Abstract
Accumulating data have revealed the pivotal function of tripartite motif protein 38 (TRIM38) in tumors. In view of this, this investigation aims to explore the function and potential mechanism of TRIM38 in non-small cell lung cancer (NSCLC). A xenotypic tumor model was established in vivo by subcutaneously injecting NSCLC cells (2 × 106 cells) in tail vein of each mouse. Relative expression of TRIM38 mRNA was detected via quantitative real-time polymerase chain reaction (qRT-PCR). For exploring the role of TRIM38 in vivo and in vitro, mice or NSCLC cells were divided into two groups: the vector group and the TRIM38 overexpression group. Also, protein expression levels of TRIM38, Vimentin, E-cadherin, and N-cadherin were determined using western blotting and immunohistochemistry staining. Tumor nodules of mouse lung tissues were assessed via performing H&E staining. Moreover, proliferation of NSCLC cells was evaluated through colony formation and CCK-8 assays. Further, migration and invasion of NSCLC cells were assessed through wound healing and transwell assays. Protein levels of pathway-related proteins including p-p65, p65, IκB, p-IκB, p-AMPK, AMPK, and NLRP3 were examined through western blotting analysis. Tumor lung tissues of mice and NSCLC cells showed low protein and mRNA expression of TRIM38. Functionally, up-regulation of TRIM38 reduced the number of tumor nodules and suppressed epithelial-to-mesenchymal transition (EMT) in lung tissues of mice. Furthermore, up-regulation of TRIM38 in NSCLC cells inhibited migration, invasion, EMT, and proliferation. With respect to the mechanism, in vivo experiments, the inhibitory effects of TRIM38 overexpression on tumor nodules, and EMT were reversed by AMPK inhibitor. In vitro experiments, TRIM38 overexpression caused down-regulation of p-IκB and p-p65 as well as up-regulation of p-AMPK. The inhibitory effects of TRIM38 overexpression on migration, proliferation, invasion, and EMT of NSCLC cells were reversed by overexpression of NLRP3. Concurrently, AMPK inhibitor enhanced the TRIM38-overexpressed NSCLC cell’s abilities in migration, clone formation, invasion, and proliferation. TRIM38 regulated the AMPK/NF-κB/NLRP3 pathway to suppress the NSCLC’s progression and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer has emerged as a leading cause of death worldwide, in which non-small cell lung cancer (NSCLC) is an important subtype [1, 2]. Currently, NSCLC therapy is challenged by the fact that more than 70% patients have been diagnosed at advanced stages, with a 5-year overall survival rate of less than 5% [3, 4]. Although advances have been made in chemotherapy and molecular targeted therapy [5,6,7], the survival time of NSCLC patients is still limited [8]. Therefore, it is important to explore NSCLC’s pathogenesis and identify potential targets for NSCLC treatment.
Tripartite motif protein 38 (TRIM38) refers to ubiquitin ligase E3, with PRY-SPRY domain, B box-type zinc finger, and RING-type zinc finger [9, 10]. It is a member of TRIM protein family that plays pivotal part in many biological functions such as cardiac fibrosis, degeneration of chondrocytes, innate immunity, and inflammation response [11,12,13]. Recently, TRIM38 is reported to be one of the tumor microenvironment-related factors, which has the ability to repress the progression of bladder cancer [14, 15]. More importantly, data from gene expression profiling sequencing have showed that TRIM38 expression is decreased in dermatomyositis patients [16], and dermatomyositis is identified as an independent risk factor in lung cancer [17, 18]. Current findings suggest that TRIM38 may have connection with NSCLC’s occurrence.
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a common transcription factor, is crucial for modulating inflammation and immunity [19,20,21]. NOD-like receptor pyrin domain-containing 3 (NLRP3) is a classical downstream inflammatory complex of NF-κB, which has been proved to promote inflammasome development and affect pathogenesis of breast cancer and lung cancer [22,23,24,25]. Also, NSCLC metastasis is associated with activation of the NF-κB/NLRP3 pathway [26, 27]. Adenosine monophosphate-activated protein kinase (AMPK) is an upstream negative regulator for NF-κB, which can inactivate NF-κB [28, 29] and AMPK activation is related to the suppression of NSCLC cell’s growth [30]. These findings propose the possible implication of the AMPK/NF-κB/NLRP3 pathway in NSCLC. In addition, TRIM38 is demonstrated to negatively regulate NF-κB in many cells such as chondrocytes, cardiomyocytes, and osteoclasts [12, 31, 32]. Meantime, a study has reported that TRIM38 promotes downstream glucose transporter-1 (GLUT1) degradation of AMPK in bladder cancer [15], suggesting that AMPK may be a potential mechanism of TRIM38 in regulating NF-κB activity. Thus, we speculated that TRIM38 may inhibit NF-κB/NLRP3 activity by activating AMPK, thereby controlling the progression of NSCLC.
Hereon, we intended to explore the function of TRIM38 in NSCLC and related molecular mechanisms. This study may lay theoretical basis foundation for NSCLC treatment via molecular therapy.
Methods
Cell culture techniques
Human NSCLC cell lines (HCC827, H1299, A549, H460, H1975, and H1650) and a BEAS-2B cell line derived from mouse bronchiolar epithelial cells were bought from the Academy of Sciences Cell Bank (Beijing, China). Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS) and 1% solution of antibiotic (penicillin and streptomycin) was used to culture NSCLC cells. For culturing BEAS-2B cells, Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS was used. All cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Transfection and treatment of cells
Recombinant lentivirus for overexpressing TRIM38 or NLRP3 along with corresponding empty vector was bought from GenePharma (Shanghai, China). H1299 cells at a density of 3 × 106 cells were plated into a 6-cm dish, and further transfected with 2 μg TRIM38 overexpression plasmid, 2 μg NLRP3 overexpression plasmid, and corresponding empty vectors through Lipofectamine 3000 (Invitrogen, Carlsbad, California, USA). Following 48 h of transfection, partially transfected cells were treated with compound C (AMPK inhibitor; Selleck Chemicals, Houston, TX, USA) in concentration of 3 μM for 24 h [33].
In vivo tumorigenesis assay
BALB/c nude mice (male, 4–5-week-old) were bought from Shanghai Laboratory Animals Co., Ltd. (Shanghai, China). All mice were housed in controlled condition (a 12-h dark and 12-h light period of cycle with 60% humidity at 22–24 °C), and had free access to water and food. Animal experiments were performed in accordance with Institutional Animal Care and Use Committee’s guidelines from Beijing Viewsolid Biotechnology Co. LTD (VS212601456).
To determine the expression of TRIM38 in NSCLC in vivo, H1299 cells (2 × 106 cells suspended in 200 μL of PBS) or 200 μL PBS (as a control) were injected in each mouse via tail vein. Mice were divided into two groups (n = 6/group): the normal group and the tumor group. After 56 days, mice were anesthetized with sodium pentobarbital (50 mg/kg), followed by euthanizing them through cervical dislocation. Lung tissues of mice were collected and preserved for further analysis.
To explore the influence and mechanism of TRIM38 in NSCLC in vivo, H1299 cells (2 × 106 cells) transfected with TRIM38 overexpression lentivirus or empty vector lentivirus were injected each mouse with or without intraperitoneal injection of the AMPK inhibitor compound C (Selleckchem Chemicals, 20 mg/kg/day) via tail vein. Mice were divided into three groups (n = 6): the empty vector, the TRIM38 overexpression group, and the TRIM38 + Compound C group. After 56 days, mice were anesthetized and euthanized as mentioned above. The lung tissues were collected and preserved for further analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Lung tissues of mice were used to RNA extraction via Trizol (Sangon Biotech, Shanghai, China). Complementary DNA (cDNA) was synthesized via reverse transcription based on protocols of a PrimeScript™ RT reagent Kit (Takara, Beijing, China). In 20 μL reaction mixture containing 2 μL cDNA, PCR amplification was conducted using SYBR® Premix Ex Taq™ II (Takara). Amplification steps were displayed as follows: 1 min of 98 °C, 10 s of 98 °C with 30 cycles, 15 s of 55 °C, and 1 min of 72 °C. Amplification primers were provided in Table 1. Relative mRNA expression of TRIM38 normalized to GAPDH was calculated with the 2−ΔΔct method.
Western blotting
RIPA lysis buffer (Solarbio, Beijing, China) was used for protein extraction. A BCA kit (Pierce, Waltham, MA, USA) was used to measure the protein concentration. Subsequently, 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate 50 μg protein samples, followed by transferring on polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, USA). After being blocked with 5% nonfat milk, the membranes were incubated with primary antibodies at 4°Covernight, including anti-TRIM38 (1:200, MA5-26235, Invitrogen), anti-E-cadherin (1:5000, ab40772, Abcam, Cambridge, CA, USA), anti-N-cadherin (1:5000, ab76011, Abcam), anti-NLRP3 (1:1000, ab263899, Abcam), anti-Vimentin (1:2000, ab92547, Abcam), anti-IκB (1:5000, ab32518, Abcam), anti-p-IκB (1:1000, ab92685, Abcam), anti-p65 (1:2000, ab32536, Abcam), anti-p-p65 (1:1000, ab76302, Abcam), anti-AMPK (1:1000, ab32047, Abcam), and β-actin (1:1000, ab8224, Abcam). Then the membranes were washed using Tween-20 + Tris-Buffered Saline (TBST), followed by incubation with the anti-rabbit secondary antibody (1:2000, ab6721, Abcam). Finally, visualization of protein bands was obtained by enhanced chemiluminescence (ECL) kit (TFS).
Immunohistochemistry (IHC) staining
Protein expression of TRIM38, Vimentin, E-cadherin, and N-cadherin in lung tissues was examined via IHC staining. Lung tissues fixed with 10% formalin were embedded using paraffin, cut into 4 μm sections, and dewaxed using xylene, followed by rehydration using alcohol. After antigen repair and block of nonspecific sites were performed, these sections were incubated with primary antibodies overnight at 4 °C. Primary antibodies were listed as follows: anti-TRIM38 (1:200, PA5-83,204, Invitrogen), anti-N-cadherin (1:500, ab76011, Abcam), anti-E-cadherin (1:500, ab40772, Abcam), and anti-Vimentin (1:200, ab92547, Abcam). Next, the horseradish anti-rabbit secondary antibody (1:500, ab6112, Abcam) was added to incubate for 30 min, and the diaminobenzidine substrate solution was added to these sections. At last, hematoxylin was used to stain these sections and staining areas were observed through a microscope (Olympus, Tokyo, Japan).
Hematoxylin–eosin (H&E) staining
The tumor nodules of mouse lung tissues were determined via H&E staining. Lung tissues of mice were exposed to paraformaldehyde (4%) at 4 °C overnight and paraffin-embedded. Subsequently, the sections with embedded paraffin were cut into 5 μm slices followed by graded ethanol mediated deparaffinization. After H&E staining, dehydration was performed using xylene and graded ethanol, and these sections were observed under a microscope.
Cell counting Kit-8 (CCK-8) assay
The viability of cells was assessed via a proliferation detection CCK-8 kit (Dojindo, Kumamoto, Japan). In brief, cells (5 × 103 cells/well) were seeded in 96-well plates, followed by incubation at 37 °C for 0 h, 24 h, 48 h, and 72 h. Afterward, each well was supplemented with 10 µL CCK-8 reagent to culture for additional 2 h. Finally, a microplate reader was used to measure the optical density at 450 nm.
Colony formation assay
The cells in RPMI-1640 medium were re-suspended to 1 × 104 cells/mL concentration, and were plated in a 6-well plate with 2 × 103 cells per well. After that, cells were cultured at 37 °C for 14 days, followed by fixing and staining for 30 min using paraformaldehyde and crystal violet, respectively. Then the number of colonies in each well was counted and photographed. When the number of cells reached at least 50, it is considered as a countable colony.
Wound healing assay
Cells (3 × 105 cells/well) in 6-well plates were routinely cultured in RPMI-1640 medium containing 10% FBS. When the monolayer of cell was formed, scratches were made using a pipette tip and the relative length of the scratches was recorded. After being washed with PBS, these cells were cultured for 24 h using serum-free medium. To measure the wound healing distance, cells were observed and photographed using an inverted microscope. The migration rate was calculated by the formula: 100 × (scratch width at 24 h/scratch width at 0 h).
Transwell assay
Transwell chambers (Corning Costar, Cambridge, MA, USA) with pore size of 8 μm were used to assess cell invasion. In brief, 200 μL of serum-free medium re-suspended with 1 × 105 cells was added to the upper compartment of transwell chambers coating with Matrigel Matrix (BD BioSciences, Franklin Lakes, NJ, USA). Concurrently, 600 μL of full medium containing 20% FBS was appended in lower chambers. Following 24 h of incubation, cotton swabs were used to scrape off the cells from upper chambers. Invading cells in lower chambers were counted using a light microscope (Olympus) after they were fixed and stained using 4% paraformaldehyde and 0.4% crystal violet.
Statistical analysis
All statistical analysis was performed via GraphPad Prism 7.0 tool. Mean ± standard deviation (SD) was presented as experimental data. Difference comparisons between two groups were performed by using Student's t-test. One-way Analysis of Variance (ANOVA) method was used for comparisons between multiple groups, followed by Tukey's multiple comparisons. The p < 0.05 indicated significant differences.
Results
Low expression of TRIM38 is observed in lung tissues of NSCLC mice and NSCLC cells
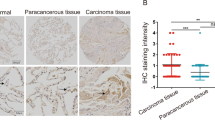
The expression pattern of TRIM38 was firstly determined in lung tissues of mice. The qRT-PCR data indicated that TRIM38 mRNA expression was decreased in lung tissues of NSCLC mice compared to lung tissues of normal mice (p < 0.001, Fig. 1A). The results from western blotting and IHC staining displayed lower protein expression of TRIM38 in lung tissues of NSCLC mice than those of normal mice (p < 0.001, Fig. 1B, C). Then we examined protein expression of TRIM38 in BEAS-2B cells and NSCLC cells (A549, H1299, HCC827, H1975, H460, and H1650). It was found that protein expression of TRIM38 was reduced in NSCLC cells compared to BEAS-2B cells (p < 0.001, Fig. 1D).
TRIM38 is lowly expressed in tumor tissues of non-small cell lung cancer (NSCLC) mice. A Relative mRNA expression of TRIM38 in lung tissues of normal mice and lung tissues of NSCLC mice was determined by quantitative real-time polymerase chain reaction (qRT-PCR). B Relative protein expression of TRIM38 was determined by western blot in lung tissues of normal mice and lung tissues of NSCLC mice. C Relative protein expression of TRIM38 was determined by immunohistochemistry staining in lung tissues of normal mice and lung tissues of NSCLC mice. D Relative protein expression of TRIM38 was determined by western blot in BEAS-2B, A549, H1299, HCC827, H1975, H460, and H1650 cells. Each experiment was conducted in three biological replicates. ***p < 0.001
TRIM38 suppresses tumor growth and epithelial-to-mesenchymal transition (EMT) in vivo, which was associated with the AMPK/NF-κB/NLRP3 pathway
Next, lentivirus overexpressing TRIM38 was transfected into H1299 cells and the overexpression efficiency was detected with western blot. As exhibited in Fig. 2A, protein expression of TRIM38 was markedly up-regulated after transfection of TRIM38 overexpressing lentivirus (p < 0.001), suggesting successful overexpression of TRIM38.
TRIM38 suppresses tumor growth and epithelial-to-mesenchymal transition (EMT) in vivo. A Relative protein expression of TRIM38 in H1299 cells was determined by western blot. B The tumor nodules in lung tissues of non-small cell lung cancer (NSCLC) mice were observed under a light microscope. C The number of tumor nodules was detected using hematoxylin–eosin staining in lung tissues of NSCLC mice. D The protein expression of E-cadherin, N-cadherin, and Vimentin in lung tissues of NSCLC mice was detected using immunohistochemistry staining. *p < 0.05. ***p < 0.001. Each experiment was conducted in three biological replicates
Previously, TRIM38 is demonstrated to negatively regulate NF-κB activity in many cells such as chondrocytes, cardiomyocytes, and osteoclasts [12, 31, 32]. Meanwhile, a study has demonstrated that TRIM38 can promote downstream GLUT1 degradation of AMPK in bladder cancer [15]. Therefore, for exploring the effects and mechanisms of TRIM38 overexpression on tumor growth and EMT in mice, H1299 cells transfected with lentivirus of overexpressing TRIM38 and corresponding empty vector were injected into mice and AMPK inhibitor was used. Under a light microscope, we observed that the number of tumor nodules was reduced after TRIM38 overexpression in lung tissues of NSCLC mice, and the reduction effect of TRIM38 overexpression on tumor nodules was reversed by AMPK inhibitor (compound C) (p < 0.001, Fig. 2B). According to the result of H&E staining, we also observed decreased tumor nodules in the TRIM38 group compared to the vector group, and the reduction effect of TRIM38 overexpression on tumor nodules was reversed by compound C (Fig. 2C). Besides, we evaluated EMT by detecting protein biomarkers of EMT in lung tissues of NSCLC mice. Decreased expression of Vimentin and N-cadherin proteins as well as increased E-cadherin expression was found after TRIM38 overexpression, suggesting the inhibitory effect of TRIM38 overexpression on EMT (Fig. 2D). And the inhibitory effect of TRIM38 overexpression on EMT was also reversed by compound C (Fig. 2D). These findings implied that anti-cancer function of TRIM38 was partly dependent on the regulation of AMPK/NF-κB/NLRP3 pathway in vivo.
TRIM38 represses proliferation, migration, invasion, and EMT of NSCLC cells in vitro
When we explored the influences of TRIM38 on NSCLC cells in vitro, we found that TRIM38 overexpression caused decreases of clone number and cell viability, migration rate, and cell invasion number in H1299 cells (p < 0.001, Fig. 3A–D). Simultaneously, TRIM38 overexpression caused suppression of EMT in H1299 cells, as evidenced by high E-cadherin expression along with low N-cadherin and Vimentin expression (p < 0.001, Fig. 3E).
TRIM38 represses proliferation, migration, invasion, and epithelial-to-mesenchymal transition (EMT) of non-small cell lung cancer (NSCLC) cells in vitro. A The number of colonies in H1299 cells was detected by colony formation assay. B Cell viability in H1299 cells was examined by CCK-8 assay. C The migration rate of H1299 cells was detected by wound healing assay. D The invasion of H1299 cells was detected by transwell assay. Invasion was evaluated by counting at least 5 randomly chosen fields. E The protein expression of E-cadherin, N-cadherin, and Vimentin in H1299 cells was detected using western blot. ***p < 0.001. Each experiment was conducted in three biological replicates
The inhibitory effects of TRIM38 on migration, proliferation, invasion, and EMT of NSCLC cells are mediated by NLRP3 in vitro
Next, we further explored downstream mechanisms of TRIM38 in vitro. Firstly, the regulatory relation between TRIM38 and NLRP3 was investigated. As displayed in Fig. 4A, TRIM38 overexpression evidently resulted in low NLRP3 expression in H1299 cells with p < 0.001, suggesting negative regulatory relation between TRIM38 and NLRP3. Then rescue assays were performed. We found that the clone formation ability and cell viability were promoted by NLRP3 overexpression, whereas NLRP3 overexpression reversed TRIM38-mediated suppression of clone formation and viability in H1299 cells (p < 0.001, Fig. 4B, C). Likewise, the migration rate and the number of invasion cells were increased by NLRP3 overexpression, whereas NLRP3 overexpression reversed TRIM38-mediated suppression of migration and invasion in H1299 cells (p < 0.001, Fig. 4, D, E). Furthermore, TRIM38 overexpression resulted in up-regulation E-cadherin protein expression along with down-regulation of N-cadherin and Vimentin expression, which were reversed by NLRP3 overexpression in H1299 cells (p < 0.01, Fig. 4F).
Suppressive influences of TRIM38 on proliferation, migration, invasion, and epithelial-to-mesenchymal transition (EMT) of non-small cell lung cancer (NSCLC) cells are mediated by NLRP3 in vitro. A Relative protein expression of NLRP3 in H1299 cells was determined by western blot. B The number of colonies in H1299 cells was detected by colony formation assay. C Cell viability in H1299 cells was detected by CCK-8 assay. D The migration rate of H1299 cells was detected by wound healing assay. E The invasion of H1299 cells was detected by transwell assay. Invasion was evaluated by counting at least 5 randomly chosen fields. F Relative protein expression of E-cadherin, N-cadherin, and Vimentin in H1299 cells was detected using western blot. vs. vector, ***p < 0.001; vs. TRIM38, ##p < 0.01, ###p < 0.001. Each experiment was conducted in three biological replicates
TRIM38 can regulate the AMPK/NF-κB/NLRP3 pathway
To explore whether TRIM38 affected the AMPK/NF-κB/NLRP3 pathway in H1299 cells, the following experiments were performed. Firstly, AMPK and NF-κB-associated protein expression levels were examined using western blot. We discovered that TRIM38 reduced protein levels of p-inhibitor of NF-κB (IκB) and p-p65, and increased the protein level of p-AMPK, implying that TRIM38 led to AMPK phosphorylation and suppressed the NF-κB/NLRP3 pathway (p < 0.01, Fig. 5A). Then we examined the role of AMPK inhibitor (compound C) in TRIM38-overexpressed H1299 cells. We found that protein levels of p-IκB, p-p65, and NLRP3 were increased after the addition of compound C in TRIM38-overexpressed H1299 cells (p < 0.001, Fig. 5B).
TRIM38 can regulate the AMPK/NF-κB/NLRP3 pathway. A Relative protein expression of p-p65, p65, p-IκB, IκB, p-AMPK, and AMPK was examined using western blot. B Relative protein expression of p-p65, p65, p-IκB, IκB, and NLRP3 was examined using western blot. **p < 0.01. ***p < 0.001. Each experiment was conducted in three biological replicates
TRIM38 represses migration, proliferation, and invasion of NSCLC cells via regulating the AMPK/NF-κB/NLRP3 pathway
Finally, we delved into whether the AMPK/NF-κB/NLRP3 pathway participated in TRIM38-induced inhibition of proliferation, migration, and invasion in H1299 cells. Our data demonstrated that the clone formation ability, cell viability, the migration ability, and the invasion ability of TRIM38-overexpressed H1299 cells were enhanced in response to the addition of compound C (p < 0.01, Fig. 6A–D).
TRIM38 represses proliferation, migration, and invasion of non-small cell lung cancer (NSCLC) cells via regulating the AMPK/NF-κB/NLRP3 pathway. A The number of colonies in H1299 cells was detected by colony formation assay. B Cell viability in H1299 cells was detected by CCK-8 assay. C The migration rate of H1299 cells was detected by wound healing assay. D The invasion of H1299 cells was detected by transwell assay. Invasion was evaluated by counting at least 5 randomly chosen fields. **p < 0.01. ***p < 0.001. Each experiment was conducted in three biological replicates
Discussion
NSCLC has seriously threatened human health, with increasing mortality rate year after year [34, 35]. Nevertheless, due to complicated pathogenesis, no effective treatment for NSCLC has been established [36]. As one of the tumor microenvironment-related factors, TRIM38 has got much attention in recent years due to its involvement in cancer-related diseases [14]. A study from Wang et al. has revealed that TRIM38 expression is reduced in patients with dermatomyositis (a key risk factor of lung cancer) [16]. Another study has demonstrated that TRIM38 is lowly expressed in patients with bladder urothelial carcinoma (BLCA) [15]. Consistently, we also observed the low expression of TRIM38 in tumor lung tissues of NSCLC mice as well as NSCLC cells, which suggested that there was a certain correlation of TRIM38 with NSCLC.
There is increasing evidence that TRIM38 plays important roles in diverse cancers. For example, TRIM38 is identified as a potential prognostic biomarker and therapeutic target in hepatocellular carcinoma (HCC) [37]. TRIM38 is found to repress proliferation and migration of BLCA cells [15]. Here, we discovered that TRIM38 inhibited tumor growth and metastasis in vivo, and repressed migration, invasion, proliferation, & EMT of NSCLC cells in vitro. Based on the above findings, we inferred that TRIM38 may serve as a valuable target for therapeutics to prevent the progression and development of NSCLC.
Except for function importance of TRIM38, we explored the mechanisms by which TRIM38-mediated tumor inhibition in NSCLC. Previously, convincing evidence has uncovered the inhibition effect of AMPK on the NF-κB pathway [38, 39], and NF-κB/NLRP3 pathway activation participates in NSCLC metastasis [27, 40]. On the other hand, TRIM38 is found to facilitate GLUT1 degradation of AMPK in bladder cancer [15]. Therefore, we assumed that TRIM38 may regulate the AMPK/NF-κB/NLRP3 pathway to affect NSCLC development, and then validated our assumption. In vitro experiments, we found that TRIM38 activated AMPK phosphorylation and inactivated the NF-κB/NLRP3 pathway, as indicated by decreased expression of p-p65 and p-IκB along with increased expression of p-AMPK. Above all, NLRP3 overexpression or AMPK inhibitor reversed suppressive effects of TRIM38 overexpression on clone formation, viability, migration, invasion of H1299 cells. Meanwhile, NLRP3 overturned the suppressive effect of TRIM38 overexpression on EMT of H1299 cells. In vivo experiments, we found that the inhibitory effects of TRIM38 overexpression on tumor nodules and EMT were reversed by AMPK inhibitor. Taken together, these outcomes implied that TRIM38 may impair tumor growth and metastasis by regulating the AMPK/NF-κB/NLRP3 pathway in NSCLC.
However, one limitation of our study is that we only used one cell line to perform in vitro function experiments. Given different origin of the other cell lines (HCC827, A549, H460, H1975, and H1650), the use of more cell lines will make our study more persuasive. In future studies, we will use different cell lines to perform function experiments.
To sum up, TRIM38 expression was decreased in tumor cells and tumor lung tissues. TRIM38 overexpression suppressed in vitro invasion, migration, proliferation, and metastasis of NSCLC cells, which also inhibited tumor metastasis and growth in vivo. In terms of mechanisms, TRIM38 may reduce NF-κB/NLRP3 activity via activating AMPK, thereafter impairing the progression and development of NSCLC. Our discoveries offered original insight to the regulatory mechanism and crucial role of TRIM38 in NSCLC, which provided new ideas for NSCLC treatment.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Boloker G, Wang C, Zhang J (2019) Erratum to updated statistics of lung and bronchus cancer in United States (2018). J Thorac Dis 11:E63
Youlden DR, Cramb SM, Baade PD (2008) The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol 3:819–831
Arbour KC, Riely GJ (2019) Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 322:764–774
Imyanitov EN, Iyevleva AG, Levchenko EV (2021) Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol 157:103194
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594
Rakaee M, Busund LT, Paulsen EE, Richardsen E, Al-Saad S, Andersen S, Donnem T, Bremnes RM, Kilvaer TK (2016) Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 7:72184–72196
Chen VW, Ruiz BA, Hsieh MC, Wu XC, Ries LA, Lewis DR (2014) Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 120(Suppl 23):3781–3792
Liu X, Lei X, Zhou Z, Sun Z, Xue Q, Wang J, Hung T (2011) Enterovirus 71 induces degradation of TRIM38, a potential E3 ubiquitin ligase. Virol J 8:61
Jia X, Zhao C, Zhao W (2021) Emerging roles of MHC class I region-encoded E3 ubiquitin ligases in innate immunity. Front Immunol 12:687102
Hu MM, Shu HB (2017) Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol Immunol 14:331–338
Hu S, Li Y, Wang B, Peng K (2021) TRIM38 protects chondrocytes from IL-1beta-induced apoptosis and degeneration via negatively modulating nuclear factor (NF)-kappaB signaling. Int Immunopharmacol 99:108048
Hu MM, Yang Q, Zhang J, Liu SM, Zhang Y, Lin H, Huang ZF, Wang YY, Zhang XD, Zhong B, Shu HB (2014) TRIM38 inhibits TNFalpha- and IL-1beta-triggered NF-kappaB activation by mediating lysosome-dependent degradation of TAB2/3. Proc Natl Acad Sci U S A 111:1509–1514
Liang H, Huang C (2020) Identification of tumor microenvironment-related genes in lower-grade gliomas by mining TCGA database. Transl Cancer Res 9:4583–4595
Wang X, He H, Rui W, Zhang N, Zhu Y, Xie X (2021) TRIM38 triggers the uniquitination and degradation of glucose transporter type 1 (GLUT1) to restrict tumor progression in bladder cancer. J Transl Med 19:508
Aljabban J, Syed S, Syed S, Rohr M, Weisleder N, McElhanon KE, Hasan L, Safeer L, Hoffman K, Aljabban N, Mukhtar M, Adapa N, Allarakhia Z, Panahiazar M, Neuhaus I, Kim S, Hadley D, Jarjour W (2020) Investigating genetic drivers of dermatomyositis pathogenesis using meta-analysis. Heliyon 6:e04866
Waldman R, DeWane ME, Lu J (2020) Dermatomyositis: Diagnosis and treatment. J Am Acad Dermatol 82:283–296
Bolko L, Gitiaux C, Allenbach Y (2019) [Dermatomyositis: new antibody, new classification]. Med Sci (Paris) 35 Hors serie n degrees 2:18–23
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Courtois G, Gilmore TD (2006) Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25:6831–6843
Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB (2003) TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol 326:105–115
Faria SS, Costantini S, de Lima VCC, de Andrade VP, Rialland M, Cedric R, Budillon A, Magalhaes KG (2021) NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci 28:26
Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, Zhao W, Geng B (2020) Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res 39:176
Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, Feng Y, Yang S (2021) Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res 170:105748
Platnich JM, Chung H, Lau A, Sandall CF, Bondzi-Simpson A, Chen HM, Komada T, Trotman-Grant AC, Brandelli JR, Chun J, Beck PL, Philpott DJ, Girardin SE, Ho M, Johnson RP, MacDonald JA, Armstrong GD, Muruve DA (2018) Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep 25:1525-1536.e7
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R, Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, Long HA, Xiao XL, Zhang F, Wu JM, Qin DL, Wu AG (2020) Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-kappaB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers (Basel) 12(1):193
Wang Y, Liu F, Chen L, Fang C, Li S, Yuan S, Qian X, Yin Y, Yu B, Fu B, Zhang X, Li Y (2022) Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-kappaB/NLRP3 inflammasome pathway. Front Immunol 13:867516
Uthman L, Kuschma M, Romer G, Boomsma M, Kessler J, Hermanides J, Hollmann MW, Preckel B, Zuurbier CJ, Weber NC (2021) novel anti-inflammatory effects of canagliflozin involving hexokinase II in lipopolysaccharide-stimulated human coronary artery endothelial cells. Cardiovasc Drugs Ther 35:1083–1094
Poma P (2020) NF-kappaB and disease. Int J Mol Sci 21(3):9181
Dong J, Peng H, Yang X, Wu W, Zhao Y, Chen D, Chen L, Liu J (2020) Metformin mediated microRNA-7 upregulation inhibits growth, migration, and invasion of non-small cell lung cancer A549 cells. Anticancer Drugs 31:345–352
Kim K, Kim JH, Kim I, Seong S, Kim N (2018) TRIM38 regulates NF-kappaB activation through TAB2 degradation in osteoclast and osteoblast differentiation. Bone 113:17–28
Lu Z, Deng M, Ma G, Chen L (2022) TRIM38 protects H9c2 cells from hypoxia/reoxygenation injury via the TRAF6/TAK1/NF-kappaB signalling pathway. PeerJ 10:e13815
Zheng Z, Bian Y, Zhang Y, Ren G, Li G (2020) Metformin activates AMPK/SIRT1/NF-kappaB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 19:1089–1104
Chen R, Manochakian R, James L, Azzouqa AG, Shi H, Zhang Y, Zhao Y, Zhou K, Lou Y (2020) Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol 13:58
Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D’Amico TA, Dilling TJ, Dobelbower M, Gettinger S, Govindan R, Gubens MA, Hennon M, Horn L, Lackner RP, Lanuti M, Leal TA, Lin J, Loo BW Jr, Martins RG, Otterson GA, Patel SP, Reckamp KL, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer KW, Yang SC, Gregory K, Ocn HM (2019) NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 17:1464–1472
Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M, Prelaj A, Zilembo N, Ganzinelli M, Pallavicini LM, De Simone I, Colombo MP, Sica A, Torri V, Garassino MC (2019) Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 75:39–51
Cao J, Su B, Peng R, Tang H, Tu D, Tang Y, Zhou J, Jiang G, Jin S, Wang Q, Wang A, Liu R, Deng Q, Zhang C, Bai D (2022) Bioinformatics analysis of immune infiltrates and tripartite motif (TRIM) family genes in hepatocellular carcinoma. J Gastrointest Oncol 13:1942–1958
Bess E, Fisslthaler B, Fromel T, Fleming I (2011) Nitric oxide-induced activation of the AMP-activated protein kinase alpha2 subunit attenuates IkappaB kinase activity and inflammatory responses in endothelial cells. PLoS ONE 6:e20848
Zhang Y, Qiu J, Wang X, Zhang Y, Xia M (2011) AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol 31:2897–2908
Haneklaus M, O’Neill LA, Coll RC (2013) Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol 25:40–45
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
KHZ and MRL designed the study; GHL, ZKN, and SJ collected the data; XHB, ZTL, and MRL analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
For conducting animal experiments, Institutional Animal Care and Use Committee’s guidelines from Beijing Viewsolid Biotechnology Co. LTD were followed (VS212601456).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, K., Lin, G., Nie, Z. et al. TRIM38 suppresses migration, invasion, metastasis, and proliferation in non-small cell lung cancer (NSCLC) via regulating the AMPK/NF-κB/NLRP3 pathway. Mol Cell Biochem 479, 2069–2079 (2024). https://doi.org/10.1007/s11010-023-04823-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04823-y