Abstract
Autism research continues to receive considerable attention as the options for successful management are limited. The understanding of the autism spectrum disorder (ASD) etiology has now progressed to encompass genetic, epigenetic, neurological, hormonal, and environmental factors that affect outcomes for patients with ASD. Glycosaminoglycans (GAGs) are a family of linear, sulfated polysaccharides that are associated with central nervous system (CNS) development, maintenance, and disorders. Proteoglycans (PG) regulate diverse functions in the central nervous system. Heparan sulfate (HS) and chondroitin sulfate (CS) are two major GAGs present in the PGs of the CNS. As neuroscience advances, biochemical treatments to correct brain chemistry become better defined. Nutrient therapy can be very potent and has minimal to no side effects, since no molecules foreign to the body are needed. Given GAGs are involved in several neurological functions, and that its level can be somewhat modulated by the diet, the present study aimed to evaluate the role of GAGs levels in ASD symptoms. Both tGAG and its different fractions were evaluated in the urine of ASD and healthy control childrens. As levels differed between groups, a second trial was conduted evaluating if diet could reduce tGAG levels and if this in turn decrease ASD symptoms. The present study found that tGAG concentration was significantly higher in the urine of children with ASD compared to healthy control children and this was also evident in all GAG fractions. Within groups (controls and ASD), no gender differences in GAG excretion were found. The use of a 90 days elimination diet (casein-free, special carbohydrates, multivitamin/mineral supplement), had major effects in reducing urinary tGAG excretion in children with ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a heterogeneous group of behaviorally defined neurodevelopmental disorders. This group of disorders is presented from birth or very early during development and affect essential human behaviours such as imagination, the ability to communicate ideas and feelings, social interaction, and the establishment of relationships with others (Bjørklund 2013). Although there is strong evidence for genetic factors in ASD, it appears to be a polygenic condition (Betancur 2011), and also responsive to a range of environmental events. Although, its precise etiology is unknown, ASD is clearly a complex human genetic disorder that involves interactions between genes and environment (Freitag et al. 2010; Li et al. 2014). Future integrative epigenomic analyses of genetic risk factors for environmental exposures and methylome analyses are expected to be important for understanding the complex etiology of ASD (LaSalle 2014).
Autism was once considered as a rare disorder, however, the reported incidence and prevalence have drastically increased during the last decades. Although, this estimate could be affected by a change in diagnosis, increased awareness, new therapeutic options and specific approaches to the education of children with ASD (Macedoni-Lukšič et al. 2015), today the prevalence of ASD in the US is estimated to be at least 1 in 88 births (CDC 2012; Blumberg et al. 2013).
Nutrition affects multiple aspects of neuroscience including neurodevelopment, neurogenesis and functions of neurons, synapses and neural networks in specific brain regions. Nutrition-gene interactions play a critical role in these responses, leading in turn to major effects on brain health, dysfunction, and disease. Individual differences in multiple gene variants, including mutations, single nucleotide polymorphism (SNPs) and copy number variants (CNVs), significantly modify the effects of nutrition on gene expression. A further layer of regulation is added by differences in the epigenome, and nutrition is one of many epigenetic regulators that can modify gene expression without changes in DNA sequence (Dauncey 2013).
Epigenetic marks are modifications of DNA and histones (Jalili et al. 2013). They are considered to be permanent within a single cell during development and are hereditable across cell division. Programming of neurons through epigenetic mechanisms is believed to be critical in neural development. Mutation of genes encoding proteins that translate epigenetic markings (MBDs) may lead to ASD. Copy number variations, which are identified in a significant percentage of patients with developmental disorders, may also lead to alterations in the fine structure of the brain through epigenetic effects (as illustrated by maternal 15q duplication in the regulation of specific candidate genes that are critical for brain development). Better understanding of epigenetic mechanisms in ASD and neurodevelopmental disorders will ultimately help in the development of novel treatment options (Rangasamy et al. 2013).
Heparan sulfate (HS) regulates diverse cell-surface signaling events, and its roles in the development of the nervous system recently have been increasingly uncovered by studies using genetic models carrying mutations of genes encoding enzymes for its synthesis. Roles of HS in neural development have been studied in animal models carrying mutations in Ext1 and other genes encoding enzymes involved in HS synthesis. These genetic studies revealed that HS is necessary for the specific functioning of certain brain structures, such as the cerebellum and the olfactory bulbs, cortical neurogenesis, and a variety of axon path-finding processes (Kantor et al. 2004). Pearson et al. (2013) suggest that aberrant function extracellular matrix glycosaminoglycan (GAG) localized to the subventricular zone of the lateral ventricles may be a biomarker for ASD, and potentially involved in the etiology of the disorder.
Considering that several GAG play an active role in some areas of the brain and that the extracellular matrix function of GAG in the lateral ventricles has been proposed as an ASD biomarker, the present study aimed to: 1) evaluate potential differences in GAG levels in ASD patients compared to control subjects, and 2) explore the potential role of diet on GAG modulation, and its effects on ASD symptoms. We hypothesized that GAG levels will differ between ASD and control patients and that diet play a major role in regulating GAG levels. These findings may have important implications for ASD patients and their treatment.
Materials and methods
Patients and sampling
The present study was approved by the local ethics committee (Jósa András County Hospital, Nyíregyháza, Hungary), and informed consistent was obtained from the parents of each child.
A total of 106 children (26 females and 80 males; range 2–14 years) with a diagnosis of ASD were recruited for this study from the Pediatric Department at Jósa András County Hospital. Each child was evaluated to ensure that they met the criteria for autistic disorder based on the American Psychiatric Association (APA 1994). Their diagnosis was also confirmed using the Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994). None of the children with ASD had any additional medical conditions such as epilepsy, cerebral palsy or motor deficiencies.
Thirty one (31) age-matched controls (seven females and 27 males; range 2–14 years) from kindergarten and elementary schools in Eastern Hungary were also included in the present study. These children were recruited to the study by physicians and other healthcare professionals, and were examined to ensure that they didn’t had any neurological or neurodevelopmental issues.
GAG (total and fractions) analytical methods
Urine samples were collected to quantify total glycosaminoglycan (tGAG). Since some preservatives may interfere with analyses (Di Ferrante 1967), samples were quickly frozen after collection and kept frozen until analysis (including transport to the laboratory).
Total glycosaminoglycan was measured as the amount of Alcian Blue precipitated from 50 μl of urine at pH 5.8 in the presence of 50 mmol l−1 MgCl2 using the technique described by Whiteman (1973). In order to determine the percentage ratio of the different aGAG (acid glycosaminoglycan) fractions, the method of Di Ferrante (1967) was used with minor modifications (Endreffy and Dicsö 1988). Urine samples were precipitated with CPC (N-cetylpyridinium chloride) at pH 4.0, stabilized (so that timing of the reaction is less critical) and stored at 4 °C until analyzed (Endreffy and Dicsö 1988). The aGAG reference standard solutions (HA = hyaluronic acid, HS = heparan sulfate, Co-4/6-S = chondroitin-4/6-sulfate, DS = dermatan sulfate, KS = keratan sulfate, and UA = uronic acid) were obtained from the Pediatric Clinics of Pécs and Szeged Medical Universities, Hungary.
Twenty milliliter first-morning urine sample was mixed with 0.5 ml 5 % (v/w) aqueous CPC, and acetic acid 10 % (v/w) was then added at pH 4. Samples were then incubated at 25 °C for 4 h and then at 4 °C for 12 h. At the end of the incubation, samples were centrifuged at 8000xg for 10 min. All supernatant was removed, and the pellet was washed with alcohol (96 % v/w) twice. Samples were centrifuged at 8,000xg for 10 min and the excess alcohol removed. This second precipitate was dissolved in 2 ml of 0.6 M NaCl, following centrifugation at 8,000xg for 10 min. 2 ml of this supernatant was treated with 8 ml of absolute alcohol and stored at 4 °C for 12 h. The sample was subsequently centrifuged at 8,000xg for 10 min, and the resulting precipitate dissolved in 0.1 ml 0.6 M NaCl. GAG fractions without hyaluronidase digestion were then quantified in 30 μl. Samples were placed on a thin layer of silica gel, and the plates were developed at 23 °C for 90 min in a n-propanol, ammonium, water solvent, at 40: 60: 5 (v:v:v).
GAG fractions were also quantified following hyaluronidase digestion. Briefly, 10 μg hyaluronidase (1000 USP/U/mg) was added to the aGAG test (Kieselgel 60, Merck), incubated at 37 °C for 6 h and centrifuged at 8,000xg for 10 min. 30 μl of the supernatant was placed on a thin layer of silica gel and was developed at 25 °C as above.
The dried plate was sprayed with a 2 % (v/w) orcinol ethyl acetate solution, dried, and sprayed with 20 % (v/v) sulfuric acid. The plate was kept at 100 °C until the appearance of the HS blue, DS and KS violet spots. Hyaluronidase digests hyaluronic acid and chondroitin-4/6-sulfate, the spots were then quantitated by densitometry (Clinisan-2, Philips).
Elimination diet
A second experiment was conducted to test if GAG (total or fractions) excretion could be modulated by the diet. Twenty ASD patients aging between 3 and 11 years were selected to follow a special diet for 3 months. The diet was adjusted to suit the taste and habits of the family, and to avoid any foods suspected of causing detrimental consequences for patient’s appetite, palatability or dislike. A control group of 6 ASD patients with similar characteristics was maintained with a free diet during the same 3 month period. No patient was receiving stimulant medication or antipsychotic drugs. The study began with an initial physical evaluation conducted by a physician to determine that the children were in reasonable physical health. Twenty children took the elimination diet and supplement for 3 months (lactose-free and specific carbohydrate diet, vitamins, minerals, and omega-3 oils). On the last day of the 3 month period on the elimination and free diet, the mothers of the patients evaluated qualitative changes in sleep patterns, gastrointestinal symptoms, receptive language, general behaviour, eye contact, expressive language, and sociability after the study (Table 2). Urine samples for tGAG analysis were taken, handled, and analyzed as described before, at the beginning and at the end of the 3 month period for each single patient.
Statistical analysis
Values are presented as mean ± SEM. Potential differences in the tGAG excretion rates and their different fractions between ASD patients and controls were tested by a t-test. The potential effect of the 3 month restriction and free diets, in ASD patients, on tGAG excretion rates was tested by paired t-tests within each group (restriction and free diets). Normal distribution and homogeneity of variances were checked before any further analysis, and all meet these assumptions. Differences were considered significant with a p-value less than 0.05).
Results
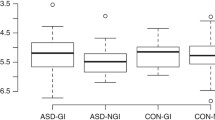
The present study found a significantly elevated tGAG excretion in 106 ASD affected children compared to 31 controls (Fig. 1). The mean value of the excretion in the patients was: 183.3 ± 5.9 mg tGAG g creatinine−1, 95 % confidence interval 132.7 to 176.5. On the other hand 28.71 ± 3.6 mg tGAG g creatinine−1 excretion was found in the controls. The main GAG fractions were: heparan sulfate and chondroitin-4/6-sulfate. But the excretions of all GAG fractions were significantly increased in the patients with ASD (Fig. 2). There was no significant difference between ASD affected boys and girls in excretion of tGAG and GAG fractions (Table 1).
Nutrients and ASD: dietary factors
Patients that followed the elimination diet (lactose-free, special carbohydrates, multivitamin/mineral supplement), occupational therapy and adaptive sports, showed significant reduction in urinary tGAG excretion compared to the beginning of the experiment (p < 0.0001; Fig. 3). Before the elimination diet, ASD patients showed an average tGAG excretion of 203.7 ± 12.7, a value that decreased to 106.8 ± 12.8 after 3 months on an elimination diet. tGAG excretion rate remained unchanged in the control group (free diet) after the 3 months period, with an average value of 205.0 ± 22.3 (p = 0.095, Fig. 3).
Results from the parent’s evaluation of several behavioral and physical changes after the elimination diet showed minor improvements after the 3 month period (Table 2).
Discussion
Epigenetic mechanisms such as DNA methylation may be able to explain some of the variability observed with both environmental and genetic risk factors in ASD (Rangasamy et al. 2013). Epigenetic modifications to nucleotides or chromatin provide long-lived effects on gene expression and phenotype without modifying the DNA sequence. Epigenetic mechanisms such as DNA methylation can be altered by environmental changes (Dolinoy et al. 2007; Jirtle and Skinner 2007). Several environmental exposures have been correlated with reduced global DNA methylation in humans (James et al. 2004; Dolinoy et al. 2007; Rusiecki et al. 2008). Deficiencies in methylation and oxidative stress pathways have been implicated in ASD (James et al. 2004).
Sokol et al. (2006) reported a high level of total plasma amyloid-β precursor protein (sAPP), including sAPPα, in a small sample of young children with severe ASD and aggression. These children presented sAPP levels ≥ 2 fold higher than those of children without ASD.
APP is a large (695–770 amino acid) glycoprotein produced in brain microglia, astrocytes, oligodendrocytes, and neurons. Proliferation, migration, differentiation, myelination, and synaptogenesis are all steps involved in neuronal development and maturation (Ray et al. 2011). Ray et al. (2011) showed that levels of sAPPα, sAPPβ (proteolytic cleavage products of APP by α- and β-secretase, respectively) are unbalanced in ASD and may contribute to an anabolic environment leading to brain overgrowth.
The Aβ-amyloid fibrils are composed predominantly of fragments of a larger transmembrane glycoprotein, the β-amyloid precursor protein (βAPP). The molecular modification in βAPP and tau that give rise to the isoforms found in the Aβ-amyloid fibrils and paired helical filaments remain unknown (Pérez et al. 1998). The demonstration that GAGs induced the polymerization of full-length tau under physiological conditions follows on from earlier studies reporting on the effects of these sulfated polysaccharides on the polymerization of βAPP fragments and suggests that GAGs may play a specific role in the amyloidosis seen in Alzheimer’s disease (AD) (Pérez et al. 1998).
Analysis of this neuroprotective effect of the sulfated glycans revealed different efficiencies, with heparan sulfate (HS) being the most effective, followed by keratan sulfate (KS) and then the chondroitin sulfates (Co-4/6-S and DS). The role of GAGs has also been suggested in neural development or regeneration. It has also been indicated that aberrant extracellular matrix GAG function localized to the subventricular zone of the lateral ventricles could be used as a biomarker for ASD, and potentially involved in the etiology of the disorder (Pearson et al. 2013).
There have been several studies of the nutritional and metabolic status of children with ASD, but each focused on the study of only a few biomarkers. The studies have demonstrated that children with ASD have impaired methylation, decreased glutathione and increased oxidative stress, and those studies demonstrated that nutritional supplementation with methylcobalamin, folinic acid, and trimethylglycine is beneficial (James et al. 2004, 2006, 2009). Many significant differences were observed in the ASD group compared to the neurotypical group, including low levels of biotin, plasma glutathione, plasma uridine, plasma ATP, red blood cell NADH, plasma sulfate (free and total), and plasma tryptophan (Chauhan et al. 2004; Adams et al. 2011).
Alterations in GAGs have been reported in a variety of neurological disorders, including ASD, epilepsy, Parkinson’s disease and schizophrenia. The present results suggest that GAG excretion could be relevant to interpreting and directing future investigations into the elusive etiology of ASD. The sulfate-dependent developmental process affects neurons in brain regions whose function appears to be intrinsic to the disabilities found in ASD. Sulfate is also necessary for the integrity of the gastrointestinal lining.
Levels between 30 and 60 mg GAG g creatinine−1 of acid glycosaminoglycan-uria are considered normal, while levels of 100–200 mg GAG g creatinine−1 have been classified as mildly elevated. Our results confirm and agree with previous findings, as our control group presented levels of 28.71 ± 3.6 mg tGAG g creatinine−1, while the ASD group presented 6.4 fold higher levels of tGAG than the control group (183.3 ± 5.9 mg tGAG g creatinine-1). This is in agreement with what is considered mildly elevated.
Research using knockout of genes involved in HS biosynthesis have provided more physiologically relevant information about the role of HS in mammalian neural development (Yamaguchi et al. 2010). To define the role of HS in brain physiology, were generated conditional Ext1-knockout mice targeted to postnatal neurons. These conditional Ext1 mutant mice normally develop without any detectable morphological changes in the brain. Remarkably, these mice recapitulate numerous autism-like behavioral phenotypes encompassing the three core deficits of ASD. Results from electrophysiological analyses indicate that removal of HS compromises glutamatergic synaptic transmission by affecting the synaptic localization of AMPA receptors. These results demonstrate that HS is required for normal functioning of glutamatergic synapses. Moreover, the development of several autism-like deficits in these mice suggests that the cellular and molecular conditions resulting from the elimination of neuronal HS recapitulate critical parts of the pathogenic mechanisms of ASD (Irie et al. 2012). These studies implicate that a decrease in N-sulfated HS in specific regions of the brain could be contributing to the etiology of ASD and may serve as a biomarker for the disorder.
The excretion of GAG, as detected by thin layer chromatography, before and after special diet in ASD children is evidence for the role of GAGs in ASD (Yamaguchi et al. 2010; Irie et al. 2012). In the present study, the excretion of GAG in children with ASD was ≥3 folds higher than that of the healthy control children.
References
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S, Barnhouse S, Lee W (2011) Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond) 8:34. doi:10.1186/1743-7075-8-34
APA - American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. American Psychiatric Association, Washington
Betancur C (2011) Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380:42–77. doi:10.1016/j.brainres.2010.11.078
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73:225–236
Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR (2013) Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. National health statistics reports; no 65. National Center for Health Statistics, Hyattsville
CDC - Centers for Disease Control and Prevention (2012) Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ 61(3):1–19
Chauhan A, Chauhan V, Brown WT, Cohen I (2004) Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci 75:2539–2549
Dauncey MJ (2013) Genomic and epigenomic insights into nutrition and brain disorders. Nutrients 5:887–914. doi:10.3390/nu5030887
Di Ferrante NM (1967) The measurement of urinary mucopolysaccharides. Anal Biochem 21:98–106
Dolinoy DC, Weidman JR, Jirtle RL (2007) Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol 23:297–307
Endreffy I, Dicsö F (1988) Glycosaminoglycan excretion in connective tissue diseases. Clin Biochem 21:135–138
Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R (2010) Genetics of autistic disorders: review and clinical implications. Eur Child Adolesc Psychiatry 19:169–78. doi:10.1007/s00787-009-0076-x
Irie F, Badie-Mahdavi H, Yamaguchi Y (2012) Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Acad Sci U S A 109:5052–5056. doi:10.1073/pnas.1117881109
Jalili M, Pati S, Rath B, Bjørklund G, Singh RB (2013) Effect of diet and nutrients on molecular mechanism of gene expression mediated by nuclear receptor and epigenetic modulation. Open Nutraceuticals J 6:27–34. doi:10.2174/1876396020130419002
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80:1611–1617
James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW (2006) Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 141B:947–956
James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, Hubanks A, Gaylor DW (2009) Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr 89:425–430. doi:10.3945/ajcn.2008.26615
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8:253–262
Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL (2004) Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44:961–975
LaSalle JM (2014) Epigenomic strategies at the interface of genetic and environmental risk factors for autism. J Hum Genet 58:396–401. doi:10.1038/jhg.2013.49
Li SO, Wang JL, Bjørklund G, Zhao WN, Yin CH (2014) Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 25:1216–1220. doi:10.1097/WNR.0000000000000251
Lord C, Rutter M, Le Couteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685
Macedoni-Lukšič M, Gosar D, Bjørklund G, Oražem J, Kodrič J, Lešnik-Musek P, Zupančič M, France-Štiglic A, Sešek-Briški A, Neubauer D, Osredkar J (2015) Levels of metals in the blood and specific porphyrins in the urine in children with autism spectrum disorders. Biol Trace Elem Res 163:2–10. doi:10.1007/s12011-014-0121-6
Pearson BL, Corley MJ, Vasconcellos A, Blanchard DC, Blanchard RJ (2013) Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav Brain Res 243:138–145. doi:10.1016/j.bbr.2012.12.062
Pérez M, Wandosell F, Colaço C, Avila J (1998) Sulphated glycosaminoglycans prevent the neurotoxicity of a human prion protein fragment. Biochem J 335(Pt 2):369–374
Rangasamy S, D’Mello SR, Narayanan V (2013) Epigenetics, autism spectrum, and neurodevelopmental disorders. Neurotherapeutics 10:742–756. doi:10.1007/s13311-013-0227-0
Ray B, Long JM, Sokol DK, Lahiri DK (2011) Increased secreted amyloid precursor protein-α (sAPPα) in severe autism: proposal of a specific, anabolic pathway and putative biomarker. PLoS One 6, e20405. doi:10.1371/journal.pone.0020405
Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC (2008) Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect 116:1547–1552. doi:10.1289/ehp.11338
Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, Zimmer JA, Lahiri DK (2006) High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J Child Neurol 21:444–449
Whiteman P (1973) The quantitative determination of glycosaminoglycans in urine with Alcian Blue 8GX. Biochem J 131:351–357
Yamaguchi Y, Inatani M, Matsumoto Y, Ogawa J, Irie F (2010) Roles of heparan sulfate in mammalian brain development current views based on the findings from Ext1 conditional knockout studies. Prog Mol Biol Transl Sci 93:133–152. doi:10.1016/S1877-1173(10)93007-X
Acknowledgments
The authors thank the children with ASD and their parents for their consensus, collaboration, and hard work as participants in this research study. Further, the physicians Denes Kovendi and Eva Satorhegyi are thanked for help with diagnosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Endreffy, I., Bjørklund, G., Dicső, F. et al. Acid glycosaminoglycan (aGAG) excretion is increased in children with autism spectrum disorder, and it can be controlled by diet. Metab Brain Dis 31, 273–278 (2016). https://doi.org/10.1007/s11011-015-9745-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9745-2