Abstract
Tourette syndrome (TS) is a chronic neuropsychiatric disorder with clinical manifestations of involuntary and repeated muscle twitching and vocal twitching. The drugs used to treat TS are relatively limited. The aim of this study was to investigate the effects of rhynchophylline (RH) and the underlying mechanism in 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)–induced neurotoxicity in a TS rat model. A TS model was induced with DOI. The rats were divided into control, TS, TS + tiapride (25 mg/kg), and TS + RH (20 and 40 mg/kg) groups. Behavioral tests were performed 24 h after the last administration by nodding and stereotype experiments. Interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α) levels in striatum and serum were detected with an enzyme-linked immunosorbent assay (ELISA). Western blot analysis was used to detect the expression levels of Toll-like receptor (TLR)/nucleotide-binding domain (NOD)–like receptor protein 3 (NLRP3)/nuclear factor kappa B (NF-κB) signal proteins in the striatum. The expression of TLR2 and NF-κB p65 subunit was detected with immunohistochemical analysis. RH may significantly improve behavioral changes in rats with DOI-induced TS and reduce the levels of inflammatory factors in serum and striatum. RH inhibited the activation of TLR/NLRP3/NF-κB signaling proteins in the striatum of TS rats. In BV2 cells, DOI-induced inflammation mediated through TLR/NLRP3/NF-κB was significantly inhibited following RH administration. The therapeutic effect of RH in TS was studied and its mechanism of action mediated via the TLR/NLRP3/NF-κB pathway was clarified in vitro and in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourette syndrome (TS) is a neuropsychiatric disease common in childhood. It is mainly manifested in the form of an involuntary, repetitive, rapid, and aimless muscle twitch or vocal twitch in one or more parts and may be accompanied with emotional disorders, hyperactivity, inattention, forced movements or thinking, and other behavioral symptoms (Leckman 2002). In recent years, the incidence of TS has increased and is at the peak of age among children. It is mostly observed in men and the ratio of male to female morbidity is 3–4:1. Although this disease is not a critical illness, it is recurrent and difficult to recover from prolonged illness. TS is accompanied with a variety of coexisting diseases, which seriously affect the physical and mental health as well as the growth and development of children (Peterson and Leckman 1998). Patients with TS are often accompanied with behavioral disorders such as behavioral or speech disorders, impulsion, hyperactivity, distraction, and obsessive-compulsive disorder. These symptoms may reduce the function of patients and affect family life and social acceptance (Bloch et al. 2011). Although the etiology of TS has been incompletely understood, it is strongly associated with the genetic components and abnormalities of central inflammation.
Inflammation participates in the pathophysiological process of TS. Recent studies have demonstrated the increase in the level of inflammatory factors such as interleukin (IL)-1β, interferon-γ, and IL-2 in the basal ganglia of patients with TS (Morer et al. 2010). Longitudinal imaging recorded indirect evidence of inflammation of basal ganglia accompanied with acute basal ganglia swelling and obsessive-compulsive disorder (Giedd et al. 1996).
NOD-like receptors (NLRs) patrol cellular cytoplasm. A group of NLRs, including multi-protein complexes called inflammasomes assembled by nucleotide-binding domain (NOD)–like receptor protein (NLRP)-1b, NLRP3, and NLRC4, plays an important role (Gan and Mucke 2008; Libert and Guarente 2012). The assembly of inflammasomes is crucial for the activation of caspase-1. Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature bioactive forms. As IL-1β and IL-18 exert strong inflammatory activities, the expression of these molecules is regulated at various levels. In particular, the activation of inflammasome and the release of biologically active IL-1β and IL-18 require two signals. The first step involves identification of PAMPs through TLRs, such as lipopolysaccharide (LPS), and poly(I:C), to induce upregulation in the expression of inflammatory components and activation of signals necessary for pre-IL-1β expression (Schug et al. 2010; Feng et al. 2011). In the second step, NLRs recognize the cytoplasmic danger signal and induce the assembly of the inflammatory bodies that activate pre-IL-1β and pre-IL-18 (Chen et al. 2005; Ma et al. 2015).
Gambir plant (Gouteng), a traditional Chinese medicinal plant belonging to the family Rubinaceae, has many pharmacological properties. It is mainly used to treat cardiovascular diseases (Yao et al. 2006; Deibel et al. 1996; Gorman et al. 2006) and disorders of the central nervous system, such as dizziness, convulsions, numbness, and hypertension (Peterson et al. 1994; Truong Do et al. 2014; Shin et al. 2013; Zhang et al. 2008). Rhynchophylline (RH) is considered as the most effective pharmacological component in Gouteng. Considering the pharmacological activity of Gouteng, RH is thought to be beneficial for the treatment of TS. Here, we aimed to clarify the mechanism of action and anti-inflammatory activity of RH against TS.
Materials and Methods
Reagents
The selective 5-HT2A/2C receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) was purchased from Sigma-Aldrich (Shanghai, China) and RH was obtained from Tianjin Chase Sun Pharmaceutical Co., Ltd. (Tianjin, China). All antibodies were procured from Cell Signaling Technology (MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-6, IL-1β, and tumor necrosis factor (TNF)-α were purchased from Nanjing KeyGEN Biotech Co., Ltd. (Nanjing, China).
Animals
Fifty male SD rats (8 weeks, 180–200 g) were purchased from Beijing Vital River Co., Ltd. and maintained under specific pathogen-free conditions in GLP laboratories according to the guidelines of the organization. These animals were housed in conventional animal facilities under a constant temperature of about 22–24 °C. The rats had free access to standard water and food particles in a 12-h light/dark cycle environment.
Experimental Protocols
Rats were randomly divided into five groups (10 rats in each group) as follows: control group, DOI treatment group, DOI + tiapride (25 mg/kg) group, and DOI + RH (20 and 40 mg/kg) group. DOI was intraperitoneally injected at 1 mg/kg for 21 days to induce TS in rats. The control group and DOI group were intraperitoneally injected with normal saline or DOI. RH (20 and 40 mg/kg) and tiapride (25 mg/kg) were orally administered for 21 days.
Nodding Experiment
Rats were raised in cages of 42 × 28 × 20. After acclimation to the environment for 3 min, the nodding movements of rats were statistically recorded on day 21. The time was 3 min.
Stereotype Behavior Evaluation
The animals were placed in a large observation cage. The scoring method of stereotype behavior in TS animal model was a double-blinded observation for 1 h and 5 min after the last administration, and the total score was recorded. Scoring criteria and methods were as follows: 0 points, no rigid behavior; 1 point, rotation behavior; 2 points, excessive up and down movement of the head and neck; 3 points, excessive head and neck movement plus rotation; 4 points, head swinging sideways and excessive up and down movement of the head and neck.
Determination of Dopamine in Striatum
Striatum tissue samples were homogenized with 1:3 (g:mL) of normal saline. After centrifugation at 12,000 rpm for 20 min, the supernatant was collected. A total of 100 μL of supernatant was mixed with 10 μL of internal standard solution (2 g/mL, paracetamol). The mixture was deproteinized with 300 μL of acetonitrile, vortexed for 5 min, and centrifuged at 15,000 rpm for 10 min. About 150 μL of water was added to dilute 150 μL of the supernatant. After vortex mixing for 5 s, the mixture was transferred to an autosampler vial, and 10 μL of the sample was injected into a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system for analysis.
Cell Culture
BV2 cells were cultured in a moist incubator in the presence of 95% air and 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic (penicillin/streptomycin).
Cell Viability Assay
Cell viability was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. BV2 cells were treated with different concentrations of RH (1, 2, 4, 8, 16, 32, and 64 μM) for 2 h, followed by incubation with 1 μM DOI for 4 h. The cells were incubated with 20 μL MTT (5 mg/mL) solution for 4 h, and 150 μL dimethyl sulfoxide (DMSO) was used to terminate the reaction. The absorbance value was measured with a microplate spectrophotometer at a test wavelength of 570 nm. The data were expressed as the percentage of the average absorbance of the control. Cell survival rate (%) = (A treatment/A control) × 100%.
Inflammatory Cytokines
The levels of IL-6, IL-1β, and TNF-α in serum, striatum, and cell supernatant were detected with ELISA kits according to the manufacturer’s instructions.
Western Blot Analysis
Striatum tissue and BV2 cells were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing 0.1% phenylmethylsulfonyl fluoride. The bicinchoninic acid (BCA) protein analysis kit (Beyoncé, Nanjing, China) was used to quantify the total protein content. Equal levels of proteins were loaded on 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels. The separated bands were transferred onto polyvinylidene fluoride (PVDF) membranes and the membranes were blocked with skim milk at room temperature for more than 2 h. The PVDF membrane was incubated overnight at 4 °C with an appropriate concentration of a specific antibody. The membranes were washed thrice with TBST and incubated with a secondary antibody at room temperature for 1 h. The protein bands were visualized with an enhanced chemiluminescence (ECL) kit on a gel imaging system (Talon Technology Co., Ltd).
Immunohistochemistry
The expression of TLR2 and p-NF-κB p65 in striatum tissue was detected with an immunohistochemical method. In short, striatum tissue was fixed with 4% paraformaldehyde (PFA), embedded in paraffin, and sliced. Paraffin sections were dewaxed in xylene and absolute ethanol, microwaved in sodium citrate buffer, and washed with phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 min. Each sample was blocked with 5% goat serum for 20 min and treated with primary antibodies against TLR2 (1:1000) or p-NF-κBP65 (1:200) overnight at 4 °C. After incubation, the samples were washed thrice with PBS and treated with a goat anti-rabbit IgG secondary antibody for 20 min, followed by incubation with horseradish peroxidase–labeled streptavidin working solution for 20 min. The samples were washed thrice with PBS and stained with 3–3′ diaminobenzidine (DAB) and hematoxylin. After dehydration and drying, the samples were fixed with neutral glue and observed under a microscope.
Immunofluorescence
The expression of TLR2 and p-NF-κBp65 in BV12 cells was detected by immunofluorescence. Briefly, the cells were washed twice with PBS, fixed with 4% PFA for 30 min, and incubated with 0.5% Triton X-100 in PBS for 5 min. The samples were blocked with 5% bovine serum albumin (BSA) for 1 h and incubated overnight at 4 °C with primary antibodies against TLR2 (1:800) and p-NF-κBP65 (1:200). The samples were washed thrice with PBS and incubated with a goat anti-rabbit IgG secondary antibody conjugated to Alexa Fluor® 488 (1:400) for 1 h. The samples were washed thrice with PBS and stained with DAPI at room temperature followed by washing for 5 min. Images were acquired under a fluorescence microscope.
Results
Effects of RH on Nodding and Stereotype Behavior
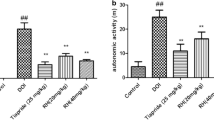
In comparison with the control group, the TS group showed a significant increase in the number of nods as well as in the stereotype behavior scores. The RH (20 and 40 mg/kg) and tiapride (25 mg/kg) groups showed a significant decrease in the number of nods and stereotype behavior scores (Fig. 1).
Effects of RH on Inflammatory Cytokine Levels in Serum, Striatum, and Cell Supernatant
The concentrations of IL-1β, IL-6, and TNF-α in serum, striatum, and cell supernatant were detected. In comparison with the rats from the control group, those from the TS group showed a significant increase in the levels of IL-1β, IL-6, and TNF-α in serum, striatum, and cell supernatant. RH treatment significantly reduced the concentrations of IL-1β, IL-6, and TNF-α in serum, striatum, and cell supernatant induced by DOI exposure (Fig. 2).
Effects of RH on Cell Viability
The effects of RH on the viability of BV2 cells were assayed by MTT assay. Treatment with 0.1–100 μM RH for 24 h had no effect on the viability of BV2 cells, while 200–1000 μM RH remarkably decreased the viability of BV2 cells (Fig. 3a). The MTT results showed that DOI significantly reduced the viability of BV2 cells and increased the cell viability to varying degrees. The results of the MTT assay show that DOI treatment decreased the viability of BV2 cells and RH could reverse this effect (2, 4, and 8 μM) (Fig. 3b).
Effects of RH on TLR/NLRP3/NF-кB Pathway in the Striatum
In comparison with the control group, the TS group showed an increase in the levels of TLR2, myeloid differentiation primary response 88 (MyD88), NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, IL-1β, p-N-κB p65, and p-IκBα (Fig. 4) in the striatum. Treatment with RH (20 and 40 mg/kg) or tiapride (25 mg/kg) reversed this effect on the expression of TLR2, MyD88, NLRP3, ASC, caspase-1, IL-1β, p-N-κB p65, and p-IκBα in the striatum.
Effects of RH on TLR/NLRP3/NF-кB Pathway in BV2 Cells
As shown in Fig. 5, the levels of TLR2, MyD88, NLRP3, ASC, caspase-1, IL-1β, p-N-κB p65, and p-IκBα increased in BV2 cells treated with DOI as compared with the control cells. RH (2, 4, and 8 μM) treatment reduced the levels of TLR2, MyD88, NLRP3, ASC, caspase-1, IL-1β, p-N-κB p65, and p-IκBα in the striatum.
Effects of RH on TLR2 and p-NF-кB p65 Pathway in the Striatum
As shown in Fig. 6, DOI treatment increased the levels of TLR2 and p-NF-κB p65 in the striatum of TS rats as compared with the striatum of control rats. Treatment with RH (20 and 40 mg/kg) reduced the levels of TLR2 and p-NF-κB p65 in the striatum.
Effects of RH on TLR2 and p-NF-кB p65 Pathway in BV2 Cells
In comparison with the control group, DOI-treated groups showed an increase in the levels of TLR2 and p-NF-κB p65 in BV2 cells (Fig. 7). Treatment with RH resulted in a decrease in the levels of TLR2 and p-NF-κB p65 in BV2 cells.
Effects of RH on Dopamine in the Striatum
As shown in Fig. 8, the content of dopamine in striatum increased in DOI-induced TS rats, while RH (20 and 40 mg/kg) and tiapride (25 mg/kg) treatment reduced the content of dopamine in the striatum.
Discussion
TS is related to dopamine signal dysfunction, and dopamine is closely related to learning mechanism. Other biochemical pathways, including histamine neurotransmission and amino acid neurotransmission, may be involved in TS (Zhang et al. 2012; Cox et al. 2016). In this study, we focused on neuroinflammation in a TS rat model. Several studies have reported the pharmacological effects of RH related to the regulation of nervous system diseases. The present study further proves that RH exerts therapeutic effects on TS in vivo and in vitro. The therapeutic advantage of RH may be related to its effects on neuroinflammation, providing further evidence for its clinical application.
Neuroinflammation refers to the activation of immune responses in the brain (Long et al. 2010; Zhang et al. 2010), characterized with the activation of the immune cells, resulting in excessive production of inflammatory cytokines such as IL-6 and TNF-α (Streit 2006; Dobos et al. 2010). Neuroinflammation is involved in central nervous system diseases such as depression, Alzheimer’s disease, and stroke (Chen et al. 2016; Deng et al. 2015a; Zhu et al. 2015; Chen et al. 2015). However, little is known about the inflammatory response of the central nervous system in TS. The lack of data on brain inflammation in patients with TS is likely owing to the ethical restrictions on obtaining cerebrospinal fluid from children (Morer et al. 2010). The results of the present study show that DOI treatment may increase the levels of IL-1β, TNF-α, and IL-6 in serum and striatum, thereby causing brain inflammation. Peripheral and central inflammation were observed during the occurrence and development of TS. The results of the in vitro experiment show that DOI stimulation increases the concentration of inflammatory cytokines and that RH significantly reduced DOI-induced inflammatory cytokine levels in BV2 cells.
TLR pathway is a conservative mechanism that maintains the stability of the cellular environment. TLR may activate NLRP3 to stimulate the inflammatory response and promote the inflammatory response induced by NLRP3. Considering this upstream-downstream relationship, TLR-mediated NLRP3 signal transduction plays an important role in many diseases, especially nervous system diseases. In this study, TLR was shown to be activated in DOI-induced TS, while RH treatment resulted in a significant reduction in the levels of TLR and NLRP3. In BV2 cells, RH treatment restored the expression of TLR and NLRP3. These observations suggest that TLR-mediated NLRP3 signaling may serve as a therapeutic target for TS treatment.
In general, NF-κB is distributed in the cell cytoplasm and binds to the inhibitor IκBα. After activation, IκBα is degraded by IκBα kinase (IKK) complex, which promotes transportation of NF-κB to the nucleus and induces transcription and expression of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α (Rutledge and Adeli 2007; Deng et al. 2015b). The NF-κB signaling regulated by NLRP3 was observed in this study. The results show that RH inhibited the degradation of IκBα. In addition, RH administration inhibited DOI-induced NF-κB p65 nuclear translocation. In BV2 cells, RH inhibited the activation of NF-κB signaling. These observations are consistent with the results reported in previous studies, wherein the response of NLRP3/NF-κB pathway to the stimulus was regulated by TLR.
Thus, RH treatment significantly ameliorated TS caused by DOI, suggestive of its application in TS therapy. RH significantly reduced TS and neuroinflammation in vivo and in vitro. This study expands the scope of new treatment strategies for TS.
References
Bloch M, State M, Pittenger C (2011) Recent advances in Tourette syndrome. Curr Opin Neurol 24:119–125
Chen J, Deng X, Liu N, Li M, Liu B, Fu Q, Qu R, Ma S (2016) Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J Funct Foods 22:463–476
Chen J, Zhou Y, Muellersteiner S, Chen LF, Kwon H, Yi S et al (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374
Chen T, Ma Z, Zhu L, Jiang W, Wei T, Zhou R et al (2015) Suppressing receptor-interacting protein 140: a new sight for salidroside to treat cerebral ischemia. Mol Neurobiol 53:6240–6250 Ahead of Print
Cox JH, Seri S, Cavanna AE (2016) Histaminergic modulation in Tourette syndrome. Expert Opin Orphan Drugs 4(2):205–213
Deibel MA, Ehmann WD, Markesbery WR (1996) Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci 143:137–142
Deng XY, Chen JJ, Li HY, Ma ZQ, Ma SP, Fu Q (2015a) Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem Biol Interact 240:22–28
Deng XY, Li HY, Chen JJ, Li RP, Qu R, Fu Q, Ma SP (2015b) Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res 291:12–19
Dobos N, Korf J, Luiten PGM, Eisel ULM (2010) Neuroinflammation in Alzheimer’s disease and major depression. Biol Psychiatry 67:503–504
Feng Z, Suping W, Li G, Vosler PS, Yanqin G, Zigmond MJ (2011) Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol 95:373–395
Gan L, Mucke L (2008) Paths of convergence: sirtuins in aging and neurodegeneration. Neuron 58:10–14
Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE (1996) Case study: acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry 35:913–915
Gorman DA, Zhu H, Anderson GM, Davies M, Peterson BS (2006) Ferritin levels and their association with regional brain volumes in Tourette's syndrome. Am J Psychiatr 163:1264–1272
Leckman JF (2002) Tourette’s syndrome. Lancet 360:1577–1586
Libert S, Guarente L (2012) Metabolic and neuropsychiatric effects of calorie restriction and sirtuins. Annu Rev Physiol 75:669–684
Long, Hongyan, Zhangpiao, Tan Xuanzhong, (2010) Effect of Jian oral liquid on Tourette’s behavior and content of 5-HT in brain and plasma in rats of Tourette syndrome. Chinese J Basic Med Trad Chinese Med. 16. 8
Ma C-H, Kang L-L, Ren H-M, Zhang D-M, Kong L-D (2015) Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. J Ethnopharmacol 172:108–117
Morer A, Chae W, Henegariu O, Bothwell ALM, Leckman JF, Kawikova I (2010) Elevated expression of MCP-1, IL-2 and PTPR-N in basal ganglia of Tourette syndrome cases. Brain Behav Immun 24:1069–1073
Peterson BS, Gore JC, Riddle MA, Cohen DJ, Leckman JF (1994) Abnormal magnetic resonance imaging T 2 relaxation time asymmetries in Tourette’s syndrome. Psychiatry Res 55:205–221
Peterson BS, Leckman JF (1998) The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry 44:1337–1348
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23
Shin IS, Hong J, Jeon CM, Shin NR, Kwon OK, Kim HS, Kim JC, Oh SR, Ahn KS (2013) Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem Toxicol An Int J Publ Br Ind Biol Res Assoc 62:506–513
Streit WJ (2006) Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci 29:506–510
Truong Do M, Gyun Kim H, Ho Choi J, Gwang Jeong H (2014) Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med 74:21–34
Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X et al (2010) myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 30:4712–4721
Yao Y, Walsh WJ, McGinnis WR, Praticò D (2006) Altered vascular phenotype in autism: correlation with oxidative stress. Arch Neurol 63:1161–1164
Zhang B, Kong Q, Xu H, Tan X (2008) Study on pharmacodynamic of Jing’an oral liquid for treating multiple tics in children. Zhongguo Yaoshi (Wuhan, China) 11:878
Zhang B, Long H-Y, Zhang J-C (2012) Effects of Jing’an Oral liquid on the central neurotransmitter of multiple tics children. Zhongguo Zhong Xi Yi Jie He Za Zhi 32:926–929
Zhang, Piao, Long Hongyan, Xuanzhong Tan, Duan Lingling, (2010) Effect of Jinan oral liquid on dopamine and homovanillic acid in plasma and brain tissue of rats with Tourette syndrome. Chinese J Exp Trad Med Formulae. 16.1
Zhu L, Wei T, Gao J, Chang X, He H, Luo F, Zhou R, Ma C, Liu Y, Yan T (2015) The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis 20:1433–1443
Grant Support
This work was supported by the National Natural Science Foundation of China (81774364).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hongyan, L., Mengjiao, Z., Chunyan, W. et al. Rhynchophylline Attenuates Neurotoxicity in Tourette Syndrome Rats. Neurotox Res 36, 679–687 (2019). https://doi.org/10.1007/s12640-019-00059-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00059-1