder President, STES, Pune, and Dr. R.N. Kane, Principal, Sinhgad Institute of Pharmaceutical Sciences, Lonavala for providing necessary infrastructural facility, and support in the completion of present research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thakare, V.N., Patil, R.R., Suralkar, A.A. et al. Protocatechuic acid attenuate depressive-like behavior in olfactory bulbectomized rat model: behavioral and neurobiochemical investigations. Metab Brain Dis 34, 775–787 (2019). https://doi.org/10.1007/s11011-019-00401-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00401-8