Abstract
More than 80% of maturity-onset diabetes of the young (MODY) in Chinese is genetically unexplained. To investigate whether the insulin gene (INS) mutation is responsible for some Chinese MODY, we screened INS mutations causing MODY10 in MODY pedigrees and explored the potential pathogenic mechanisms. INS mutations were screened in 56 MODY familial probands. Structure–function characterization and clinical profiling of identified INS mutations were conducted. An INS mutation, at the position 2 alanine-to-threonine substitution (A2T), was identified and co-segregated with hyperglycemia in a MODY pedigree. The A2T mutation converted an α-helix into a β-sheet at the N-terminal of the signal peptide (SP) of preproinsulin. The A2T mutation did not affect preproinsulin translocation across endoplasmic reticulum (ER) membrane, but impaired its SP cleavage within the ER. In INS-1 cells transfected with an A2T mutant, glucose-stimulated insulin secretion (GSIS) was significantly decreased, while BiP luciferase activities were significantly increased compared to that of wild type (WT). We identified an INS-A2T mutation cosegregating with diabetes in a Chinese MODY pedigree. This mutation severely impaired SP cleavage and thus blocked the formation of proinsulin, resulting in enhanced ER stress, which may be responsible for decreased insulin secretion and subsequently, the onset of MODY10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maturity-onset diabetes of the young (MODY) with autosomal dominant mode of inheritance constitutes 1–5% of diabetes cases in the USA and other industrialized countries [1]. A recent population study demonstrated that 3% of diabetes diagnosed under 30 in the UK was due to MODY [2]. Clinically, MODY is frequently misdiagnosed and precision treatment thereof is strongly dependent on genetic diagnosis and genetic counseling [3]. It has been determined that abnormalities in at least 13 genes on different chromosomes contribute to the development of MODY [4]. Mutations in the six classic MODY causative genes, i.e., HNF4A, GCK, HNF1A, PDX1, HNF1B, and NEUROD1/BETA2, have been recognized as the underlying cause for 80% of MODY in Caucasians [3]. In contrast, more than 80% of MODY in Chinese cannot be attributed to the above six MODY pathogenic genes, especially MODY types 1–3 [5]. Insulin, the uniquely hypoglycemic hormone in vivo, is secreted from the pancreatic β cells in a tightly regulated manner to maintain glucose homeostasis. Mutations in INS do not merely result in permanent neonatal diabetes (PNDM) [6, 7], but also lead to insulinopathies [8], autoantibody-negative type 1 diabetes, and MODY10 [9].
The human INS gene spans 1431 bp and is located at 11p15.5, contains three exons and two introns, and encodes a single-chain precursor—preproinsulin composed of 110 amino acids [10]. From the amino- (N) to carboxyl- (C) terminus, preproinsulin is comprised sequentially of the signal peptide, B-chain, C-peptide with its two sets of flanking dibasic cleavage sites, and the A-chain [11]. The newly synthesized preproinsulin in the rough ER is translocated to the luminal side of the ER, wherein signal peptidase cleaves the signal peptide to form proinsulin. Within the ER lumen, proinsulin undergoes oxidative folding, forming three evolutionarily conserved disulfide (S–S) bonds that are essential for insulin stability and bioactivity [12]. The proinsulin is then transported to the Golgi apparatus, where the C-peptide is excised to form mature bioactive insulin stored in secretory granules [12].
The dominant/recessive mutations in the INS gene that affect (pre)proinsulin biosynthesis and proinsulin folding are associated with a spectrum of diabetes phenotypes, ranging from severe PNDM to mild MODY [7, 13, 14]. Dominant mutations in the coding regions of the INS result in PNDM and MODY via proinsulin misfolding, ER stress, and/or β cell failure [14], whereas recessive mutations lead to PNDM by affecting insulin biosynthesis at transcriptional and/or translational level [7].
Here, we used PCR-direct sequencing and multiplex ligation-dependent probe amplification (MLPA) to screen for INS mutations in 56 probands with MODY who were negative for the six classic MODY genes, and a missense mutation, A2T, was identified in INS from a MODY family. The genotype-clinical phenotype correlation of patients bearing A2T mutation was analyzed, and the pathogenesis of MODY10 caused by the A2T mutation was investigated.
Materials and methods
Subjects
From 2015 to 2017, unrelated MODY probands who fulfilled the conventional MODY criteria (non-obesity, absence of autoantibodies, at least one patient with onset age < 25 years, a family history of diabetes for at least three consecutive generations) [1] and their family members were referred to or recruited by the Department of Endocrinology & Metabolism, Shanghai Diabetes Institute, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Subsequently, 56 MODY probands who were negative for HNF4A, GCK, HNF1A, PDX1, HNF1B, and NEUROD1/BETA2 gene mutations underwent INS genetic testing, genetic counseling, and standardized clinical and laboratory evaluation [15, 16]. In addition, we enrolled 201 unrelated, non-diabetic control subjects of Han Chinese descent according to the following criteria: age ≥ 60 years, no diabetic family history, normal glucose tolerance (NGT) [17].
All participants completed medical and family history questionnaires, and their information was supplemented with information from the medical records. The American Diabetes Association criteria (2015) were used for diagnosing diabetes, impaired fasting glucose (IFG), and impaired glucose tolerance (IGT) [18]. Written informed consent was obtained from all participants. This study was approved by the Institutional Review Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
Identification of INS mutations
Genomic DNA was extracted from the peripheral leukocytes of the 56 MODY pedigrees, and the coding regions and flanking regions of INS were sequenced using PCR-direct sequencing and MLPA [16, 17]. Two primer pairs, i.e., exon 1 and 2: forward, 5′-GGGTTGAGAGGTAGGGGAGA-3′ and reverse, 5′-ACAGGGAGCTG-GTCACTTTT-3′; exon 3: forward, 5′-AGAGAGCGTGGAGAGAGCTG-3′ and reverse, 5′-CCCTGACTGTGTCCTCCTGT-3′, were used [6]. Sequences were compared with the published sequence (NM_000207.2) using Sequence Navigator (Applied Biosystems). The identified mutations were examined for co-segregation with hyperglycemia in other family members, and the genotypes of mutations in the 201 control subjects were tested. The mutations and variants were numbered according to the Human Genome Variation Society (https://www.hgvs.org/).
Assessment of conservation and deleterious/pathogenic prediction for mutations
Conservation across species implicates functional critical in organisms and substitution of a significant amino acid can lead to the pathogenicity of missense mutations [19]. Specific regions of insulin protein from different mammals were aligned using ClustalX to evaluate conservation across species. The pathogenicity of the identified missense variants was predicted using the online prediction programs SIFT, based on amino acid conservation (https://sift.jcvi.org/www/SIFT_seq_submit2.html), and PolyPhen-2, which is based on the protein structure and function (https://genetics.bwh.harvard.edu/pph2/).
Secondary structure prediction
Since no existing structural information is available for the signal peptide of human preproinsulin, we used the PSIPRED web server (https://bioinf.cs.ucl.ac.uk/psipred/) to predict the secondary structure of wild type and mutated signal peptide using the default parameter. We use the Jnet reliability score in the tool (PSIPRED, https://bioinf.cs.ucl.ac.uk/psipred) to evaluate the confidence of secondary structure prediction. Based on the predicted results, the hypothesis was formed and verified using CFSSP (Chou &Fasman Secondary Structure Prediction Server).
Plasmid construction
The human INS cDNA with or without a Myc tag was subcloned into a pcDNA3.3 vector. A2T mutation was introduced using the QuikChange site-directed mutagenesis kit (Stratagene, USA) and confirmed by DNA sequencing.
Cell transfection, partial plasma membrane permeabilization with digitonin, and western blot analyses
HEK293T cells (1 × 106 cells) (Cell bank, Chinese Academy of Sciences, Shanghai, China) transfected with human pcDNA3.3-hINS-WT-Myc or pcDNA3.3-hINS-A2T -Myc were washed, resuspended, and incubated in 50 μL of 150 mmol/L NaCl, 2 mmol/L CaCl2, 50 mmol/L HEPES, pH 7.5, ± 0.01% digitonin on ice for 10 min. The cells were spun at 14,000 rpm for 10 min at 4 °C; the supernatant was transferred to a new tube containing 50 μL lysis buffer and the pellet lysed in 150 μL lysis buffer. Both supernatant and pellet were detected by western blot with anti-Myc-tag or anti-GFP antibody, and analyzed using 12% SDS-PAGE [20].
Dual luciferase reporter gene assay
The promoter of BiP was designed, synthesized, and subcloned at the XhoI/HindIII sites of the pGL3-basic vector. Negative control (NC), i.e., empty pcDNA3.3, pcDNA3.3-hINS-WT, or pcDNA3.3-hINS-A2T, was co-transfected with pGL3-BiP promoter into INS-1 cells (Cell bank, Chinese Academy of Sciences, Shanghai, China), respectively. The pRL-TK plasmid (Promega, USA) containing the Renilla luciferase gene was used as an internal control. The luciferase activity was measured 48 h after transfection according to manufacturer’s instructions. Each reaction was run in triplicates.
Glucose-stimulated insulin secretion (GSIS)
Rat INS-1 cells (Cell bank, Chinese Academy of Sciences, Shanghai, China) were plated onto 12-well plates 1 day before transfection with Lipofectamine (Invitrogen, USA) using 2 μg pcDNA3.3, pcDNA3.3-hINS-WT, or mutant pcDNA3.3-hINS-A2T. 48 h after transfection, cells were incubated with DMEM containing 25.5 mmol/L glucose and 10% FBS (Gibco, Thermo Fishier, USA) for 16 h. The media were then collected and measured by commercially available insulin ELISA Kit. In parallel, duplicated wells of each transfection were used for total RNA isolation. Human insulin mRNA levels were determined by RT-PCR using forward primer: 5′-GCAG CCTTTGTGAACCAACAC-3′ and reverse primer: 5′-CCCCGCACACTAGGTAGA GA-3′.
Statistical analysis
All clinical and laboratory values were presented as means ± SEM unless otherwise stated. Comparison of the clinical and laboratory parameters between genotypic groups was performed using unpaired Student's t-tests and Pearson χ2 tests as appropriate. P < 0.05 was considered to be significant. SPSS19.0 (SPSS Inc.) was used for data analysis and processing.
Results
Genetic and clinical phenotype analysis
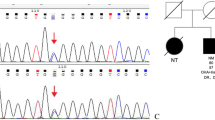
A heterozygous missense mutation in the INS gene, A2T (c.4G>A, p.Ala2Thr), was identified in one of the MODY families (Fig. 1a). This mutation was co-segregated with diabetes in four carriers in three generations of the pedigree, but was not detected in other probands or in the 201 control subjects with NGT, indicating that the mutation is not a simple polymorphism.
Identification of INS-A2T mutation. a Pedigree, genotypes, and clinical characteristics of INS-A2T family. Black circles and squares indicate participants diagnosed with MODY. White circles and squares indicate normal glucose tolerance (NGT); red arrow indicates the index case for the family. Individuals treated with insulin did not undergo the oral glucose tolerance test (OGTT). The numbers under the symbols are the family members’ identification numbers, followed below by the genotype at codon 2 in the family, age at diabetes diagnosis for affected family members and age at examination, and finally treatment for diabetes. nd, not determined. At codon 2, N indicates a normal allele (Ala); m indicates a mutant allele (Thr). b Schematic illustration of preproinsulin and the corresponding domains in insulin. Numbers refer to the amino acids bordering the domains. Filled arrows indicate the A2T mutation identified in INS. c ClustalX alignment of specific preproinsulin regions from different mammals
The A2T mutation co-segregated with diabetes, as it was found in the proband, the proband’s mother, maternal uncle, and maternal grandfather, who were diagnosed with diabetes at 22, 39, 33, and 50 years of age, respectively (Fig. 1a). Compared to their unaffected relatives (n = 3), A2T carriers (n = 4) had significantly elevated fasting plasma glucose (FPG), 2 h plasma glucose (2 h PG) and HbA1c, and decreased fasting insulin (FINS)/FPG and 2 h insulin (2hINS)/2hPG (P < 0.05 or 0.01, Table 1), indicating the defect of endogenous insulin secretion. The body mass index (BMI) of the mutation carriers in this family was < 25 kg/m2, and the patients were negative for serum antibodies against glutamate decarboxylase (GAD) and protein tyrosine phosphatase-like protein (IA-2), which is consistent with the diagnosis of MODY.
The proband had been diagnosed with diabetes at the age of 22 with polyuria and polydipsia, and his glycated hemoglobin (HbA1c) was 8.9%. Subsequently, he had been prescribed metformin and gliclazide; however, plasma glucose control was not satisfactory, with HbA1c > 8%. The proband’s treatment was switched from oral hypoglycemic agents (OHA) to low-dose insulin (0.2 U/kg/day), and the HbA1c decreased to 7.6%. His mother, maternal uncle, and maternal grandfather had been on OHA treatment since their diagnosis of type 2 diabetes.
Bioinformatics prediction
As shown in Fig. 1b and c, the A2T mutation is located at the N-terminal of the signal peptide, and the Ala2 residue is highly conserved across mammalian species (rhesus monkey, mouse, dog, horse, platypus) and in Xenopus. The substitution of Thr for Ala results in a non-polar hydrophobic alanine changing to a polar hydrophilic threonine at the preproinsulin signal peptide. The SIFT results indicated that A2T may be deleterious (score = 0.998); another computer program PolyPhen-2 also predicted that it may be pathogenic (damaging) (score = 0.5).
As shown in Fig. 2a, the 2ALWMRLLPLLALLALW17 peptide fragment was predicted as an α-helix with high confidence (blue bars). The Proline 9 was also predicted as in an α-helix conformation. To our knowledge, prolines should structurally be helix terminators rather helix formers. We therefore believe that the PSIPRED-predicted helix (2ALWMRLLPLLALLALW17) should be kinked or separated into two short helixes by the Pro9 residue. Moreover, the confidence scores of the secondary structure of the 1–24 amino acids in the WT-preproinsulin and A2T-preproinsulin SP were 478999999999875267888999 and 828999999999875267 888999, respectively (range 0–9, bigger is better), which were evaluated by the Jnet reliability score in the tool (PSIPRED, https://bioinf.cs.ucl.ac.uk/psipred). The CFSSP results supported our assumption (Fig. 2b).
The secondary structure prediction of WT and A2T-preproinsulin signal peptide. a PSIPRED prediction for secondary structure of preproinsulin signal peptide. Conf: confidence of prediction. Pred: predicted secondary structure. AA: target sequence. Magenta cylinder: helix. Arrows: strand. Straight line: coil. b CFSSP prediction for secondary structure of preproinsulin signal peptide. c CFSSP secondary structure prediction of the A2T-substituted sequence of preproinsulin signal peptide
According to the Chou-Fasman parameters, individual amino acids have strong tendency to prefer one type of secondary structure over others. Empirically, alanine, along with glutamate, leucine, and methionine, are strong helix formers, with helix-forming tendencies of 1.42, 1.39, 1.41, and 1.45, respectively (https://swift.cmbi.ru.nl/teach/aainfo/chou.shtml). The helix-forming tendency of threonine was 0.83 and was definitely helix-unfavorable. We therefore speculate that the A2T substitution may alter the local conformation of the signal peptide. To support our assumption, the A2T-substituted sequence was resubmitted to CFSSP, and the results are shown in Fig. 2c. According to the prediction, the A2T mutation changes the α-helix into a β-sheet structure at the beginning of two amino acids, and such conformation change may affect the local conformation of the signal peptide, which supports our hypothesis.
A2T mutation does not affect translocation of A2T-preproinsulin across the ER membrane, but impaired the formation of proinsulin
Several key steps in the insulin secretory pathway involve synthesis, folding and maturation, and any defective in these steps could reduce or block completely insulin production. The translocation of newly synthesized preproinsulin polypeptide into the ER is one of the early events in the secretory pathway. To investigate whether A2T mutation affects the translocation of preproinsulin across ER membrane, we conducted a protein localization assay using transfected HEK293T cells with a recombinant A2T mutant expression vector. Digitonin was used to partially permeabilize the plasma membrane of HEK293T cells while maintaining organelle membranes intact [21] in cells coexpressing recombinant preproinsulin and cytosolic green fluorescent protein (GFP). Upon permeabilization and centrifugation, about 50% of cytosolic GFP was relocated from pellet to supernatant, while (pre)proinsulin from WT and A2T remained exclusively in the pellet. Strikingly, we observed that completely cleaved proinsulin from WT, but predominantly uncleaved preproinsulin with a minor cleaved band from A2T mutant were detected using an anti-Myc antibody (Fig. 3). These results demonstrated that the products of A2T mutant are not free in the cytosol and its localization with the ER; however, A2T mutation severely compromised the efficiency of SP cleavage of preproinsulin.
Translocation of A2T-preproinsulin across the ER membrane. After transfection, HEK293T cells coexpressing cytosolic GFP and pcDNA3.3-hINS-WT-Myc or pcDNA3.3-hINS-A2T-Myc, were treated with digitonin on ice to permeabilize the plasma membrane, which releases a major fraction of cytosolic GFP. The cells were then centrifuged and sedimented, and each pellet (P) and supernate (S) was sequentially analyzed by anti-Myc and anti-GFP immunoblotted at 12% SDS-PAGE under reducing conditions
Impact of A2T mutation on ER stress
A2T mutation leads to defective in the cleavage off the pre-peptide; therefore, uncleaved preproinsulin might be stuck in the ER and caused ER stress. To assess whether the mutants induce ER stress, we performed firefly luciferase assays driven by a BiP promoter in INS-1 cells. BiP/GRP78 is an essential class of chaperones that plays a central role in the ER to prevent protein aggregation and provide folding assistance. The up-regulation of BiP is an indicator of ER stress. We have observed that the BiP luciferase activities in A2T mutant-transfected cells were markedly increased compared to that of WT-transfected cells (Fig. 4), indicating that A2T mutation leads to heightened ER stress.
Dual luciferase reporter assay for WT and A2T mutant of hINS. The BiP promoter-driven firefly luciferase assay in INS-1 cells 48 h after transfection of WT or A2T mutants of hINS (normalized to Renilla luciferase activity). NC, negative control. Results are expressed as means ± SEM from three independent experiments. **P < 0.01
Glucose-stimulated insulin secretion (GSIS)
Our clinic data showed that A2T mutation carriers secreted insulin at only 50% levels compared to that of their unaffected relatives. Our biochemical studies demonstrated that the A2T mutation in the insulin signaling peptide could affect insulin maturation specifically at the cleavage of signaling peptide off, leading to less or no insulin produce from this allele. To further prove this assumption, we assessed the insulin production and insulin secretion in A2T transfected INS-1 cells, a rat β-cell line. WT, and A2T mutation of human insulin cDNAs were cloned in pcDNA3.3 expression vector and transfected into INS-1 cells individually. We assayed the insulin expression at the mRNA and proteins levels and found that the mRNA expression levels of mutant and WT INS genes were comparable (Fig. 5a). This suggests that the A2T mutation does not cause any defective at the transcriptional level. When transfected cells incubated with 25.5 mmol/L glucose, the insulin production in WT positive control cells was greatly enhanced; however, the insulin production in A2T mutant samples remains at the basal levels, significantly reduced compared to that of WT (Fig. 5b).
Impact of the INS-A2T mutation on glucose-stimulated insulin secretion from INS-1 cells. a Real-time PCR measurements of human insulin mRNA expression in transfected INS-1 cells. Gene expression was normalized to endogenous actin. NC, negative control. b Insulin secretion in cell culture medium from wild type and mutant A2T stimulated with high glucose (25.5 mmol/L) was detected by ELISA. NC, negative control. Results shown are means ± SEM from three independent experiments. **P < 0.01
Discussion
To our knowledge, this is the first INS/MODY10 family of A2T mutation with systemic functional analysis, which was identified in the Chinese MODY population. A2T mutation was co-segregated with diabetes in this pedigree but was not detected in 201 control subjects. Furthermore, decreased FINS/FPG and 2hINS/2hPG in mutation carriers indicated the deficiency of fasting and postprandial endogenous insulin secretion (Table 1). These results suggest that this mutation could be clinically pathogenic.
How does the A2T mutation lead to diabetes? We performed deleterious/pathogenic prediction and secondary structure analysis of the mutation, revealing that the Ala2 residue is highly conserved across mammalian species (Fig. 1c). Moreover, the substitution of Thr for Ala results in the change of a non-polar hydrophobic alanine to a polar hydrophilic threonine with a larger molecular weight, which may significantly change the spatial conformation of preproinsulin. SIFT and PolyPhen-2 predicted that the A2T mutation would be deleterious (score = 0.998) and pathogenic (damaging) (score = 0.5), respectively.
Secondary structure analysis with high confidences further indicated that the A2T mutation leads to a conformational change from α-helix to β-sheet in the preproinsulin signal peptide (Fig. 2). Preproinsulin translocation across the ER membrane occurs mainly through signal recognition protein (SRP)-dependent co-translational translocation [22, 23], and N-terminal region positive charge of signal peptide is thought to facilitate its orientation at the ER membrane. Previous study indicated that R6C mutation resulted in a misorientation of newly synthesized R6C molecules due to the loss of the positive charge, leading to the failure of preproinsulin translocation into the ER lumen [24]. Because the A2T does not involve in the change of N-terminal region positive charge, we speculated that the local conformational change of A2T may not influence SRP recognition and binding, nor affecting preproinsulin elongation and translocation across the ER membranes. As expected, our Western blotting results confirmed that A2T mutation does not hinder preproinsulin translocation across the ER membrane, but only prolonged A2T-preproinsulin ER retention due to failed signal peptide cleavage (Fig. 3), similar to that of previously reported A24D mutation (20). In addition, present study confirmed that high glucose-stimulated insulin secretion of A2T transfected INS-1 cells was significantly lower than that of WT, similar to that of NC (Fig. 5). Therefore, barely additional insulin secretion other than endogenous (basal) insulin was detected in A2T transfected INS-1 cells. We proposed that the A2T mutation blocked the formation of proinsulin, without or with minimal ER exit of proinsulin.
BiP, commonly referred to as GRP78, is an ER chaperone and function as a central regulator for ER stress signaling. In unstressed cells, ER stress transducers such as ATF6, IRE1, and PERK were kept in an inactive state by interaction with BiP. Under ER stress or formation of misfolded protein in the ER, BiP is released from these transducers and becomes activated, triggering the UPR [25]. BiP luciferase reporter activity was increased by the A2T mutation (Fig. 4), indicating unfolded protein response (UPR) and increased ER stress in the INS-1 cells.
During the treatment period, except for treatment of the proband was switched from OHA to low-dose insulin, the other three A2T mutation carriers in the MODY family were treated with OHA (Table 1). In these patients, it is possible to control glycemia with low insulin doses or OHA because one functional allele of insulin gene is usually sufficient for maintaining insulin secretion and glucose homeostasis [26]. Thus, insulin haploinsufficiency itself cannot account for early-onset insulin-deficient diabetes. Therefore, it is highly likely that the development of monogenic diabetes caused by INS mutations may be attributed to a gain-of-toxic function from the mutant gene product [27, 28]. Interestingly, aberrant conformational change of A24D-preproinsulin induced thiol attack, resulting in A24D blocking ER exit of coexpressed WT-proinsulin through dominant negative behavior, thereby leading to decreased insulin secretion [20]. Further studies are needed to confirm whether A2T leads to a decrease in insulin secretion like A24D through the similar mechanism (Fig. 5, Table 1).
The same mutation in INS may cause different clinical phenotypes in different individuals. For example, the INS gene heterozygous intronic mutation c.188-31G>A can cause mild MODY [29] and severe neonatal diabetes [30], suggesting that additional mechanisms such as other genetic or environmental factors may be involved in the pathogenesis and clinical manifestation of diabetes [13]. Interestingly, in addition to the A2T mutation found in the MODY family in this study, this mutation was also identified in a study on monogenic mutation screening of type 2 diabetes patients in China [31]. A2T mutation may cause both MODY and monogenic T2DM, which is similar to the pathophysiology of Chinese MODY2 [17] and T2DM [32] caused by mutations in GCK.
Although our present study is exciting, but it has some limitations. First, more Chinese MODY families need to be recruited to identify additional INS mutations, especially the mutations in SP, including A2T, which will further support the causal relationship between INS mutations and MODY phenotypes. Second, the studies on transgenic animal models bearing A2T mutation will provide definitive evidence whether this mutation causes the development of diabetes mellitus and its pathogenesis in vivo. Third, the precise diagnosis of MODY based on genetic and phenotypic characteristics can lead to informed decisions in treatment and improved prognosis [33]; therefore, pharmacogenomics study with expanded samples may guide precision medication of diabetes patients carrying INS mutations, such as A2T.
In summary, we identified the first MODY10 pedigree in Chinese, carrying the INS-A2T mutation. Secondary structure analyses predicted that A2T mutation in the signal peptide changed the α-helix into a β-sheet structure at the N-terminal of the signal peptide. The aberrant conformational change of A2T-preproinsulin severely impaired its signal peptide cleavage and blocked the formation and trafficking of proinsulin, although no defected in the translocation across the ER membrane. Large-scare retention of A2T-preproinsulin in ER induced enhanced ER stress, which may be responsible for the reduction of insulin secretion in MODY10 patients bearing A2T mutation.
Abbreviations
- MODY:
-
Maturity-onset diabetes of the young
- INS :
-
Insulin gene
- SP:
-
Signal peptide
- ER:
-
Endoplasmic reticulum
- GSIS:
-
Glucose-stimulated insulin secretion
References
Fajans SS, Bell GI, Polonsky KS (2001) Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 345:971–980. https://doi.org/10.1056/NEJMra002168
Shields BM, Shepherd M, Hudson M et al (2017) Population-based assessment of a biomarker-based screening pathway to aid diagnosis of monogenic diabetes in young-onset patients. Diabetes Care 40:1017–1025. https://doi.org/10.2337/dc17-0224
Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S (2010) Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 53:2504–2508. https://doi.org/10.1007/s00125-010-1799-4
American Diabetes Association (2019) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42(Suppl 1):S13–S28. https://doi.org/10.2337/dc19-S002
Xu JY, Dan QH, Chan V, Wat NM, Tam S, Tiu SC, Lee KF, Siu SC, Tsang MW, Fung LM, Chan KW, Lam KS (2005) Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet 13:422–427. https://doi.org/10.1038/sj.ejhg.5201347
Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI, Neonatal Diabetes International Collaborative Group (2007) Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA 104:15040–15044. https://doi.org/10.1073/pnas.0707291104
Garin I, Edghill EL, Akerman I et al (2010) Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci USA 107:3105–3110. https://doi.org/10.1073/pnas.0910533107
Gabbay KH (1980) The insulinopathies. N Engl J Med 302:165–167
Molven A, Ringdal M, Nordbø AM, Raeder H, Støy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, Undlien DE, Joner G, Søvik O, Bell GI, Njølstad PR, Norwegian Childhood Diabetes Study Group (2008) Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes 57:1131–1135. https://doi.org/10.2337/db07-1467
Bell GI, Pictet RL, Rutter WJ, Cordell B, Tischer E, Goodman HM (1980) Sequence of the human insulin gene. Nature 284:26–32
Dodson G, Steiner D (1998) The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol 8:189–194. https://doi.org/10.1016/s0959-440x(98)80037-7
Weiss MA (2009) Proinsulin and the genetics of diabetes mellitus. J Biol Chem 284:19159–19163. https://doi.org/10.1074/jbc.R109.009936
Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S, Neonatal Diabetes International Collaborative Group (2008) Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes 57:1034–1042. https://doi.org/10.2337/db07-1405
Meur G, Simon A, Harun N, Virally M, Dechaume A, Bonnefond A, Fetita S, Tarasov AI, Guillausseau PJ, Boesgaard TW, Pedersen O, Hansen T, Polak M, Gautier JF, Froguel P, Rutter GA, Vaxillaire M (2010) Insulin gene mutations resulting in early-onset diabetes: marked differences in clinical presentation, metabolic status, and pathogenic effect through endoplasmic reticulum retention. Diabetes 59:653–661. https://doi.org/10.2337/db09-1091
Liu L, Furuta H, Minami A, Zheng T, Jia W, Nanjo K, Xiang K (2007) A novel mutation, Ser159Pro in the NeuroD1/BETA2 gene contributes to the development of diabetes in a Chinese potential MODY family. Mol Cell Biochem 303:115–120. https://doi.org/10.1007/s11010-007-9463-0
Liu L, Nagashima K, Yasuda T, Liu Y, Hu HR, He G, Feng B, Zhao M, Zhuang L, Zheng T, Friedman TC, Xiang K (2013) Mutations in KCNJ11 are associated with the development of autosomal dominant, early-onset type 2 diabetes. Diabetologia 56:2609–2618. https://doi.org/10.1007/s00125-013-3031-9
Liu L, Liu Y, Ge X, Liu X, Chen C, Wang Y, Li M, Yin J, Zhang J, Chen Y, Zhang R, Jiang Y, Zhao W, Yang D, Zheng T, Lu M, Zhuang L, Jiang M (2018) Insights into pathogenesis of five novel GCK mutations identified in Chinese MODY patients. Metabolism 89:8–17. https://doi.org/10.1016/j.metabol.2018.09.004
American Diabetes Association (2015) (2) Classification and diagnosis of diabetes. Diabetes Care 38(Suppl):S8–S16. https://doi.org/10.2337/dc15-S005
Ellard S, Bellanné-Chantelot C, Hattersley AT, European Molecular Genetics Quality Network (EMQN) MODY Group (2008) Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia 51:546–553. https://doi.org/10.1007/s00125-008-0942-y
Liu M, Lara-Lemus R, Shan SO, Wright J, Haataja L, Barbetti F, Guo H, Larkin D, Arvan P (2012) Impaired cleavage of preproinsulin signal peptide linked to autosomal-dominant diabetes. Diabetes 61:828–837. https://doi.org/10.2337/db11-0878
Miyamoto K, Yamashita T, Tsukiyama T, Kitamura N, Minami N, Yamada M, Imai H (2008) Reversible membrane permeabilization of mammalian cells treated with digitonin and its use for inducing nuclear reprogramming by Xenopus egg extracts. Cloning Stem Cells 10:535–542. https://doi.org/10.1089/clo.2008.0020
Eskridge EM, Shields D (1983) Cell-free processing and segregation of insulin precursors. J Biol Chem 258:11487–11491
Okun MM, Shields D (1992) Translocation of preproinsulin across the endoplasmic reticulum membrane. The relationship between nascent polypeptide size and extent of signal recognition particle-mediated inhibition of protein synthesis. J Biol Chem 267:11476–11482
Guo H, Xiong Y, Witkowski P, Cui J, Wang LJ, Sun J, Lara-Lemus R, Haataja L, Hutchison K, Shan SO, Arvan P, Liu M (2014) Inefficient translocation of preproinsulin contributes to pancreatic beta cell failure and late-onset diabetes. J Biol Chem 289:16290–16302. https://doi.org/10.1074/jbc.M114.562355
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. https://doi.org/10.1038/nrm3270
Leroux L, Desbois P, Lamotte L, Duvillié B, Cordonnier N, Jackerott M, Jami J, Bucchini D, Joshi RL (2001) Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes 50(Suppl 1):S150–S153. https://doi.org/10.2337/diabetes.50.2007.s150
Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T (1999) A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103:27–37. https://doi.org/10.1172/JCI4431
Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R (2007) Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 56:1268–1276. https://doi.org/10.2337/db06-0658
Dusatkova L, Dusatkova P, Vosahlo J, Vesela K, Cinek O, Lebl J, Pruhova S (2015) Frameshift mutations in the insulin gene leading to prolonged molecule of insulin in two families with Maturity-Onset Diabetes of the Young. Eur J Med Genet 58:230–234. https://doi.org/10.1016/j.ejmg.2015.02.004
Garin I, Perez de Nanclares G, Gastaldo E, Harries LW, Rubio-Cabezas O, Castaño L (2012) Permanent neonatal diabetes caused by creation of an ectopic splice site within the INS gene. PLoS ONE 7:e29205. https://doi.org/10.1371/journal.pone.0029205
Yan J, Jiang F, Zhang R, Xu T, Zhou Z, Ren W, Peng D, Liu Y, Hu C, Jia W (2017) Whole-exome sequencing identifies a novel INS mutation causative of maturity-onset diabetes of the young 10. J Mol Cell Biol 9:376–383. https://doi.org/10.1093/jmcb/mjx039
Ma Y, Han X, Zhou X, Li Y, Gong S, Zhang S, Cai X, Zhou L, Luo Y, Li M, Liu W, Zhang X, Ren Q, Ji L (2019) A new clinical screening strategy and prevalence estimation for glucokinase variant-induced diabetes in an adult Chinese population. Genet Med 21:939–947. https://doi.org/10.1038/s41436-018-0282-3
Thanabalasingham G, Owen KR (2011) Diagnosis and management of maturity onset diabetes of the young (MODY). BMJ 343:d6044. https://doi.org/10.1136/bmj.d6044
Acknowledgements
We thank Lin He for his assistance. We apologize to many authors whose works could not be cited due to space limitations. This work is supported by the National Natural Science Foundation of China [Grant Numbers 81970686, 81770791, 81471012, 81270876], the Interdisciplinary Program of Shanghai Jiao Tong University [Grant Number YG2019ZDA08], the Shanghai Leading Talent [Grant Number SLJ15055], and the National Institute of Diabetes and Digestive and Kidney Diseases [Grant Number SC1DK104821 to Y. Liu].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, Y., Li, M. et al. Identification of Ala2Thr mutation in insulin gene from a Chinese MODY10 family. Mol Cell Biochem 470, 77–86 (2020). https://doi.org/10.1007/s11010-020-03748-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03748-0