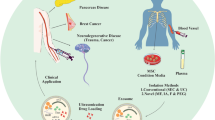
Abstract
Exosomes are special extracellular vesicles secreted by cells, which are of great significance in the basic research of life science and clinical application and has become a hot research field with rapid development in recent 10 years. Therefore, the isolation and separation of exosomes is particularly important for the research and application of exosomes. This paper aims to review the research progress of exosome isolation and separation methods in recent years, including ultracentrifugation, ultrafiltration, size‑exclusion chromatography, precipitation, immunomagnetic bead capture method, aptamer-based isolation, and isolation methods based on microfluidic technology. It is generally accepted that most of the existing methods have limitations, for example, ultracentrifugation is time-consuming and laborious, and immunomagnetic bead capture method and aptamer-based separation method have small sample processing capacity and high cost. As a result, we also introduce some common situations in which two or more methods are combined for use. Finally, the separation and isolation methods including all those presented in this review were compared and summarized.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term exosome was first used in 1989 to describe the extracellular vesicle subgroup of endosomal origin. However, the existence of exosomes was first discovered by Rose Johnstone et al. (1983, 1987) in the supernatant of sheep reticulocyte culture medium in 1983. And in 1987, they reported that exosomes were heterogenous and highly diversified and had various active molecules derived from the cells from which they originated (Hendrix 2021). Tulken et al. (2020) subsequently combined the isolation technique with sensitive detection and unbiased multiomics to confirm that exosomes have specific biomarkers, which further explains the heterogeneity of EVs and their unique functions. Since the beginning of the twenty-first century, especially in the past 10 years, the progress of exosomes has highlighted their importance in cell biology, pathology, clinical medicine, and other fields. Nowadays, the emerging field of exosome research is undergoing a phase of rapid development, with the number of exosome publications increasing rapidly after 2010 (Fig. 1).
In this paper, firstly, exosomes, their characteristics and applications are introduced. Then main methods applied in the field of exosome separation are stated. Finally, comparation of exosome isolation methods and summary of their advantages and disadvantages are presented.
Biology of Exosomes
Exosome is a class of extracellular vesicles. Extracellular vesicles are vesicle-like bodies with bilayer membrane structure and are cells derivatives. The diameter of extracellular vesicles ranges from 30 nm to 10 μm. Extracellular vesicles can be divided into the following subgroups: exosomes, which are released after the fusion of intracellular vesicles and plasma membrane, and have a diameter of 30–150 nm; micro vesicles, which is released directly from the plasma membrane and has a diameter of 100–1000 nm; apoptotic body, which is produced by apoptosis and has a diameter of 50 nm–2 μm; large oncosomes, which are produced from tumor cells and are 1–10 μm in diameter. Among these subgroups, exosomes are the most special ones. Exosomes widely exist in supernatant of cell culture and various body fluids (blood, lymph, urine, etc.), containing a variety of proteins, DNA, mRNA, miRNA, lipids, signal molecules and other substances. The way how exosomes are formed makes them different from other extracellular vesicles. Exosomes originate from the endocytosis of cells. In the process of endocytosis, plasma membrane invagination encloses part of the extracellular components and membrane proteins to form early endosomes, which then exchange substances with other organelles or fuse with other endosomes to form late endosomes and further form multivesicular endosomes (MVEs), which contain many intracellular vesicles. Exosomes are released from the cell when multivesicular endosomes fuse with plasma membrane. Mathieu et al. (2021) proposed that EVs bearing CD63 together with one or the two of CD9 and CD81 correspond to exosomes derived from multivesicular endosomes.
It is generally believed that exosomes have specific functions, such as assisting the transport and transmission of proteins, RNA and other molecules between cells, to realize the transfer of materials and information between cells (Rashed et al. 2017). Exosomes play important roles in antigen presentation and in information transmission between cells (Bang and Thum 2012). These characteristics of exosomes make them of great significance in basic research of life science and clinical application. For example, they may be used as biomarkers to detect cancer, tumor, or other diseases clinically. But now there are still many difficulties in the isolation and extraction of exosomes. Obviously, there are little difference in size and density between extracellular vesicle subgroups, which led to a problem: exosome subgroup could not be completely purified if relying on their difference in size and density from other EVs. Therefore, isolation and separation technology of exosomes has become one of the limiting factors in the research and application of exosomes.
According to the physical, biological, and chemical properties of exosomes, the commonly used methods for isolation of exosomes include ultracentrifugation, ultrafiltration, size-exclusion chromatography (SEC), polymer precipitation, and immunoaffinity separation. In addition, in recent years, the use of microfluidic technology and other new methods to isolate exosomes are also being studied and developed.
Methods to Isolate Exosomes
Ultracentrifugation
Ultracentrifugation is a relatively common method to separate exosomes.
Johnstone et al. (1983) initially isolated exosomes from the supernatant of sheep reticulocyte culture medium by differential centrifugation. The improved differential centrifugation method has been widely adopted after optimization by Thery et al. (Bobrie et al. 2012), including application of size-exclusion techniques to exclude proteins prior to ultracentrifugation and use of ultrafiltration membranes to eliminate large vesicles (including small aggregated vesicles) during exosome isolation. Differential centrifugation method is low cost, simple operation, and can acquire more EVs, which is suitable for preparing large-volume samples. However, this method is time-consuming, expensive equipment requiring, unstable recovery. In addition, repeated high-speed centrifugation may cause damage to exosomes, thereby reducing their quality.
Gardiner et al. 2016; Doyle et al. 2019 found that ultracentrifugation is the most widely used exosome separation method so far, accounting for 85% or even higher, and is usually used to separate exosomes from conditioned cell culture medium. Lobb et al. (2015) also pointed that ultracentrifugation is the most widely used method for exosome separation and has been considered as the gold standard for separating exosome populations of relatively the same size for a long time. Tian et al. (2019) evaluated the quality and efficiency of commonly used exosome separation methods by using nano-flow cytometry, and found that when exosomes were separated from plasma samples, the purity of exosomes separated by ultracentrifugation was significantly higher than that of other methods. However, the centrifugation process of ultracentrifugation is long, and the recovery rate is often variable. Purity of isolated exosomes cannot be guaranteed by ultracentrifugation alone, for many substances including other extracellular vesicles have the same density as exosomes. In addition, Cvjetkovic et al. (2014) found that due to the different types of rotors used by researchers, results would be different, and repeated centrifugation steps also had an impact on the quality of isolated exosomes. Studies have shown that (Anderson 1966), the coupling of differential centrifugation and density gradient ultracentrifugation effectively improves the purity and quality of isolated particles, but the only disadvantage is that the preparation and additional maintenance of gradients require extra time to prevent gradient damage during centrifugation.
Ultrafiltration
The method involves the use of membrane filters with specific size-exclusion limits, exosomes can be selectively separated from other constituents of sample using ultrafiltration membranes with different relative molecular weight interception (MWCO). The sample solution is continuously passed through membrane filters with different pore sizes to separate exosomes.
Vergauwen et al. (2017) separated the same sample with 5 different centrifugal membranes, then for each column 2 mL product was concentrated to 0.1 mL by centrifugation at 3000 × G at 4 °C. Then the products harvested with different methods were analyzed by NTA, electron microscopy, protein concentration measurement, Western blot and RT-qPCR. After comparison, it was found that the selection of filter and membrane (type, aperture size) had a great influence on the recovery rate of EVs in the sample. The different binding ability of EVs with various membrane filters would lead to the recovery rate change. Among them, regenerated cellulose membrane is the best membrane for EVs samples, and the adhesion of EVs to regenerated cellulose membrane can be minimized by reasonable adjustment of pore size.
Ultrafiltration is simple and efficient, requires less equipment, and does not affect the biological activity of exosomes due to operation problems. Because ultrafiltration is carried out at room temperature without the addition of chemical reagents, there is no damage to exosomes, so the purity of exosomes extracted is high (Zhang Yu-Xing et al. 2018). However, ultrafiltration is separated according to size difference between target and other components, which determines that there may be other extracellular vesicles of similar size in the isolated exosomes (Livshits et al. 2015). In addition, the pressurization process in ultrafiltration may cause some mechanical damage to exosomes.
Size‑Exclusion Chromatography (SEC)
Size-exclusion chromatography (SEC) is a technique for separating exosomes based on their size. SEC has been proven to provide high-quality EV isolation from plasma, Benedikter et al. (2017) believed that the purity of exosomes isolated by this method was sufficient for mass spectrometry analysis. SEC does not require the use of centrifuges, and purity of exosomes isolated is comparable to that of ultracentrifugation. However, SEC method has several defects. It is time consuming to concentrate for harvested exosomes are severely diluted. It is also time consuming for combining and eluting processes of components in sample.
Guan Sheng et al. (2020) isolated exosomes from urine by size-exclusion chromatography (SEC) and ultracentrifugation (UC) in their experiments respectively. Western Blot, quantitative evaluation of BCA protein, tracking analysis and evaluation of nanoparticles, fluorescence labeling and fluorescence intensity analysis were applied to characterize exosomes by these two methods. The results showed that the recovery rate of exosomes isolated with SEC was higher than that isolated with UC, and the structural integrity and biological activity of exosomes isolated with SEC were better than that of UC, which was conducive to subsequent analysis.
In addition, Yong Qin Koh et al. (2018) found in a comparison of four exosome enrichment methods that the combination of ultracentrifugation and size-exclusion chromatography (qEV column) is more effective than the two methods alone in enriching exosomes. Current commercial columns have a sample capacity limit, which reduces their possibility in high-throughput applications, such as qEV columns designed to load samples which are less than or equal to 500µL. However, EVs enrichment based on ultracentrifugation allows processing of a larger sample volume and thus greater exosome production. An exclusion column is then used to effectively remove soluble proteins and other contaminants and thus specifically enrich exosomes. The combination of the two methods not only reduced the variability of the experiment, but also reduced the time required for exosome enrichment, and improved the yield and recovery. This approach may also help to enrich exosomes from other types of body fluids, such as milk, saliva and urine. Exosomes isolated by SEC-coupled technology have high quality, which can be used for protein and RNA diagnosis, as well as drug and drug delivery system research (Sidhom et al. 2020).
Polymer Precipitation
Since this method is now rarely used alone, it is usually an auxiliary method, so it will be briefly stated here.
In the early stage of exosome research, several commercial kits have been developed and patented based on polymer precipitation method for the separation of exosomes from cell culture medium and various body fluids (Witwer et al. 2013), for example, ExoQuick from System Biosciences (Palo Alto, CA, USA) and total exosome isolation kits from Thermo Fisher Scientific. These kits allow rapid isolation and separation of exosomes, but are not suitable for large-scale sample processing. At the 2012 ISEV Symposium in New York, participants discussed the possibility of exosomes isolated by polymer precipitation for containing large amounts of non-EV contaminants such as lipoproteins, pointing that further separation is required after polymer precipitation treatment if higher exosome purity needed.
Immunomagnetic Bead Capture Method
In extracellular vesicles, only the surface of exosomes is rich in some special membrane proteins, such as CD9, CD63, CD81, CD82, and Hsp70. These special membrane proteins can be used as specific markers for the separation of exosomes. After fixing with antibodies of these marker proteins, magnetic beads and other solid phase can capture exosomes. Exosomes can be separated from the magnetic bead—exosome complex by elution step. Because the heterogeneity of exosomes results from their origin, the abundance of these markers is also different on different exosomes. Different types of exosomes can be captured with magnetic beads or other solid phase coupled with specific antibodies, among which CD63 antibody is a commonly used one.
Zarovni et al. (2015) developed nano-immunomagnetic bead, which can adsorb exosomes. ThermoFisher and other companies launched commercial exosome separation magnetic beads, Miltenyi Biotec and other companies developed immune separation kits (Jin-ge et al. 2020).
This method is suitable for the separation of exosomes from specific sources with high purity and little influence on the structure and morphology of exosomes. Therefore, it is a better method for enrichment and characterization of unique exosomes. However, the cost of immunoaffinity capture method is high. In addition, the change of pH and the addition of salt reagents in the process of exosome elution may affect the subsequent research and application.
Aptamer-Based Method for Exosome Isolation
This method isolates exosome based on specific recognition and binding between special aptamers and exosome surface proteins. There are many specific protein molecules on the surface of exosomes such as CD63, and their corresponding aptamers recognize target proteins on the surface of exosomes by conformational complementarity and bind to exosomes. By adjusting the buffer system and other conditions, the aptamer structure and shape change and the release of exosomes occurs. Chao Liu et al. (2019) developed a method of λ-DNA and DNA adaptor to separate exosomes, which achieved the size-selective separation and analysis of single exosome surface proteins simultaneously. Zhang et al. (2019) developed a method for exosome isolation based on CD63 aptamer. They labeled exosomes with biotin-modified CD63 aptamer, and streptavidin-coupled magnetic beads were added to capture the exosomes labeled with CD63 aptamer. By adding complementary oligonucleotide to aptamer DNA, exosomes are released and can be detached from the magnetic beads with high separation speed, recovery of 78%, and less protein impurities.
On account of the good specificity of aptamer, this separation method not only ensures high purity of exosomes, but also has good separation efficiency and tends to be stable, with high recovery and little damage to exosomes, which can be used for rapid and non-destructive separation of exosomes. At present, the technique of exosome isolation by aptamer is still under further research and development.
Isolation Methods Based on Microfluidic Technology
Exosome separation methods based on microfluidic technology may have advantages in the application fields of in vitro diagnosis of diseases and drug carrier development, so there are also many studies. Chen et al. (2010) first applied microfluidic chip to the separation of exosomes. In recent years, microfluidic techniques for exosome separation and their application in diagnosis in vitro have also received a lot of attention (Liao et al. 2019).
Filtration
Filtration is a proven label-free isolation method using physical holes, porous membranes, or nanoarrays to separate exosomes. Typically, there are two types: direct-flow and cross-flow filtration (Hassanpour Tamrin et al. 2021; Shirejini et al. 2022). In direct-flow, the sample flows vertically through the membrane surface with the solution, and the trapped particles accumulate on the membrane surface, which easily leads to concentration polarization and reduced filtration efficiency. However, in cross-flow filtration, the sample solution passes along the membrane surface and generates shear force to reduce the accumulation of particles on the membrane surface, thus overcoming the problem of clogging the membrane due to high pressure drop.
Liang et al. (2017) developed an integrated dual-filter microfluidic device for the separation, enrichment, and quantification of EVs in urine. Particles larger than 200 nm were excluded by the membrane with a pore size of 200 nm in the sample chamber, whereas particles smaller than 30 nm passed through the dual-filter microfluidic device. Finally, EVs with a size between 30 and 200 nm were isolated and enriched in the isolation chamber (Liang et al. 2017; Su et al. 2019).
Deterministic Lateral Displacement (DLD)
DLD is a newly developed microfluidic technology for separation based on fluid dynamics and particle size. The microfluidic device consists of tilted column arrays, which lead to fluidic bifurcations and create unique streamlines between gaps in column arrays (Iliescu et al. 2019; Mousavi et al. 2022). Particle flow in a DLD array is affected by fluid forces and cylinder barrier effects. As a result, particles with a radius smaller than the critical size follow the fluid flow direction and move in a zigzag shape, while particles larger than the critical size collide with the pillars and are displaced laterally across the array, thus arriving to the next streamline (Hassanpour Tamrin et al. 2021; Contreras-Naranjo et al. 2017; Iliescu et al. 2019). The critical size is determined by the clearance distance between the two columns and the column displacement angle due to the lateral offset of each row of the column array (Huang et al. 2004). Smith et al. (2018) designed a chip integrated with 1024 nano-DLD array to isolate EVs at a high speed of 900 μL/h and have a 50% yield for blood and urine samples. This method also has the potential to isolate exosomes.
As mentioned above, although this method can effectively separate particles, it still presents some challenges and problems for exosomes with a diameter of less than 200 nm. Due to the small size of nanoparticles, the lateral force caused by their diffusivity is quite large, which affects the separation efficiency (Hassanpour Tamrin et al. 2021). In addition, DLD, like filtration, also carries the risk of clogging, and since the DLD method uses a large number of columns, which increases fluid resistance, so that it may not be suitable for exosome isolation of large sample volumes (Shirejini et al. 2022).
Viscoelastic Flow
Viscoelastic flow is a continuous label-free microfluidic technology based on size to isolate exosomes. In this method, isolation depends on particle migration induced by size-dependent elastic lifting forces in viscoelastic media. Liu et al. (2017) developed a viscoelasticity-based microfluidic system by adding 0.1 wt% PEO to the solution to create a viscoelastic environment to provide a viscoelastic force for particles of different sizes. Large particles rapidly migrate to the centerline of the channel due to greater viscoelastic force and are collected at the middle outlet, whereas exosomes migrate slowly to the center of the channel, and finally stay near the sidewall of the downstream microchannel and are collected at two-sided outlets. The critical size in this viscoelasticity-based microfluidic device can be controlled by adjusting the PEO concentration. This method is simple to operate and achieves high purity (> 90%) and high recovery (> 80%), without causing physical damage to exosomes (Liu et al. 2017; Shirejini et al. 2022).
Acoustic-Based Microfluidic Technology
Acoustic-based microfluidic technology provides reagent-free and contact-free method to isolate exosomes (Le et al. 2021). Kyungheon Lee et al. (2015) developed a microfluidic technology based on acoustics, using acoustic nanofiltration to separate exosomes from the matrix according to the size of particles. Samples containing exosomes and other cellular components were first injected into a chamber exposed to ultrasound. Ultrasonic waves apply a radiant force to these particles, which then respond to the radiant force and migrate to the pressure node. In general, the rate of migration is proportional to the size of the particle. Ultrasonic waves are tuned in this way to separate particles in a wide range. This method is simple, fast, adjustable, and requires a low initial volume (50µL).
Current acoustic-based isolation can only deal with biological fluids, which require pre-process before exosomes isolation, and thus increasing the complexity of the operation and the risk of sample loss (Wu et al. 2017; Shirejini et al. 2022). Wu et al. (2017) from Duke University in the United States developed the sonic-microfluidic technology by using sound pressure nodes to make particles or cells of different sizes deviate from the center, so as to separate particles of different sizes at the end of the channel. This method can process 100μL blood sample in 25 min, with the recovery of more than 80%, and with the purity of more than 90%, and the fewer loss of exosomes.
Electrostatic-Adsorption
Electrostatic-adsorption is a simple, fast, label-free approach, and easy to integrate. Chen et al. (2021) integrated chitosan electrostatic adsorption, scaffold matrix, shuttle flow on a microfluidic chip, since exosomes have negatively charged phosphate groups, exosomes can be captured or released by adjusting the pH of the solution in the microchannel to change the surface charge polarity of chitosan. In addition, the Y-shaped column array and shuttle flow in the microchannel ensure sufficient contact between the sample and chitosan to improve the separation efficiency. This method achieved rapid isolation of exosomes from quantitative samples (10 μL) within 15 min, and can ensure high purity (> 90%) and high RNA recovery rate (> 84%), which cannot be achieved by traditional centrifugation methods (Chen et al. 2021; Zhang et al. 2021).
Immune-Based Microfluidic Technology
Among microfluidic technologies, the most widely used method is immune microfluidic separation, which is mainly based on immunological principles to separate exosomes. Exosomes have various specific membrane-bound proteins on their surfaces. By immobilizing specific antibodies on microfluidic chips, exosomes can be captured based on interaction between membrane-bound proteins and antibodies. The principle of this method is similar to that of the immunomagnetic bead capture method, but the advantage of the immune microfluidic separation method is that it can separate exosomes from 10–100ul of serum within 60 min (Doyle et al. 2019).
He et al. (2014) developed a new microfluidic method based on immunology, integrating magnetic beads on microfluidic chips for specific immune separation and targeted protein analysis of exosomes. This method qualified for the requirement for characterization and analysis, thus improving analytical sensitivity (LIU Na et al. 2019). Immunology-based separation methods have the advantage of specificity, but due to the disadvantages of biological antibodies, such as difficult preparation, high price, poor stability, problems with pH and changes of salt concentration or ionic strength in elution of antigen, the application of this method is limited. Antibody substitutes with high stability, low cost, and convenient elution need to be developed for microfluidic chips (Liao et al. 2019).
Aptamer-Based Microfluidic Technology
A number of the microfluidic chips are based on aptamers, which are specific pieces of DNA that recognize and bind to molecules such as proteins. Xu et al. (2018) developed a two-stage microfluidic platform sensor (Exo PCD chip) based on DNA aptamers (Zhang et al. 2019). The chips integrate functions of separation of exosomes and in situ electrochemical analysis of blood samples. It is concluded that the capture efficiency of this method is higher than the common microfluidic chip based on antigen–antibody interaction, and can separate exosomes under mild elution conditions, which is beneficial to the analysis of downstream experiments.
Isolation methods based on microfluidic technology has made remarkable progress. What makes microfluidic technology outstanding is that it can integrate different functions into the chip, such as downstream analysis to detect the main components of exosomes (Raju et al. 2022). In addition, microfluidic chips also have the advantages of continuous separation and automation. It should be emphasized that microfluidic methods have become a trend. With the development of microfluidic chips, more applicable devices will emerge.
Discussion
Comparison Between Exosome Isolation Methods
The comparison of methods mentioned above to isolate exosomes is shown in Table 1.
The isolation and separation of exosomes is an important and promising technique in the application and research of exosomes. Since the discovery of exosomes, isolation methods such as ultracentrifugation, ultrafiltration, size‑exclusion chromatography, precipitation, immunomagnetic bead capture method, aptamer-based isolation, and isolation based on microfluidic technology have been developed, all of which are based on the unique properties of exosomes such as size, density, and surface proteins respectively.
Although these technologies have been relatively mature and have played their respective advantages in the isolation of exosomes, there are still some problems in terms of convenience, rapidity, purity, recovery rate, and physiological activity. For example, the purity and recovery rate of precipitation are low. Ultracentrifugation is implemented according to the density of sample particles, however, the density of extracellular vesicles from different sources may not differ significantly. Therefore, the products obtained may contain both exosomes and some other membrane vesicles, so we can only call them extracellular vesicles rather than exosomes. Ultrafiltration and SEC methods are based on the size of the extracellular vesicles to be isolated. Since there is no strict limit on the size of exosomes and other extracellular vesicles, the obtained product may still be a mixture of various extracellular vesicles. In addition, the method of separating exosomes based on density and size may also cause some damage to exosomes in the separation process, which affect most applications and studies of exosomes. Method based on the principle of immunology and adapter body belongs to affinity separation, which has good specificity, moreover, the effect of isolation is better. However, the price of affinity materials for target objects based on immunological separation method is relatively high, and some ion or pH conditions brought by elution process are unfavorable for downstream research and application.
Conclusion
In recent years, many researchers have found that the effect of using only one traditional separation method cannot meet the expected requirements. As a result, most of them prefer to adopt combination of two or more isolation techniques to complement each other and optimize the separation results. Although ultracentrifugation has been regarded as the gold standard for exosome separation for quite a long time, it is limited in several aspects stated above so that it gradually fails to be regarded as most reliable method. We believe that affinity isolation is a better option for exosome isolation in the future considering its advantage of simple operation, few steps and ability to obtain high-quality exosomes. For single sample, magnetic affinity isolation is better. For batch samples continuous and automated separation methods may be more suitable, such as microfluidic methods.
As can be seen from Table 1, each method currently has more or less limitations. Therefore, for the significance of exosome, isolation method which adapts to more conditions or to obtain qualified exosome or large amount of exosome conveniently will be a continuous pursuit in following years.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Anderson NG (1966) An introduction to particle separations in zonal centrifuges. Natl Cancer Inst Monogr 21:9–39 (PMID: 5926674)
Bang C, Thum T (2012) Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44(11):2060–2064. https://doi.org/10.1016/j.biocel.2012.08.007 (Epub 2012 Aug 10 PMID: 22903023)
Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, Wouters EFM, Savelkoul PH, Lopez-Iglesias C, Koenen RR, Rohde GGU, Stassen FRM (2017) Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep 7(1):15297. https://doi.org/10.1038/s41598-017-15717-7
Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C (2012) Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 16:1. https://doi.org/10.3402/jev.v1i0.18397.PMID:24009879;PMCID:PMC3760636
Chen C, Skog J, Hsu CH, Lessard RT, Balaj L, Wurdinger T, Carter BS, Breakefield XO, Toner M, Irimia D (2010) Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10(4):505–511. https://doi.org/10.1039/b916199f
Chen W, Cao R, Su W, Zhang X, Xu Y, Wang P, Gan Z, Xie Y, Li H, Qin J (2021) Simple and fast isolation of circulating exosomes with a chitosan modified shuttle flow microchip for breast cancer diagnosis. Lab Chip 21(9):1759–1770. https://doi.org/10.1039/d0lc01311k (PMID: 33710183)
Contreras-Naranjo JC, Wu HJ, Ugaz VM (2017) Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17(21):3558–3577. https://doi.org/10.1039/c7lc00592j
Cvjetkovic A, Lötvall J, Lässer C (2014) The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles 25:3. https://doi.org/10.3402/jev.v3.23111 (PMID:24678386;PMCID:PMC3967015)
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7):727. https://doi.org/10.3390/cells8070727
Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF (2016) Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 31(5):32945. https://doi.org/10.3402/jev.v5.32945
Guan S, Yu H, Yan G, Gao M, Sun W, Zhang X (2020) Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J Proteome Res 19(6):2217–2225. https://doi.org/10.1021/acs.jproteome.9b00693 (Epub 2020 Apr 28 PMID: 32248692)
Hassanpour Tamrin S, Sanati Nezhad A, Sen A (2021) Label-free isolation of exosomes using microfluidic technologies. ACS Nano. https://doi.org/10.1021/acsnano.1c03469 (PMID: 34723478)
He M, Crow J, Roth M, Zeng Y, Godwin AK (2014) Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 14(19):3773–3780. https://doi.org/10.1039/c4lc00662c
Hendrix A (2021) The nature of blood(y) extracellular vesicles. Nat Rev Mol Cell Biol 22(4):243. https://doi.org/10.1038/s41580-021-00348-8 (PMID: 33568799)
Huang LR, Cox EC, Austin RH, Sturm JC (2004) Continuous particle separation through deterministic lateral displacement. Science 304(5673):987–990. https://doi.org/10.1126/science.1094567 (PMID: 15143275)
Iliescu FS, Vrtačnik D, Neuzil P, Iliescu C (2019) Microfluidic technology for clinical applications of exosomes. Micromachines (basel) 10(6):392. https://doi.org/10.3390/mi10060392.PMID:31212754;PMCID:PMC6631586
Jin-ge QIU, De-wu LIU, Bao-li SUN, Yao-kun LI, Yong-qing GUO, Ming DENG, Guang-bin LIU (2020) Research progress on animal exosome isolation methods. China Biotechnology 40(9):36–42. https://doi.org/10.13523/j.cb.2004051
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262(19):9412–9420
Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD (2018) Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (landmark Ed) 23(5):865–874. https://doi.org/10.2741/4621 (PMID: 28930577)
Le MN, Fan ZH (2021) Exosome isolation using nanostructures and microfluidic devices. Biomed Mater 16(2):022005. https://doi.org/10.1088/1748-605X/abde70.PMID:33477118;PMCID:PMC8082697
Lee K, Shao H, Weissleder R, Lee H (2015) Acoustic purification of extracellular microvesicles. ACS Nano 9(3):2321–2327. https://doi.org/10.1021/nn506538f
Liang L-G et al (2017) An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci Rep 7:46224. https://doi.org/10.1038/srep46224
Liao Z, Li Y, Gu L, Lei R, Miao Y, Lan H, Deng Y, Geng L (2019) Advances in microfluidic chip-based extracellular vesicle separation. Se Pu. 37(4):343–347. https://doi.org/10.3724/SP.J.1123.2018.11045
Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei J, Hu G, Nie G, Sun J (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11(7):6968–6976. https://doi.org/10.1021/acsnano.7b02277 (Epub 2017 Jul 7 PMID: 28679045)
Liu C, Zhao J, Tian F, Chang J, Zhang W, Sun J (2019) λ-DNA- and aptamer-mediated sorting and analysis of extracellular vesicles. J Am Chem Soc 141(9):3817–3821. https://doi.org/10.1021/jacs.9b00007 (Epub 2019 Feb 22 PMID: 30789261)
Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM (2015) Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 30(5):17319. https://doi.org/10.1038/srep17319
Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A (2015) Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 17(4):27031. https://doi.org/10.3402/jev.v4.27031
Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Théry C (2021) Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 12(1):4389. https://doi.org/10.1038/s41467-021-24384-2.PMID:34282141;PMCID:PMC8289845
Mousavi SM, Amin Mahdian SM, Ebrahimi MS, Taghizadieh M, Vosough M, Sadri Nahand J, Hosseindoost S, Vousooghi N, Javar HA, Larijani B, Hadjighassem MR, Rahimian N, Hamblin MR, Mirzaei H (2022) Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol Ther Nucleic Acids 27(28):758–791. https://doi.org/10.1016/j.omtn.2022.04.011
Na LIU, Pan-pan DU, Yang YANG, Xiao-mao LI (2019) Research progress on exosomes isolation methods based on microfluidics technology[J]. Biotechnol Bull 35(1):207–213. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2018-0571
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33(3):967–978. https://doi.org/10.1016/0092-8674(83)90040-5 (PMID: 6307529)
Raju D, Bathini S, Badilescu S, Ghosh A, Packirisamy M (2022) Microfluidic platforms for the isolation and detection of exosomes: a brief review. Micromachines 13(5):730. https://doi.org/10.3390/mi13050730
Rashed MH, Bayraktar E, Helal KG, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C (2017) Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 18(3): 538 doi: https://doi.org/10.3390/ijms18030538. PMID: 28257101; PMCID: PMC5372554.
Shirejini SZ, Inci F (2022) The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol Adv 54:107814. https://doi.org/10.1016/j.biotechadv.2021.107814
Sidhom K, Obi PO, Saleem A (2020) A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci 21(18):6466. https://doi.org/10.3390/ijms21186466
Smith JT, Wunsch BH, Dogra N, Ahsen ME, Lee K, Yadav KK, Weil R, Pereira MA, Patel JV, Duch EA, Papalia JM, Lofaro MF, Gupta M, Tewari AK, Cordon-Cardo C, Stolovitzky G, Gifford SM (2018) Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip 18(24):3913–3925. https://doi.org/10.1039/c8lc01017j (PMID: 30468237)
Su W, Li H, Chen W, Qin J (2019) Microfluidic strategies for label-free exosomes isolation and analysis Trac Trends Anal. Chem 118:686–698. https://doi.org/10.1016/j.trac.2019.06.037
Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X (2019) Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles 9(1):1697028. https://doi.org/10.1080/20013078.2019.1697028
Tulkens J, De Wever O, Hendrix A (2020) Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat Protoc 15(1):40–67. https://doi.org/10.1038/s41596-019-0236-5 (Epub 2019 Nov 27 PMID: 31776460)
Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, Van den Broecke R, Denys H, De Wever O, Hendrix A (2017) Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep 7(1):2704. https://doi.org/10.1038/s41598-017-02599-y
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 27:2. https://doi.org/10.3402/jev.v2i0.20360
Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ (2017) Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A 114(40):10584–10589. https://doi.org/10.1073/pnas.1709210114
Xu H, Liao C, Zuo P, Liu Z, Ye BC (2018) Magnetic-based microfluidic device for on-chip isolation and detection of tumor-derived exosomes. Anal Chem 90(22):13451–13458. https://doi.org/10.1021/acs.analchem.8b03272 (Epub 2018 Oct 3 PMID: 30234974)
Yu-Xing Z, Xiang Z, Wen-Jie C, Li-Guo Z (2018) Research progress on the methods of exosome isolation [J]. E-Journal of Translational Medicine 5(04):42–46
Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, Muhhina J, Fondelli C, Gavrilova J, Chiesi A (2015) Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 1(87):46–58. https://doi.org/10.1016/j.ymeth.2015.05.028 (Epub 2015 Jun 2 PMID: 26044649)
Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z (2019) Rapid capture and nondestructive release of extracellular vesicles using aptamer-based magnetic isolation. ACS Sens 4(5):1245–1251. https://doi.org/10.1021/acssensors.9b00060 (Epub 2019 Apr 29 PMID: 30915846)
Zhang H, Zhang Q, Deng Y, Chen M, Yang C (2021) Improving isolation of extracellular vesicles by utilizing nanomaterials. Membranes (basel) 12(1):55. https://doi.org/10.3390/membranes12010055.PMID:35054584;PMCID:PMC8780510
Acknowledgements
The authors thank the laboratory and the research group for their consistent research on new methods for exosome separation. The authors also thank the foundation for its support.
Funding
This work was supported by National Natural Science Foundation of China international (Regional) Cooperation and exchange project “Research on Bio-inspired New Material Additive Manufacturing (Number 5181101987) “.
Author information
Authors and Affiliations
Contributions
ES performed the conceptualization; WX, JC, LA wrote the main manuscript text; ES was in charge of review and supervise. WX prepared Fig. 1 and Table 1 and was responsible for the edit. All authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, WM., Li, A., Chen, JJ. et al. Research Development on Exosome Separation Technology. J Membrane Biol 256, 25–34 (2023). https://doi.org/10.1007/s00232-022-00260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-022-00260-y