Abstract
Extracellular vesicles (EVs) secreted by various cells offer great potential for use in the diagnosis and treatment of disease. EVs are heterogeneous membranous vesicles. Exosomes are a subtype of EVs, 40–150 nm spherical vesicles with a lipid layer derived from endosomes. Exosomes, which are involved in signal transduction and maintain homeostasis, are released from almost all cells, tissues, and body fluids. Although several methods exist to isolate and characterize EVs and exosomes, each technique has significant drawbacks and limitations that prevent progress in the field. New approaches in the biology of EVs show great potential for isolating and characterizing EVs, which will help us better understand their biological function. The strengths and limitations of conventional strategies and novel methods (microfluidic) for EV isolation are outlined in this review. We also present various exosome isolation techniques and kits that are commercially available and assess the global market demand for exosome assays.
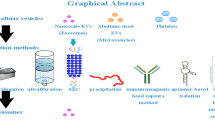
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Communication between cells in multicellular organisms involves the secretion of proteins that bind to receptors on neighboring cells and the release of membrane vesicles [1]. Membrane vesicles consist of a lipid bilayer containing soluble hydrophilic components and transmembrane proteins from the donor cell [2]. There are several types of secreted mentioned vesicles that have different biochemical and structural properties based on their intracellular site of origin, which likely influence their function. These include microvesicles, also called ectosomes, which range in size from 100 to 350 nm and are formed by vesicles that secrete directly from the plasma membrane [3, 4]. Apoptotic bodies are more large EVs than others that contain parts of dying cells, e.g., intact organelles, micronuclei, and chromatin remnants [5]. Amphisomes are intermediate organelles produced through the fusion of endosomes with autophagosomes within cells. Amphisome formation is an essential step during a sequential maturation process of autophagosomes before their ultimate fusion with lysosomes for cargo degradation [6]. Amphisomes form by single or multiple autophagosome-endosome fusions and can be recognized morphologically by their mixed autophagic-endocytic contents. Autophagy and exosome pathways are strictly interconnected at several levels. Amphisomes are then either degraded by lysosomal enzymes or released from the cell as exosomes.
Exosomes, extracellular vesicles with a diameter of 40–100 nm in which nucleic acids, proteins, and lipids are embedded, regulate many pathophysiological processes, including immune responses, metabolism, etc. [7]. Exosomes can transport biochemically active molecules and regulate expression of gene and cellular functions [8]. Therefore, the pharmaceutical industry and academia are particularly interested in exosomes as an innovative drug delivery system.
Exosomes are constituted of several molecules, such as proteins, RNA, and DNA. Exosomal proteins differ in their properties from the cells or tissues from which they are derived [9]. Chaperones, adhesion molecules, and MHCs are the most important exosomal proteins [10]. Exosome-specific proteins could serve as biomarkers for exosome identification [11]. ALG -2-Interacting protein X (ALIX), Heat shock protein 70 (HSP 70), tetraspanins, and tumor susceptibility gene 101 (TSG101) are among the proteins with higher levels in exosomes [12]. Exosomes also contain mitochondrial DNA, noncoding RNAs (ncRNAs), and metabolic enzymes, as well as signaling molecules such as G-proteins and protein kinases [13]. Exosomes are taken up by recipient cells through multiple mechanisms, including interactions between exosomal molecules and recipient cells receptors. These include binding of phosphatidylserine to lymphocyte: T cell immunoglobulin domain and mucin domain protein 1 (TIM1) or TIM4, intercellular adhesion molecule 1 (ICAM1) to lymphocyte function-associated antigen 1 (LFA1), and possibly milk fat globule EGF factor 8 protein (MFGE8) to avβ3 or avβ5 integrins. After interaction with molecules on the recipient cell, the exosome could fuse directly to the membrane [14].
Cellular communication mediated by exosomes is involved in a variety of processes, including angiogenesis, senescence, immune signaling, differentiation, and proliferation. There is also evidence that exosomes aid in the spread of pathological proteins, including viral proteins and genomic RNA, microRNA, and mRNA found in exosomes from virus-infected cells. Exosomes can play an important role in viral infections, especially retroviruses, depending on their genetic content and proteins [15]. The ability of exosomes to transmit inflammatory mediators makes exosomes important players in inflammatory responses and in the pathogenesis of diseases such as type 2 diabetes mellitus, cancer, and degenerative diseases [16]. Since exosomes are abundant in biofluids, they have great potential as a non-invasive method to study exosome-associated biomarkers to determine their diagnostic and prognostic value. However, due to the challenges posed by the heterogeneity of extracellular vesicles, researchers are increasingly focusing on improving methods to isolate and characterize diverse populations of EVs. Here, we review current exosome isolation techniques, the diagnostic and therapeutic value of exosomes, and the commercial approach of exosome-based detection methods.
Preparation and characterization of exosomes
The “Minimal Information for Studies of Extracellular Vesicles” (MISEV) guidelines were created by the International Society for Extracellular Vesicles (ISEV) with the goal of standardizing the characterization, isolation, and reporting of EVs, such as exosomes. These guidelines provide researchers with a structured approach to ensure that their studies are both reproducible and comparable to others in the field. By following these guidelines, researchers can establish a solid framework for EVs research that promotes consistency and reliability [17].
A group of professors has formed the Extracellular Vesicles Track Consortium to establish standardized protocols for the isolation and characterization of EVs. The main goal of this group is to define best practices for EV research and to promote a collaborative approach to the field. Specifically, the consortium has developed a set of protocols for the isolation of exosomes from various biological fluids such as blood, urine, and cerebrospinal fluid. These protocols have been designed to be both reproducible and scalable, allowing researchers to isolate exosomes from both small and large volumes of biological samples [18].
Conventional approaches for exosome isolation
It is difficult to obtain exosomes in complex body fluids with a high yield because they are a nanoscale vesicular component. Exosomes are obtained from cell cultures or plasma, and their morphological and physical properties allow their identification [19]. Numerous techniques have been established for the isolation of exosomes. These techniques include differential and gradient density centrifugation, precipitation, immunoaffinity capture, filtration, size exclusion chromatography, and microfluidics-based techniques (Table 1).
Differential and gradient density centrifugation
The gold standard method for separating exosomes is centrifugation, especially ultracentrifugation [20]. These techniques do not require technical skill or complex sample preparation. Differential centrifugation typically requires several steps, including removal of cells, debris, and larger vesicles, followed by precipitation of exosomes at high speed at 100 000 g [21]. Density gradient ultracentrifugation (UC) is the other alternative technique that uses a density gradient for isolation. Sucrose is a commonly used medium for density centrifugation. In this method, vesicles are separated according to their flotation density, allowing them to float upward in a sucrose gradient. Therefore, exosomes can be separated without aggregates using this technique by pelleting and easily removing impurities at the bottom of the tube [7].
Size exclusion chromatography (SEC)
SEC is a size separation technique using a porous phase. Particles with a small radius can pass through the pores, while larger particles cannot enter the pores [22]. It has been reported that SEC indicates acceptable purity for the isolation of exosomes from blood [23]. SEC could be combined with other techniques such as precipitation to improve purity and yield. Although SEC preserves vesicle structure and integrity, it is not scalable for high-throughput applications due to its long run time [24].
Filtration
Filtration-based methods have recently become known for the isolation of exosomes [25]. Originally, filtration was used as a stand-alone technique, but recently the first two centrifugation steps have been replaced by filtration with ultracentrifugation [26]. Thus, filtration can remove large debris, while ultracentrifugation allows for greater purification of filtered samples. Although filtration is easier and faster than ultracentrifugation and does not require specialized equipment, it can affect exosomes by trapping them in the pores of the filters [27]. In addition, the force exerted as the sample passes over the filter membranes can cause structural damage to large vesicles [28].
Precipitation
This is a simple and instrument-free technique for the isolation of exosomes. Precipitation is a polymer-based technique in which the sample is mixed with a polymer at low temperature and adjusted salt concentration. Polyethylene glycol (PEG) is the most commonly used polymer in this procedure [29,30,31]. Commercial kits have been reported to be more effective in isolation than ultracentrifugation and nanomembrane concentrators [25]. PEG, however, carries the risk of adding impurities to the extracted exosomes that may interfere with their biological activities. Nowadays, a non-PEG/ exosome precipitation was developed by Invent Biotechnologies (MINUTETM, Plymouth, USA) [32].
Immune-affinity capture
Proteomics of exosomes has demonstrated the existence of several protein markers on the exosome membrane [33]. These proteins are the perfect markers for immunologic based isolation of exosomes because of the immunoaffinity interactions among the antibodies and proteins [34]. Selecting the correct and specific markers such as the tetraspanin family (CD9, CD63, and CD81) is the crucial step in immunoassay techniques and can be used for effective immunocapture [35].
Microfluidics for exosome isolation and analysis
Microfluidic devices can overcome the limitations of previous methods that do not provide the high levels of purity, recovery, and yield required for routine isolation and analysis in the clinic [40]. In addition, high cost, long processing times, and difficulties in standardization are other drawbacks. Microfluidics provides parallel separation and sensing capabilities for exosome isolation, detection, and analysis on a single chip. Fast performance, high specificity, high sensitivity, and a user-friendly format enable the production of point-of-care (POC) diagnostics for noninvasive liquid biopsy of exosomes for personalized medicine and clinical applications [41]. Generally, either labeled or label-free methods are used in microfluidic systems. While label-based methods use immunoaffinity interaction to specifically separate exosomes from a mixture of other components, label-free methods use and isolate exosomes based on physicochemical differences such as size and density. Below, we present examples of each of these methods and discuss their biomedical potential for disease diagnosis, health monitoring, and therapy [42].
Label-free microfluidic methods for exosome isolation
There are several methods for sorting exosomes based on their density and size. For example, it has been reported that integrating acoustics with microfluidics results in high yield and pure exosome isolation directly from undiluted whole blood samples. By applying different acoustic forces to EVs depending on their size and density, the authors were able to separate particles of different sizes directly from whole blood [43] (Fig. 1). The cell removal unit of the microfluidic device was designed to fractionate blood components > with a diameter of 1 µm, including white blood cells (WBCs), red blood cells (RBCs), and platelets (PLTs), to obtain a cell-free plasma for the downstream exosome isolation unit that separates nanoscale bioparticles. This step requires the application of a higher frequency (∼ 40 MHz). Finally, the products of the previous phase were delivered to the exosome isolation unit, which was able to discriminate between subgroups of EVs. Due to its biocompatible, label-free, and non-contact (automated) properties, such a method offers the potential to preserve the properties, structures, and functions of the isolated exosomes. Moreover, automated exosome isolation enables short processing times, biohazard containment, and provides reproducible results with convenient integration and minimal human intervention in downstream exosome analyzers [44].
schematic diagram of a label-free acoustofluidic device for exosome isolation. A A microfluidic device composed of different units. B An optical image of the integrated acoustofluidic device. C Working principle for size-based separation using lateral deflection induced by taSSAW field [44]
Deterministic lateral displacement (DLD) column arrays are an additional label-free yet efficient technology for sorting, separation, and enrichment of micro- and nanoparticles. In this series, nano-DLD arrays are fabricated with constant slit sizes between 25 and 235 nm. At low Péclet numbers (Pe), where deterministic displacement and diffusion compete, nano-DLD arrays sort particles based on their size in the range of 20–110 nm with high resolution [45]. Another popular label-free detection method exploits the plasmon resonance properties of various novel materials to achieve nanoplasmonic phenomena. Light interacts uniquely with metallic nanoscale materials, such as gold nanoparticles, can provide unmatched sensitivity for real-time imaging and analysis [46]. Several methods use nanoplasmonic platforms to detect and characterize EVs. However, the most suitable formats are based on surface-enhanced Raman spectroscopy (SERS), surface plasmon resonance (SPR), and localized SPR [47]. Several nanoplasmon-based devices using SPR for sensing have been used for ultrasensitive label-free detection of exosomes [46, 48, 49]. Initially, imaging SPR (SPRi) was introduced as a label-free, quantitative, and real-time method for the detection and characterization of tumor-emanating exosomes without purification or enrichment. Such SPR-based microfluidic device was demonstrated for the detection of exosomes in cell culture supernatant (CCS) of B16-F1/10 (mouse melanoma cell lines) and MHCC97H/L (human hepatoma cell lines) using antibodies for tyrosine kinase receptor MET, glycoprotein CD41b, and tetraspanins (e.g., CD9), specific antibodies against exosome transmembrane proteins. Moreover, SPR-based real-time monitoring of exosomes showed a positive relation in their abundance and metastatic ability in cell lines. The working principle of SPRi (Zhu et al., 2014) is shown in Fig. 2 [50].
An antiexosome antibody microarray is used in combination with SPRi to bind and detect exosomes in CCS. Once the sample is injected into the flow cell, the exosomes and antibodies can bind to the chip. The binding of a specific ligand to the coated antibody changes the refractive index, and the CCD camera records a higher intensity of the reflections [50]
Label-based (immunoaffinity)- microfluidics for exosome separation
Lable-based methods can be used to improve the specificity of exosomes for protein profiling. The markers are antigens selectively expressed on the surface of exosomes, such as CD63 and EpCAM. As mentioned previously, combining label-free detection methods such as SPR with label-based methods to isolate exosomes has yielded promising results [50].
In one preparation, an immunoaffinity-based microfluidic device led to the identification of more up to 20 proteins of exosome expressed in serous ovarian cancer (SOC) cell lines in compared with normal cells. In addition, HGF and STAT3 were identified as top regulatory proteins by pathway analysis. The discovery of novel exosome-based biomarkers can be used for the early detection of high grade SOC and the development of new targeted therapies that target high grade SOC-specific pathways, and thus may improve clinical outcomes in women with high grade SOC [51]. Microfluidic devices such as ExoChip [52] and ExoChip are reported for commercial purposes. While ExoChip uses anti-CD63, its new format uses both CD63 and phosphatidylserine (PS)-specific protein to increase the specificity of exosome separation. The device achieves 38% for healthy exosomes and 90% capture efficiency for cancer cell exosomes. It also separates 35% more A549 exosomes than the ExoChip. Moreover, the captured exosomes are readily released by Ca2 + chelation, which enables their downstream profiling [53]. Another device called ExoSearch uses immunomagnetic beads for multiplex measurement of EpCAM, CD24, and CA -125 exosomal tumor markers in plasma of ovarian cancer patients. The device showed significant diagnostic performance equivalent to the standard Bradford assay (Fig. 3) [54].
a ExoSearch chip workflow for continuous exosome isolation, mixing, multiplexing, and in situ detection. The performance of immunomagnetic beads under a bright-field microscope (b–c). d Accumulation of exosome-bound immunomagnetic beads in a microchamber where exosomes are continuously collected and released by a magnet that can be switched on and off. e: Transmission electron microscope cross-section of an exosome-bound immunomagnetic bead [54]
In another design, MVs were extracted from packed red blood cells (pRBCs) using a filter-assisted microfluidic device. MVs were labeled with anti-CD44, anti-CD47, and anti-CD55 magnetic nanoparticles, which allowed their further detection and quantification with a miniaturized nuclear magnetic resonance (µNMR) system. The results showed that a time-dependent increase in MV can be used as an effective measure of blood aging. In addition, the ability of MVs to act as generators of oxidative stress and consumers of nitric oxide became apparent. These new insights into the biology of MV can be used to improve transfusion safety and blood product quality [55]. In another nanointerface-based format, graphene oxide/polydopamine (GO /PDA) is used to improve the efficiency and specificity of exosome immuno-capturing. The nano-interface enabled an ultrasensitive exosome ELISA assay with a very low detection limit of 50 μL(-1) and a dynamic range of 4 log, which is significantly superior to existing methods. This platform differentiated healthy controls from ovarian cancer patients by quantitatively measuring exosomes from as little as 2 μl of plasma without sample processing [56]. Similarly, immunomagnetic beads immobilized with EpCAM antibodies are reported to provide highly accurate microvalve-based fluid control, allowing on-chip isolation and direct fluorescence-based detection of circulating exosomes within 1.5 h in the blood of breast cancer patients (56). Similar to µNMR [55], immunodetection based on CD24 and EpCAM markers is used in conjunction with a nano-plasmonic exosome assay (nPLEX) to improve sensitivity over previous methods. The device is portable when equipped with miniaturized optics and can collect exosomes for further study [57]. Microfluidics-based exosome sorting is also applied for real-time monitoring of drug efficacy in APNG (alkylpurine-DNA N-glycosylase) and MGMT (O(6)-methylguanine-DNA methyltransferase), the key enzymes that can repair temozolomide-induced DNA damage in glioblastoma multiforme (GBM). A microfluidic chip was used to study the mRNA levels of these enzymes in the tumor. The results show that the original tumor and exosomes have comparable mRNA levels for these two enzymes and that these levels change significantly during treatment. Consequently, such a platform, if validated in a large population, could be applied to GBM patients to predict drug response [58].
Characterization
According to the International Society for Extracellular Vesicles (ISEV), several characterization indices are required to identify isolated exosomes, including transmembrane or GPI-anchored proteins [59]. It is also possible to determine the purity of exosomes from biological fluids by detecting the presence or absence of certain non-EV structural proteins. Three steps are usually used for the identification of isolated exosomes in research: Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) for internal and external morphology, respectively; nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS) for size; and Western blot, enzyme-linked immunosorbent assay (ELISA), and flow cytometry for surface protein markers. Methods for exosome characterization can be divided into external (particle sizing and morphology) and inclusion characterization (lipid raft and membrane protein) [59,60,61,62,63,64]. Table 2 provides an overview of the purpose, advantages, and disadvantages of the commonly used methods.
Diagnostics value of exosomes
Due to the functions of exosomes in disease progression and intercellular communication, as well as their cargo and surface proteins, they are currently used as diagnostic biomarkers in clinical trials. Exosomes have high plasma stability and can slow down the rapid degradation of nucleic acids [65]. Exosome-based liquid biopsy can be used to detect circulating tumor cells and their DNA or cell-free RNA from body fluids [66].
Cancer cell-derived exosomes contain a variety of proteins and RNAs that can serve as biomarkers for diagnosis and prognosis [67, 68]. In addition, exosomes are being used in the ongoing clinical investigation (NCT03478410) to characterize atrial fibrillation due to atherosclerosis, hypertension, and cardiac abnormalities [69]. Investigators will determine whether exosomes released from the epicardial adipose tissue of patients with and without atrial fibrillation differ as biomarkers of cardiac arrhythmias that can be used for both prevention and treatment.
Exosomes have been shown to have great potential as diagnostic biomarkers for neurodegenerative diseases such as Alzheimer's and Parkinson’s disease [70]. Exosomes are involved in the pathogenesis of Parkinson’s disease by transporting α-synuclein [71]. Mutations in Leucinerich repeat kinase 2 (LRRK2) are considered to be the cause of Parkinson's disease [72]. To develop a test to evaluate the effects of LRRK2 inhibitors, exosomal proteins, urinary and blood biomarkers from PD patients and healthy individuals were analyzed in a clinical trial (NCT01860118) [73]. As biomarkers, exosomes may also be involved in the development of Alzheimer's disease. In addition, exosome behavior in individuals at risk for AD is being investigated in two clinical trials (NCT03275363 and NCT03944603) [74, 75]. Changes in exosomal markers in blood and CSF at 2-year intervals in individuals aged 60 to 89 years are the primary outcome of the NCT03944603 study. By investigating the relationship between immune system biomarkers and aging, this study wants to find the mechanisms involved in mild cognitive impairment and Alzheimer’s disease.
Exosomes have shown promise as diagnostic and prognostic biomarkers for a number of diseases. However, to obtain accurate data, it is critical to separate exosomal RNA from contaminating nanoparticles. Fortunately, several isolation and characterization techniques have been developed to effectively isolate exosomes from other extracellular vesicles and cell debris. However, obtaining reliable exosomal RNA in the clinical setting requires quality control measures and standardized protocols that are reproducible across laboratories.
Exosomes in the clinic
A lipid bilayer and abundance of adhesion molecules in exosomes make them ideal vehicles for targeted drug delivery. Low immunogenicity and the ability to cross the blood–brain barrier make them an ideal method for drug delivery [76]. Recent advances in nanomedicine led to the development of engineered exosomes, which demonstrated the potential of exosomes for targeted drug delivery [77]. Diabetes mellitus (DM) can be regulated by exosomes miRNAs, which are known to be key regulators of insulin resistance and pancreatic b-cell damage concerning the development of DM. This suggests that DM can be treated by exogenous silencing or activation of exosomal miRNAs [78].
Exosomal gp130 from breast cancer activates the signaling pathway of IL -6/STAT3 in macrophages, causing them to produce protumor cytokines and develop into a procancer phenotype. This discovery by Hamet et al. [79] raises the possibility that exosomal proteins, particularly breast cancer-derived exosomal proteins, are critical for cancer development. The researchers discovered that chemotherapy induces the EZH2/STAT3 signaling pathway in cancerous cells of breast and releases miR-378d and miR-378a-3p-rich exosomes that are taken up by patients. These exosomes activate the NOTCH and WNT stem cell pathways by targeting NAMB and DKK3, finally conclude in drug resistance. Consequently, avoiding exosomes during chemotherapy could minimize drug resistance [80].
As well, exosomes derived from bone marrow MSCs have been shown to modify the polarization of microglia to reduce demyelination and inflammation in rats [81]. Therefore, the administration of exosomes derived from bone marrow-derived MSCs has the potential to be a therapeutic strategy for the treatment of inflammatory diseases such as MS.
Exosomes have attracted much attention in the field of regenerative medicine because of their potential therapeutic applications. These tiny membrane vesicles have been found to promote tissue regeneration by stimulating cell proliferation and differentiation. In addition, exosomes possess immunomodulatory properties, making them a potential therapy for autoimmune diseases. Recently, ongoing clinical trials have demonstrated the safety and efficacy of mesenchymal stem cell-derived exosomes in patients with acute respiratory distress syndrome (ARDS) and have shown the potential of exosomes as a novel therapeutic strategy [82].
A recent Phase I/ II clinical trial has demonstrated the safety and efficacy of exosome therapy in patients with ischemic stroke [83]. In addition, a phase I clinical trial is investigating the potential use of dendritic cell-derived exosomes as a vaccine against melanoma [84]. The results of these studies could revolutionize the field of exosome therapy and remove current barriers to widespread use at the bedside.
Global market trends in exosome investigation
In recent years, an increase in exosome-related patents has been observed. Exosome products need to be produced at large scale and low cost to be used in commercial and clinical applications. The diagnostics segment is predicted to grow at a compound annual growth rate of 58.5%, from $10.0 million in 2016 to $100.0 million in 2021, which may indicate the importance of exosomes as diagnostic biomarkers in some diseases, particularly cancer. The therapeutics segment is forecast to grow 14.9%, from $5.0 million in 2016 to $10.0 million in 2021. This sluggishness could be due to the FDA’s indecision on therapeutic authorities. However, stringent regulations and uncertainties in the therapeutic and biological definitions of EVs pose a challenge to the expanding EVs market. Overall, the global market for diagnostic and therapeutic exosome products is estimated to grow to $2.9 billion by 2030, with a projected CAGR of 29.4% between 2021 and 2030 [85]. Currently, there are several commercially developed methods for exosome enrichment and isolation. These techniques attempt to facilitate the isolation of exosomes that could be used for high-throughput biomarker studies [86]. Here, we comprehensively evaluate some companies and commercially available products for EVs (Table 3 and Table 4) [87].
Future outlook and conclusion
Exosomes have great potential as biomarkers for clinical applications in disease detection and therapy, as they not only possess signaling functions that enable communication between cells but can also be utilized for targeted drug delivery. However, their small size and heterogeneity present a challenge in isolating and detecting them at a low cost and with the desired sensitivity and selectivity in body fluids. Thus, it is crucial to develop an effective method to separate exosomes for clinical use. Conventional isolation methods have drawbacks, such as small sample volume, many processing steps, and structural damage to exosomes due to applied forces. In addition, commercially available exosome separation kits offer many advantages such as speed, high yield, and applicability, but they are also expensive, have low purity, and are not effective in separating exosomes from complex fluids. While conventional methods remain the gold standard for exosome separation, microfluidic methods reperesent a promising alternative that can overcome existing difficulties. Microfluidic devices also offer several advantages, such as low cost, relatively small size, low sample consumption, fast turnaround time, and high sensitivity, which make them suitable for clinical use, especially in the field of personalized medicine. Although several microfluidic-based isolation techniques have been developed for this purpose, it is difficult to apply these technologies on a large and industrial scale.
The choice of isolation method depends on the objective of the study and the availability of la-boratory equipment and resources. As mentioned earlier, various techniques have been used to separate different types of vesicles based on their size, shape, surface properties, and density, but inefficient separation methods, difficulties in characterization, and lack of specific bi-omarkers are still a matter of debate. On the other hand, it can be challenging to isolate exo-somes from amphisomes and ectosomes that have the same size and density [107]. To overcome these problems, it is important to carefully select appropriate controls and opti-mize experimental conditions to minimize contamination by other cellular components. The use of negative controls, such as detergent-treated samples or mock isolation procedures, can help identify potential sources of contamination. Optimization of isolation conditions such as buffer composition and centrifugation speed can also help improve the purity and specificity of isolated exosome preparations. Novel proce-dures for purification of autophagosomes and partial purification of amphisomes have allowed preliminary biochemical characterization of both organelles [108]. In addition, extensive studies are needed to confirm these properties in various disease con-texts. [11, 108].
It is expected that exosome research will continue to advance in the near future, which will likely lead to innovations in the treatment of patients. This review highlights recent scientific developments and technical obstacles in exosome isolation. It also presents a comprehensive analysis of current exosome products that provides recommendations for selecting the best commercial exosome kits based on the specific application.
Data availability
Enquiries about data availability should be directed to the authors.
References
Shen Q et al (2022) Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J Adv Res 37:221–233
Soler-Botija C et al (2022) Mechanisms governing the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles: a scoping review of preclinical evidence. Biomed Pharmacother 147:112683
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7):727
Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25(6):364–372
Battistelli M, Falcieri E (2020) Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology 9(1):21
Ganesan D, Cai Q (2021) Understanding amphisomes. Biochem J 478(10):1959–1976
Poliakov A et al (2009) Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 69(2):159–167
Mathivanan S et al (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids Res 40(D1):D1241–D1244
Mathivanan S et al (2010) Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics 9(2):197–208
Conde-Vancells J et al (2008) Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res 7(12):5157–5166
Jeppesen DK et al (2019) Reassessment of exosome composition. Cell 177(2):428-445. e18
Xie F et al (2019) Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci 6(24):1901779
Soung Y et al (2017) Exosomes in cancer diagnostics. Cancers (Basel) 9(1):8
Lia G et al (2022) Extracellular vesicles as biomarkers of acute graft-vs.-host disease after haploidentical stem cell transplantation and post-transplant cyclophosphamide. Front Immunol. https://doi.org/10.3389/fimmu.2021.816231
Brennetta JC, Brian S, Qiana LM (2018) Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. In: Muhammad Akhyar F (ed) Nanomedicines. IntechOpen, Rijeka
Scrivo R et al (2011) Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev 10(7):369–374
Witwer KW et al (2021) Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles 10(14):e12182
Van Deun J et al (2017) EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14(3):228–232
Willms E et al (2018) Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:738
Kogure T et al (2011) Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 54(4):1237–1248
Witwer KW et al (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2(1):20360
Feng Y et al (2014) Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 9(2):e88685
van Eijndhoven MA et al (2016) Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI insight. https://doi.org/10.1172/jci.insight.89631
Zeringer E et al (2015) Strategies for isolation of exosomes. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.top074476
Alvarez ML et al (2012) Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 82(9):1024–1032
Quintana JF et al (2015) Extracellular onchocerca-derived small RNAs in host nodules and blood. Parasit Vectors 8(1):58
Grant R et al (2011) A filtration-based protocol to isolate human plasma membrane-derived vesicles and exosomes from blood plasma. J Immunol Methods 371(1–2):143–151
Batrakova EV, Kim MS (2015) Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 219:396–405
Yamamoto KR et al (1970) Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40(3):734–744
Adams A (1973) Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol 20(3):391–394
Lewis GD, Metcalf TG (1988) Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol 54(8):1983–1988
Lane RE et al (2015) Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep 5:7639
Simpson RJ et al (2009) Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 6(3):267–283
Théry C et al (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. https://doi.org/10.1002/0471143030.cb0322s30
Oksvold MP, Neurauter A, Pedersen KW (2015) Magnetic bead-based isolation of exosomes. RNA Interference. Springer, pp 465–481
Kuriyama N et al (2021) Challenges for the development of extracellular vesicle-based nucleic acid medicines. Cancers (Basel) 13(23):6137
Paterna A et al (2022) Isolation of extracellular vesicles from microalgae: a renewable and scalable bioprocess. Front Bioeng Biotechnol 10:836747
Shen M et al (2020) Progress in exosome associated tumor markers and their detection methods. Mol Biomed 1(1):3
van Eijndhoven MA et al (2016) Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI insight 1(19):e89631–e89631
Lin S et al (2020) Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16(9):1903916
Contreras-Naranjo JC, Wu H-J, Ugaz VM (2017) Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17(21):3558–3577
Lane RE et al (2015) Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep 5(1):7639
Lee K et al (2015) Acoustic purification of extracellular microvesicles. ACS Nano 9(3):2321–2327
Wu M et al (2017) Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci 114(40):10584–10589
Wunsch BH et al (2016) Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat Nanotechnol 11(11):936–940
Amrollahi P et al (2019) Ultra-sensitive automated profiling of EpCAM expression on tumor-derived extracellular vesicles. Front Genet 10:1273
Rojalin T et al (2019) Nanoplasmonic approaches for sensitive detection and molecular characterization of extracellular vesicles. Front Chem 7:279
Woo HK et al (2022) Characterization and modulation of surface charges to enhance extracellular vesicle isolation in plasma. Theranostics 12(5):1988–1998
Kodam SP, Ullah M (2021) Diagnostic and therapeutic potential of extracellular vesicles. Technol Cancer Res Treat 20:15330338211041204
Zhu L et al (2014) Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem 86(17):8857–8864
Dorayappan KDP et al (2019) A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Can Res 79(13):3503–3513
Li Y et al (2022) Extracellular vesicle-mediated crosstalk between pancreatic cancer and stromal cells in the tumor microenvironment. J Nanobiotechnol 20(1):208
Kang YT et al (2019) Isolation and profiling of circulating tumor-associated exosomes using extracellular vesicular lipid-protein binding affinity based microfluidic device. Small 15(47):e1903600
Zhao Z et al (2016) A microfluidic exosearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16(3):489–496
Gandham S et al (2020) Technologies and standardization in research on extracellular vesicles. Trends Biotechnol 38(10):1066–1098
Zhang P, He M, Zeng Y (2016) Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 16(16):3033–3042
Yu D et al (2022) Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer 21(1):56
Shao H et al (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun 6(1):6999
Théry C et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101(36):13368–13373
Wu Y, Deng W, Klinke DJ 2nd (2015) Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 140(19):6631–6642
Maas SL et al (2015) Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J Control Release 200:87–96
Shao H et al (2018) New technologies for analysis of extracellular vesicles. Chem Rev 118(4):1917–1950
Pospichalova V et al (2015) Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 4:25530
Zhou X et al (2020) The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol 17(4):323–334
Kalluri R, LeBleu VS (2016) Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press, Newyork
Salehi M, Sharifi M (2018) Exosomal miRNAs as novel cancer biomarkers: challenges and opportunities. J Cell Physiol 233(9):6370–6380
Hallal S et al (2020) A comprehensive proteomic SWATH-MS workflow for profiling blood extracellular vesicles: a new avenue for glioma tumour surveillance. Int J Mol Sci 21(13):4754
S. M. Center. Role of exosomes derived from epicardial fat in atrial fibrillation. July 2021; Available from: https://ClinicalTrials.gov/show/NCT03478410.
Rastogi S et al (2021) The evolving landscape of exosomes in neurodegenerative diseases: exosomes characteristics and a promising role in early diagnosis. Int J Mol Sci 22(1):440
Yu H et al (2020) Potential roles of exosomes in Parkinson’s disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol 8:86
Kumari U, Tan E (2009) LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. FEBS J 276(22):6455–6463
U. o. A. a. Birmingham, N.I.o.N. Disorders, stroke, lrrk2 and other novel exosome proteins in parkinson’s disease. July 2021; Available from: https://ClinicalTrials.gov/show/NCT01860118
T. U. o. H. Kong, D.P.U. The university of hong kong neurocognitive disorder cohort. July 2021; Available from: https://ClinicalTrials.gov/show/NCT03275363
D. University of Colorado, N.I.o. Aging, longitudinal innate immunity and aging study. July 2021; Available from: https://ClinicalTrials.gov/show/NCT03944603
Cho E et al (2018) Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Control Release 279:326–335
Zhang X et al (2022) Research progress in the application of exosomes in immunotherapy. Front Immunol. https://doi.org/10.3389/fimmu.2022.731516
He X et al (2021) Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med 11(6):e468
Ham S et al (2018) Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front Immunol 9:871
Yang Q et al (2021) Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J Exp Clin Cancer Res 40(1):120
Li Z et al (2019) Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67:268–280
ClinicalTrials.gov. (2020) Safety and effectiveness of placental derived exosomes and umbilical cord mesenchymal stem cells in moderate to severe acute respiratory distress syndrome (ards) associated with the novel corona virus infection (covid-19). May 24; Available from: https://clinicaltrials.gov/ct2/show/NCT05387278.
ClinicalTrials.gov. (2017) Allogenic mesenchymal stem cell derived exosome in patients with acute ischemic stroke. December 27; Available from: https://clinicaltrials.gov/ct2/show/NCT03384433.
Escudier B et al (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3(1):10
Linu Dash, V.W., Onkar Sumant. Exosome diagnostic and therapeutic market statistics-2030. April 2022; Available from: https://www.alliedmarketresearch.com/exosome-diagnostic-and-therapeutic-market.
Muller L et al (2014) Isolation of biologically-active exosomes from human plasma. J Immunol Methods 411:55–65
Muthu S et al (2021) Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig 8:7
Labs K. July 2020; Available from: https://kimeralabs.com/
Therapeutics C. July 2020; Available from: http://capricor.com/
Inc N. July 2020. Available from: http://www.nanosomix.com/technology
Diagnostics E. Personalized precision healthcare. July 2020; Available from: http://www.exosomedx.com/
Biomedical A. July 2020; Available from: https://www.arunabiomedical.com/
Slyusarenko M et al (2021) Formation and evaluation of a two-phase polymer system in human plasma as a method for extracellular nanovesicle isolation. Polymers 13(3):458
Martínez-González E et al (2020) Comparison of methods and characterization of small RNAs from plasma extracellular vesicles of HIV/HCV coinfected patients. Sci Rep 10(1):1–13
Liu C et al (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11(7):6968–6976
Wang Q-L et al (2018) Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways. Theranostics 8(18):4912
Gao F et al (2019) A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO 2. Chem Sci 10(6):1579–1588
Sun L et al (2020) Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis 11(8):1–16
Smith JT et al (2018) Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip 18(24):3913–3925
Sjoqvist S, Otake K, Hirozane Y (2020) Analysis of cerebrospinal fluid extracellular vesicles by proximity extension assay: a comparative study of four isolation kits. Int J Mol Sci. https://doi.org/10.3390/ijms21249425
Kim YB et al (2020) Evaluation of exosome separation from human serum by frit-inlet asymmetrical flow field-flow fractionation and multiangle light scattering. Anal Chim Acta 1124:137–145
Han P et al (2020) Salivary small extracellular vesicles associated miRNAs in periodontal status-a pilot study. Int J Mol Sci. https://doi.org/10.3390/ijms21082809
Koliha N et al (2016) A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J Extracell Vesicles 5:29975
Gao F et al (2019) A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO(2). Chem Sci 10(6):1579–1588
Nakai W et al (2016) A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep 6:33935
Kanwar SS et al (2014) Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14(11):1891–1900
Witwer KW et al (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.20360
Berg TO et al (1998) Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem 273(34):21883–21892
Acknowledgements
We would like to thank Department of Neurosciences and Cognition, Tabriz University of Medical Sciences, Tabriz, Iran, for its support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. JW and AE-k: conceptualized the content. Material preparation, data collection and analysis were performed by MO, HB-B, and RJ-E. NA-G: designed and generated the figures. MT, MR, ZA-S,and SK: helped in the discussion and editing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
This study was extracted from the dissertation registered at Tabriz University of Medical Sciences, Tabriz, Iran (Grant No: IR.TBZMED.VCR.REC.1398.241).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omrani, M., Beyrampour-Basmenj, H., Jahanban-Esfahlan, R. et al. Global trend in exosome isolation and application: an update concept in management of diseases. Mol Cell Biochem 479, 679–691 (2024). https://doi.org/10.1007/s11010-023-04756-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04756-6