Abstract
Tourette syndrome (TS) is a neurological disorder characterized by highest familial recurrence rate among neuropsychiatric diseases with complicated inheritance. Recurrence of Tourette syndrome was frequently observed in clinical. Unexpectedly, the mechanism of recurrence of Tourette syndrome was failure to elucidate. Here, we first shown that lipopolysaccharide(LPS) may played an important role in the recurrence of Tourette syndrome. The TS model in rats was induced by DOI (the selective 5-HT2A/2C agonist 1-(2, 5-dimethoxy-4-iodophenyl) -2- aminopropane). The rats were randomly divided into 4 groups:(1)Control;(2) Control + LPS; (2)TS; (3)TS + LPS. The results demonstrated that the LPS treatment significantly increased stereotypic score and autonomic activity. LPS treatment also significantly increased inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in serum and striatum. Also, highly expressed TLR4, MyD88, P-NF-κBp65, P-IκBα in TS rats were increased respectively by LPS treatment as indicted in western blot analysis and immunohistochemistry analysis. Thus, it was supposed that lipopolysaccharide(LPS) may played an important role in the recurrence of Tourette syndrome and its mechanism was related to TLR/NF-κB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourette syndrome is a severe, recurrently neurological disorder that occurs in patients before the age of 18 years. The illness is characterized by chronic muscle movements and phonic tics for over a year (Jankovic et al. 2010). Sometimes, patients with Tourette syndrome are associated with sensory symptoms, such as premonitory urges, which incessantly promote tics and feelings of momentary relief that follow representation of tic expression (Peterson and Leckman 1998). More often, a large proportion of patients with Tourette syndrome have co-occurring symptoms of behavioral difficulties, such as dis-inhibited conduct or speech, impulsivity, motoric hyperactivity, distractibility, and obsessive–compulsive symptoms, which worse functional outcomes and impair family life and social acceptance (Bloch et al. 2011). Although the etiology of Tourette syndrome has not yet fully elucidated, a genetic component and abnormalities of central inflammation and oxidative stress are strongly suggested.
Inflammation has been suggested to participate in the pathophysiological process during Tourette syndrome. Elevated inflammatory factors, such as interleukin-1β, interferon-γ, and interleukin-2 in basal ganglia of patients with Tourette syndrome were reported in recent research (Morer et al. 2010). The indirect evidence of inflammation was recorded in basal ganglia by a longitudinal imaging method, with acute basal ganglia enlargement and obsessive-compulsive symptoms (Giedd et al. 1996). It has been known that the TLR pathway is implicated in the positive regulation of NF-κB activation and expression inflammatory cytokines which is believed to be implicated in the development of neuroinflammations and neuropathology of central nervous system diseases (Gan and Mucke 2008; Libert and Guarente 2012). LPS, bacterial endotoxin, is believed to be implicated in the development of nervous system diseases (Schug et al. 2010; Feng et al. 2011).
Recurrence of Tourette syndrome was frequently observed in clinical. Unexpectedly, the mechanism of recurrence of Tourette syndrome was failure to elucidate. This work was aimed to further illustrated the mechanisms underling of recurrence of Tourette syndrome.
Material and methods
Chemicals and reagents
LPS was purchased from Sigma Chemical Co. (St. Louis, MO, USA). DOI (the selective 5-HT2A/2C agonist 1-(2,5-dimethoxy-4-iodophenyl) -2-aminopropane) was obtained from Sigma-Aldrich (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for the detection of IL-6, IL-1β and TNF-α were produced by Nanjing KeyGEN Biotech. CO., LTD. (Nanjing, China).
Animals
Fourty male Wistar rats, weighting 180–200 g, were purchased from Beijing vital river co., LTD (License number: SCXK (Jing) 2012–0001) and housed under a 12 h/12 h light/dark cycle environment. Rats were allowed to adapt the environment for 1 week at a temperature of 22 ± 1 °C and 40–70% humidity, with free to standard food and water. All of the experiments were performed in full compliance with the guidelines of the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH Publication No. 85–23, revised 1996). Animal care and experimental protocols were approved by the Nanjing University of Chinese Medicine Committee.
Experimental protocols
Rats were randomly separated into 4 groups (with 10 rats in each group): control group, control + LPS; DOI treated group, DOI treated with LPS (5 mg/kg) group. Tourette Syndrome was induced in rats by DOI intraperitoneal injection at dosage of 1 mg/kg, once daily for 21 days continuously. Control group and DOI group were intraperitoneal injection with normal saline or DOI, while LPS group was treated with LPS intracerebroventricularly injected at dosage of 5 mg/kg.
Behavioral testing
Stereotypy recording
Stereotypy recording was conducted by two trained observers who were familiar with the measurements but blind to the group condition. For evaluating the stereotypy, each animal was observed for 2 min after DOI injection and drug administrations. The average score was calculated for each rat.
Autonomic activity test
Autonomic activity test was conducted. We connected the animal behavior analysis system with a spontaneous activity video analysis system. The sequence of each group was random. One rat was placed in every autonomic activity box. Before recording, the rat was allowed to adapt to the environment for 5 min. Then, the activity of each rat was recorded for 5 min. We chose the total distance as the objective indicator to judge the autonomic activity of the rat. The box was kept in shade, and the environment was quiet.
Evaluations of inflammatory cytokines in serum and striatum
Rats striatum were carefully dissected out under magnifying glass on ice. The level of IL-1β, IL-6, TNF-α in serum and striatum were detected by enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Nanjing KeyGEN Biotech. CO., LTD., Nanjing, China). The results of the concentrations of inflammatory cytokines were expressed as pictograms per milligram protein.
Western blot
Striatum tissue and primary neuron cultures were homogenized in ice-cold RIPA buffer containing 0.1% phenylmethylsulfonyl fluoride. The total protein content was quantified by Bicinchoninic acid (BCA) protein assay kits (Beyotime, Nanjing, China) Equal amounts of protein were loaded on 8% to 12% SDS-polyacrylamide gel electrophoresis. The transferred PVDF membranes from SDS-polyacrylamide gel electrophoresis were blocked in skim milk at room temperature over 2 h. Then the PVDF membranes were incubated with the appropriate concentration of specific antibodies overnight at 4 °C. On the second day, PVDF membranes were incubated with second antibody at room temperature for 1 h after washing three times by TBST. The immunoreactive bands were interacted with an enhanced chemiluminescence (ECL) kit and visualized on a gel imaging system (Tanon Science & Technology Co., Ltd., China).
Immunohistochemistry
The expressions of TLR4 and p-NF-κBp65 in the striatum were evaluated by immunohistochemistry staining. In brief, the striatum tissues were embedded in paraffin and sectioned. Then, the paraffin sections were deparaffinized in xylene, rehydrated by ethanol and incubated with 3% hydrogen peroxide. Striatum samples were blocked with 3% BSA and incubated with respective primary antibody at 4 °C overnight. After incubated with secondary and three antibodies, samples were stained with DAB and observed under a microscope.
Results
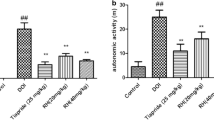
The effects of LPS on stereotypy score
As shown in Fig.1a, Rat model groups with TS induced by DOI showed abnormal stereotypes in different degrees compared with the control group, LPS showed a significant increase compared with the model group.
The effects of LPS on autonomic activity
As shown in Fig.1b, the total distance of rat model groups with TS induced by DOI showed a significant increase compared with the control group, LPS showed a significant increase compared with the model group.
Effects of LPS on inflammatory cytokines in serum and striatum
To investigate inflammatory responses triggered by DOI stimulation, we detected the concentrations of IL-1β, IL-6, TNF-α both in serum and striatum. As expected, the release of inflammatory cytokines IL-1β, IL-6, TNF-α were significantly increased serum and striatum in DOI-treated rats compared with the control group. LPS significantly increased the levels of inflammatory cytokines in serum and stratum induced by DOI exposure compared with the model group (Fig. 2).
Effects of LPS on TLR/NF-κB pathway in striatum
To further investigate the possible mechanisms of LPS, the expressions of TLR/NF-κB pathway were detected in striatum. As shown in Fig. 3, increased levels of TLR4, MyD88, p-NF-κBp65, p-IκBα were observed in DOI rats compared with those in the control group. In contrast, administration of LPS treatment were able to increased inflammation related proteins compared with the model group.
Effects of LPS on TLR4 and P-NF-кB pathway in striatum
The data depicted in Fig. 4. demonstrated the highly expressed TLR and p-NF-κBp65 in DOI-stimulated rats from immunohistochemistry. LPS increased the expressions of TLR4 and p-NF-κBp65.
Effects of LPS on TLR4 and P-NF-кB pathway in striatum. 1:Control; 2: Control + LPS; 3:TS; 4:TS + LPS. The data are expressed as mean values ± SDs. ##p < 0.01 compared with control group, ##p < 0.05 compared with control group; **p < 0.01 compared with model group. *p < 0.05 compared with model group
Discussion
Tourette Syndrome is a childhood-onset, relapsing disorder diagnosed by involuntary motor and phonic tics, with a high comorbidity with obsessive-compulsive disorder. Although up to 0.1 to 1% of the population has been affected by Tourette syndrome, especially males diagnosed more often than females (Editor n.d.), the scientific evidence and research of Tourette syndrome is relative scare compared with other central neural system diseases. The lack of information about Tourette syndrome possibly because this illness is a non-fatal disorder, mostly outgrown in late adolescence (Bos-Veneman et al. 2011). TS has been associated with dysfunctional signaling by the neuromodulator dopamine, which is strongly linked to mechanisms of reinforcement learning. Other biochemical pathways, including histaminergic neurotransmission and amino acid neurotransmission, are likely to be involved TS. In this study (Kanaan et al. 2017), we first shown that lipopolysaccharide (LPS) may played an important role in the recurrence of Tourette syndrome.
It mainly has four duplication methods of the model on TS: intraperitoneal injection iminodipropionitrile (IDPN) to establish the TS rat model (Zhang and Li 2015), intraperitoneal injection amphetamine (AMP) to establish the highly active mouse model (Simmler et al. 2016), intraperitoneal injection apomorphine (APO) to establish the to establish the dopamine receptor excited mouse model (Johnstone et al. 2015) and intraperitoneal injection DOI (the selective 5-HT2A/2C agonist 1-(2, 5-dimethoxy-4-iodophenyl) -2- aminopropane) to establish the head switch response (HTR) rat and mouse model (Schreiber et al. 1995). In this study, the selective 5-HT2A/2C agonist DOI was chosen as the model of TS because it could accurately reflect the behavioural signs of TS and was able to simulate humans better.
Lipopolysaccharides (LPS) is the key component of cell wall on Gram-Negative bacillus. Previous studies have reported that LPS could induce the inflammatory response model (Weng et al. 2017), and inflammation has been suggested to participate in the pathophysiological process during Tourette syndrome (He et al. 2015). In this study, we detected the treatment effect of LPS on DOI induced TS in rat. During our study, LPS significantly increased the abnormal stereotypes on stereotypy score and the total distance on autonomic activity in different degrees compared with the model group. Besides, after treatment with LPS, inflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in serum and striatum were significantly increased (Shen et al. 2017). A considerable amount of literature has been publish on these inflammatory cytokines. TNF-α is produced by mononuclear macrophage, which has the function of immunoregulation and inflammation regulation. IL-1β is regarded as a kind of proinflammatory cytokine, which participate in destruction or edema of tissue. IL-6 possesses various immune regulation functions which can enhance the immune function (Chunhui et al. 2017). Our research indicated that LPS could increase the symptom of DOI.
Finally, we chose western blot to discuss the mechanism of LPS on DOI-induced TS. In this study, we focus on toll-like receptors-mediated signaling pathways, which can directly active the NF-κB signaling pathway. During this signaling transdution process, MyD88 is one of the most important adaptor receptor proteins. The present studies have shown that numerous external stimuli signal could lead to the activation of NF-κB signaling pathway including LPS (Duan et al. 2014). The main function of IκB protein is to prevent the NF-κB protein enter the nucleus and combine with the DNA, which keep the NF-κB protein stay in the cytoplasm. Therefore, the research of IκB protein seems so important when explore the mechanism of NF-κB signaling pathway (Qin et al. 2016). Hence, the western blot analysis indicated that the mechanism of recurrence of Tourette syndrome might be attributed partly to the TLR/MyD88/ NF-κB pathway.
In conclusion, our study indicated that LPS could exert negative effect on DOI-induced Tourette syndrome, which might be attributed to the TLR/MyD88/ NF-κB pathway.
References
Bloch M, State M, Pittenger C (2011) Recent advances in Tourette syndrome. Curr Opin Neurol 24(2):119–125
Bos-Veneman NGP et al (2011) Altered immunoglobulin profiles in children with Tourette syndrome. Brain Behav Immun 25(3):532–538
Chunhui Y et al (2017) Pilose antler peptide protects osteoblasts from inflammatory and oxidative injury through EGF/EGFR signaling. Int J Biol Macromol 99:15
Duan D et al (2014) Activation of the TLR/MyD88/NF-κB signal pathway contributes to changes in IL-4 and IL-12 production in piglet lymphocytes infected with porcine circovirus type 2 in vitro. PLoS One 9(5):e97653
Editor T, (n.d.) Recognition and treatment of Tourette's syndrome. Emerging Infectious Diseases
Feng Z et al (2011) Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol 95(3):373–395
Gan L, Mucke L (2008) Paths of convergence: sirtuins in aging and neurodegeneration. Neuron 58(1):10–14
Giedd JN et al (1996) Case study: acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry 35(7):913–915
He F et al (2015) Association of IL-1α rs17561 and IL-1 RN rs315952 polymorphisms with Tourette syndrome: a family-based study. Int J Clin Exp Pathol 8(4):4182–4185
Jankovic, J., R. Gelineau-Kattner, and A. Davidson, Tourette's syndrome in adults. Movement Disorders Official Journal of the Movement Disorder Society, 2010. 25(13): p. 2171–5
Johnstone DM, et al (2015) Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer's and Parkinson's disease. Front Neurosci, 9(Pt B). doi: 10.3389/fnins.2015.00500
Kanaan AS et al (2017) Pathological glutamatergic neurotransmission in Gilles de la Tourette syndrome. Brain A Journal of Neurology 140(Pt 1):218
Libert S, Guarente L (2012) Metabolic and neuropsychiatric effects of calorie restriction and Sirtuins. Annu Rev Physiol 75(1):669–684
Morer A et al (2010) Elevated expression of MCP-1, IL-2 and PTPR-N in basal ganglia of Tourette syndrome cases. Brain Behav Immun 24(7):1069–1073
Peterson BS, Leckman JF (1998) The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry 44(12):1337–1348
Qin Y, Li H, Qiao J (2016) TLR2/MyD88/NF-κB signalling pathway regulates IL-8 production in porcine alveolar macrophages infected with porcine circovirus 2. J Gen Virol 97(2):445
Schreiber R et al (1995) (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther 273(1):101–112
Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB et al (2010) Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 30(19):4712–4721
Shen B, et al (2017) Picroside II protects rat lung and A549 cell against LPS-induced inflammation by the NF-κB pathway. Inflammation, 1-10
Simmler LD et al (2016) In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther 357(1):134
Weng L et al (2017) Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol 44:1–8
Zhang F, Li A (2015) Dual restoring effects of gastrodin on dopamine in rat models of Tourette’s syndrome. Neurosci Lett 588(1):62–66
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by Program for the Human Resources and Social Security Department of Jiangsu Province–“Six Talent Summit”(WSN-061).
Rights and permissions
About this article
Cite this article
Hongyan, L., Zhenyang, S., Chunyan, W. et al. Lipopolysaccharide aggravated DOI-induced Tourette syndrome: elaboration for recurrence of Tourette syndrome. Metab Brain Dis 32, 1929–1934 (2017). https://doi.org/10.1007/s11011-017-0084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0084-3