Abstract
IgA nephropathy (IgAN), the most common glomerulonephritis, has an unclear pathogenesis. The role of Th22 cells, which are intimately related to proteinuria and progression in IgAN, in mediating infection-related IgAN is unclear. This study aimed to characterize the association between intrinsic renal cells (tubular epithelial cells and mesangial cells) and Th22 cells in immune regulation of infection-related IgAN and to elucidate the impact of Th22 lymphocytosis; the proinflammatory cytokines IL-1, IL-6, and TNF-α; and CCL chemokines on kidney fibrosis. Hemolytic streptococcus infection induced an increase in IL-1, IL-6, and TNF-α, resulting in Th22 cell differentiation from T lymphocytes obtained from patients with IgAN, and the CCL20–CCR6, CCL22–CCR4, and/or CCL27–CCR10 axes facilitated Th22 cell chemotaxis. The increased amount of Th22 cells caused an increase in TGF-β1 levels, and anti-CD80, anti-CD86, and CTLA-4Ig treatment reduced TGF-β1 levels by inhibiting Th22 lymphocytosis and secretion of cytokines and chemokines, thus potentially relieving kidney fibrosis. Our data suggest that Th22 cells might be recruited into the kidneys via the CCL20–CCR6, CCL22–CCR4, and/or CCL27–CCR10 axes by mesangial cells and tubular epithelial cells in infection-related IgAN. Th22 cell overrepresentation was attributed to stimulation of the B7–CTLA-4Ig antigen-presenting pathway and IL-1, IL-6, and TNF-α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis, but its exact mechanism is complex and remains unclear. Infection, inflammation, and lymphocyte disorders are involved in IgAN [1,2,3,4]. T helper type 22 (Th22) cells, a newly identified T helper (Th) lymphocyte subset that is characterized by secretion of interleukin-22 (IL-22), is important in inflammation, infection, and autoimmune disease [5]. Studies have suggested that Th22 lymphocytosis plays a role in IgAN and correlates with proteinuria and disease prognosis [6, 7], but its mechanism of action is still unclear. It is well known that lymphocytes can be activated by antigen-presenting cells through the TCR–MHCII and CD28/CTLA4–B7 pathways, and that intrinsic renal cells express B7 proteins (CD80 and CD86). Therefore, this study was designed to characterize the relationship between intrinsic renal cells and Th22 lymphocytosis in immune regulation of infection-related IgAN.
Chronic inflammation is a common hallmark of chronic fibrotic disease, and fibrosis is a common pathological change in IgAN. Chronic inflammation is characterized by recruitment of inflammatory cells, and almost all immune cell types including Th cells are involved in this process. These immune cells, together with the injured intrinsic renal cells, release various cytokines, especially TGF-β1, which is the central driver of kidney fibrosis [8, 9]. Therefore, we also explored the possible influence of Th22 lymphocytosis on kidney fibrosis in this study.
Materials and methods
Ethics statement
This study protocol was approved by the Medical Ethics Committee of the Xiangya Hospital of Central South University for Human Studies (approval number 201403270), and all subjects provided signed informed consent.
Subjects
Twenty-one adults, aged between 16 and 46 years, with IgAN that was recently confirmed by biopsy were included in the study. Patients were excluded if they had acute infection, glucocorticoid or immunosuppressant treatments, or other complications. The demographic, clinical, and biochemical characteristics and pathology of patients with IgAN are shown in Table 1.
Th22 cell differentiation induced by mesangial cells and tubular epithelial cells through the B7–CTLA-4 pathway
Experiments were independent biological repeats. CD4+ T lymphocytes of patients with IgAN were not pooled. CD4+ T lymphocytes drawn from one patient with IgAN at a single time point were divided into different treatment groups in one repeat experiment. CD4+ T lymphocytes of patients with IgAN were isolated and purified using a CD4+ T cell isolation kit according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). LD columns with manual separators were used for magnetic separation. Human kidney 2 cells (HK2) or human mesangial cells (HMCs) (purchased from Central South University Advanced Research Center, Changsha China) and purified CD4+ T cells were mixed and cocultured at a ratio of 5:1 in RPMI-1640 supplemented with 12% fetal bovine serum (FBS) for 5 days. Anti-CD80 monoclonal antibody (mAb) (10 μg/mL), anti-CD86 mAb (10 μg/mL), CTLA-4-Ig mAb (5 μg/mL), and inactivated alpha-hemolytic streptococcus (α-HS) were used. All antibodies were purchased from eBioscience, Vienna, Austria. Suspension-cultured cells were harvested at day 5 and analyzed by flow cytometry. The concentrations of IL-1, TNF-α, IL-6, CCL20, CCL22, and CCL27 were quantified by enzyme-linked immunosorbent assay (ELISA) (R&D System Inc., Minneapolis, USA). The number of CD3+CD4+IFN-γ−IL-17−IL-22+ cells was determined by flow cytometry. α-HS was isolated from human tonsils, purified, and diluted to 1 × 108 CFU/mL in sterile phosphate-buffered saline. All bacteria were formalin-inactivated. The vaccine did not contain any viable microorganisms, as confirmed by sterility test.
Th22 cell differentiation
Purified IgAN CD4+ T cells were cultured in RPMI-1640 medium containing IL-2 (2 ng/mL) in 24-well plates and stimulated with CD3 and CD28 antibodies (1 μg/mL each) for 7 days. The exogenous cytokines used to modulate cell differentiation were IL-1β (20 ng/mL), IL-6 (100 mg/mL), and TNF-α (50 ng/mL). All reagents used for cell differentiation assays were purchased from PeproTech, USA.
Th22 cell chemotaxis assays
Purified IgAN CD4+ T lymphocytes were added to the upper chambers of a 24-well transwell plate (Corning Costar,NY, USA) in RPMI-1640 medium with 0.5% FBS in a final volume of 100 μL, and the lower chambers were filled with 600 μL of the supernatant of cultured HK2 cells or HMCs, which were treated with inactivated α-HS, anti-CD80 (10 μg/mL), anti-CD86 (10 μg/mL), or CTLA-4Ig (5 μg/mL). The transwell chambers were incubated at 37 °C in 5% CO2. The cells in the lower chamber were assigned a chemotaxis index (chemotaxis index = number of migrated Th22 cells in each experiment group ÷ number of migrated Th22 cells in response to medium alone) and analyzed by flow cytometry.
Flow cytometry
CD3 (PerCP-Cyanine5.5; eBioscience), CD4 (FITC; eBioscience), CCR4 (APC; eBioscience), CCR6 (PE-Cyanine7; eBioscience), and CCR10 (PE; BioLegend, California, USA) on T cells isolated from the blood of patients with IgAN, and IFN-γ (APC; eBioscience), IL-22 (PE; eBioscience), IL-17A (PE-Cyanine7; eBioscience), and Ki67 (Alexa 700 MAB; BD Biosciences, California, USA) were stained using the fixation/permeabilization concentrate kit (BD Biosciences) according to the manufacturer’s instructions and analyzed by flow cytometry.
ELISA
IL-1, TNF-α, IL-6, CCL20, CCL22, and CCL27 levels were quantified by using ELISA kits (R&D) according to the manufacturer’s instructions. Immunoreactivity was determined by using an ELISA reader at 450 nm.
Western blot analysis
Transforming growth factor beta 1 (TGF-β1) in HK2 cells and HMCs was analyzed by western blot. Adherent cells in the coculture system were harvested, lysed for 10 min on ice with 80 μL of RIPA buffer (Well Biology, Peking, China), and centrifuged (12,000×g for 15 min). Lysate samples (50 μg) were boiled in sample buffer for 5 min and separated on 10% SDS-PAGE, followed by transfer onto a nitrocellulose membrane (Millipore, Massachusetts, USA). The membrane was incubated with a monoclonal rabbit anti-human TGF-β1 antibody (Proteintech, Chicago, USA) and reacted with an anti-mouse IgG-HRP antibody (Proteintech). TGF-β1 levels were normalized to β-actin levels and quantified using ECL chemiluminescence system (Thermo Scientific Pierce, Alabama, USA). Films were scanned and images were analyzed using Quantity One 4.62 software (Bio-rad, California, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) or median with minimum and maximum values. Data comparisons were performed using Kruskal–Wallis one-way analysis or the Mann–Whitney U test. Variables in HK2 and HMC culture systems were compared using Student’s t test or the Wilcoxon signed-rank test. Correlations among variables were determined by calculating the Spearman rank correlation coefficients. P < 0.05 was defined as statistically significant. Statistical analyses were performed using SPSS 19.0 software (Chicago, IL, USA).
Results
HK2 cells and HMCs induce Th22 lymphocytosis via the B7–CTLA-4 antigen presentation pathway
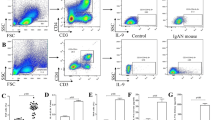
As assessed by flow cytometry, inactivated α-HS infection induced a remarkable increase in CD3+CD4+IFN-γ−IL-17−IL-22+ T lymphocytes in both HK2 and HMC coculture systems compared with controls (4.17 ± 0.69% vs 0.96 ± 0.07% and 4.54 ± 0.46% vs. 1.71 ± 0.06%, P < 0.01) (Fig. 1). By contrast, the proportions of CD3+CD4+IFN-γ−IL-17−IL-22+ cells in coculture systems were reduced when CD80, CD86, and CTLA-4 antibodies were used to block the antigen-presenting pathway (The proportion of Th22 cells in the HK2 coculture system was 2.37 ± 0.33, 2.43 ± 0.12, and 1.24 ± 0.09%, respectively; the proportion of Th22 cells in the HMC coculture system was 3.47 ± 0.28, 3.14 ± 0.20, and 2.79 ± 0.23%, respectively) (Fig. 1). Cocultures treated with a combination of CD80 and CD86 antibodies had fewer Th22 cells (HK2: 1.63 ± 0.14%, HMC: 2.71 ± 0.23%) than those treated with CD80 or CD86 antibodies alone (P < 0.01).
Th22 cell differentiation is stimulated by HK2 cells and HMCs via the antigen-presenting pathway in vitro. CD4+ T lymphocytes of patients with IgAN were cocultured with HK2 cells or HMCs for 5 days. Inactivated α-HS was applied to the medium to simulate a set of infection-related IgAN conditions. CD80, CD86, and CTLA-4 antibodies were used alone or in different combinations to block the antigen-presenting pathway. a Th22 cell proportions in cocultures treated with inactivated α-HS, CD80, CD86, and CTLA-4 antibodies alone or in different combinations. b The histogram presents the Th22 cell proportions (n = 4). *P < 0.01
Inactivated α-HS infection induces secretion of proinflammatory cytokines
IL-1 levels exhibited a significant increase in coculture systems of purified CD4+ T lymphocytes from IgAN patients and HK2 cells or HMCs under inactivated α-HS infection compared with controls (HK2: 171.72 ± 1.60 vs. 93.50 ± 5.45 pg/mL, HMC: 173.44 ± 1.21 vs. 96.36 ± 5.45 pg/mL; P < 0.01). Increases were also observed in the levels of IL-6 (HK2: 66.73 ± 1.79 vs. 35.97 ± 0.42 pg/mL, HMC: 65.87 ± 9.61 vs. 33.92 ± 0.17 pg/mL; P < 0.01) and TNF-α (HK2: 301.58 ± 1.01 vs. 102.59 ± 1.26 pg/mL, HMC: 336.95 ± 10.7 vs. 112.74 ± 0.92 pg/mL; P < 0.01). IL-1, IL-6, and TNF-α levels were decreased to varying degrees when CD80, CD86, and CTLA-4 antibodies were applied alone to partially block the antigen-presenting pathway. The difference between the antibody treatment groups and HS groups was significant at P < 0.01 except for TNF-α in the HK2-CD4+ lymphocyte coculture system treated with anti-CD80 or anti-CD86: 287.41 ± 1.01 and 284.77 ± 3.24, respectively, vs. 301.58 ± 1.01 pg/mL; P = 0.03 and 0.01 (Fig. 2). Treatment with CTLA-4Ig or a combination of CD80 and CD86 antibodies induced the most significant reduction in IL-1, IL-6, and TNF-α levels.
α-HS infection stimulates the secretion of IL-1, IL-6, and TNF-α. CD4+ T lymphocytes of patients with IgAN were cocultured with HK2 cells or HMCs for 5 days. Inactivated α-HS was applied into the medium to simulate a set of infection-related IgAN conditions. CD80, CD86, and CTLA-4 antibodies were used alone or in different combinations to block the antigen-presenting pathway. Histograms show IL-1 (a), IL-6 (b), and TNF-α (c) levels in coculture systems (n = 4). *P < 0.01
IL-1, IL-6, and TNF-α promote Th22 cell differentiation in vitro
Because levels of the proinflammatory cytokines IL-1, IL-6, and TNF-α were increased and correlated with the number of Th22 cells in coculture systems stimulated with HS, we evaluated their effects on Th22 cell differentiation in vitro. As shown in Fig. 3, IL-1, TNF-α, and IL-6 significantly promoted Th22 cell differentiation compared with controls. IL-6 had the most significant effect on Th22 cell differentiation. The effect of TNF-α was stronger than that of IL-1 but slightly weaker than that of IL-6 (3.99 ± 0.79, 2.52 ± 0.23, and 4.49 ± 0.45%, respectively; P < 0.01).
Differentiation of Th22 cells from CD4+ T lymphocytes in vitro. Proportion of Th22 cells that were differentiated from purified CD4+ T lymphocytes of IgAN patients by stimulation with different combinations of cytokines for 7 days. The medium contained anti-CD3, anti-CD28, and IL-2 to ensure T cell activation (n = 4). *P < 0.01
Th22 cells are recruited by HK2 cells and HMCs through CCL–CCR pathways
In inactivated α-HS-infected HK2 or HMC coculture systems, expression of CCR4, CCR6, and CCR10 on Th22 cells was substantially increased (Fig. 4a). CCL20, CCL22, and CCL27 levels also significantly increased accordingly (Fig. 4b). As CCL20, CCL22, and CCL27 are specific ligands of CCR6, CCR4, and CCR10 receptors that are expressed on Th22 cells, it is possible that Th22 cells might be recruited into the kidneys through the CCL-CCR pathway. Chemotaxis assays were conducted to confirm Th22 cell migration in vitro. As expected, both HK2 cells and HMCs treated with HS increased Th22 cell chemotactic indexes compared with controls (10.31 ± 0.52 vs. 4.29 ± 0.60% and 6.21 ± 1.00 vs. 3.21 ± 1.09%, respectively; P < 0.01). By contrast, treatment with anti-CD80, anti-CD86, and CTLA-4Ig alone or in combination decreased Th22 cell chemotactic indexes (Fig. 4c).
Th22 cells are recruited by HK2 cells and HMCs via the CCL–CCR pathway. CD4+ T lymphocytes of patients with IgAN were cocultured with HK2 cells or HMCs for 5 days. Inactivated α-HS was applied to the medium to simulate a set of infection-related IgAN conditions. CD80, CD86, and CTLA-4 antibodies were used alone or in different combinations to block the antigen-presenting pathway. a Expression of CCR4, CCR6, and CCR10 on Th22 cells in coculture systems. b CCL20, CCL22, and CCL27 levels in coculture systems. c Th22 cell chemotactic index (n = 4). *P < 0.01
Increase in TGF-β1 correlates with Th22 cells from IgAN patients
As illustrated in Fig. 5, inactivated α-HS infection induced a significant increase in TGF-β1 levels compared with controls (HK2: 0.88 ± 0.01 vs. 0.26 ± 0.03, HMC: 0.53 ± 0.03 vs. 0.11 ± 0.02; P < 0.01). Western blot analysis showed that after treatment with CD80, CD86, and CTLA-4 antibodies, TGF-β1 levels were suppressed in HK2 cells (0.35 ± 0.07, 0.29 ± 0.06, and 0.31 ± 0.06) and HMCs (0.14 ± 0.02, 0.17 ± 0.03, and 0.20 ± 0.02; P < 0.01 respectively). Additionally, TGF-β1 levels were significantly correlated with Th22 cell proportions in both coculture systems (HK2: r = 0.950, HMC: r = 0.892; P < 0.01).
Blockade of the antigen-presenting pathway reduces TGF-β1 levels in HK2 cells and HMCs. CD4+ T lymphocytes of patients with IgAN were cocultured with HK2 cells or HMCs for 5 days. Inactivated α-HS was applied to the medium to simulate a set of infection-related IgAN conditions. CD80, CD86, and CTLA-4 antibodies were used to block the B7–CTLA-4 antigen-presenting pathway. a, b TGF-β1 protein levels in HK2 cells and HMCs analyzed by western blot. c Correlation between TGF-β1 levels and Th22 cell proportions (n = 4). *P < 0.01
Discussion
IgAN is characterized by mucosal infection, inflammatory cell infiltration, and IgA1-circulating immune complex formation and deposition in the glomerular mesangium. Exacerbation of IgAN is closely related to inflammation and autoimmune disorder [3, 7, 10]. The mechanism of innate immunity in IgAN was revealed recently, but the role of inflammation and adapted immunity remain unclear. Previous studies have reported Th cell disorders in IgAN; for example, Peng et al. demonstrated that Th cell disorder is correlated with proteinuria in IgAN [11]. However, the relevant mechanism remains unclear. HS infection is an acknowledged cause of IgAN, and IgAN Th22 cell-lymphocytosis induced by HS infection was found in this study. Meng et al. also described Th22 cell overrepresentation in a mouse model of IgAN, where the Th22 overrepresentation was correlated with Th17 cell disorder [1], but the reason underlying the increase in Th22 cells was not explained. It is well known that T cells are activated and proliferate in infection through the TCR–MHCII and CD28/CTLA-4–B7 pathways, which are also known as antigen-presenting pathways [12], and that intrinsic renal cells express MHCII and B7 proteins [13,14,15,16]. It is possible that the Th22 lymphocytosis in infection-related IgAN is induced by intrinsic renal cells through the antigen-presenting pathway. Abnormal expression of the CTLA4 gene has been reported to increase the risk of several autoimmune diseases, including rheumatoid arthritis, transplantation, infectious diseases, and asthma [17,18,19]. Gorgi et al. have demonstrated that CTLA-4 gene polymorphisms exist in IgAN patients and are involved in the development of disease [20]. Wang et al. revealed that the CTLA-4 rs231726 gene was associated with a significant risk of developing IgAN [21]. Kim et al. demonstrated that the CTLA-4 rs231779 gene was significantly associated with the presence of proteinuria, podocyte foot process effacement, and advanced mesangial proliferation, suggesting that CTLA-4 may play key roles in the pathogenesis of IgAN [22]. These findings suggest that the CTLA-4–B7 axis is involved in the development of IgAN, but the underlying mechanism is unclear. Our results show that inactivated α-HS infection significantly increased Th22 cell differentiation and that blockade of the antigen-presenting pathway with CD80, CD86, and CTLA-4 antibodies dramatically suppressed the overrepresentation of Th22 cells. Although previous studies have reported that extraglomerular mesangium and podocytes could serve as non-hematopoietic professional antigen-presenting cells in immune-mediated renal injury [14, 16], no data exist on the role of intrinsic renal cells in IgAN based on antigen presentation. To the best of our knowledge, this study is the first to demonstrate that tubular epithelial cells and mesangial cells can act as non-hematopoietic professional antigen-presenting cells to induce Th22 cell proliferation and infiltration in IgAN.
Proinflammatory cytokines such as IL-1, IL-6, IL-21, and TNF-α have been reported to be elevated in IgAN and to be closely related to disease activity [23]. Here, in cocultures of IgAN lymphocytes with intrinsic renal cells, IL-1, IL-6, and TNF-α levels increased under inactivated α-HS infection. Moreover, the changes in Th22 cell proportions in this coculture system corresponded to changes in IL-1, IL-6, and TNF-α levels. Additionally, we found that IL-1, TNF-α, and particularly IL-6 promoted Th22 cell differentiation. Thus, Th22 cell differentiation in infection-related IgAN results from proinflammatory cytokine stimulation and/or antigen presentation from intrinsic renal cells.
It is well known that local cell overabundance may be caused by cell differentiation and/or cell infiltration. It is possible that the Th22 lymphocytosis found in IgAN may be partly due to cell infiltration. We found that levels of CCL20, CCL22, and CCL27, which are also known as Th22 cell-attractive chemokines, increased when HK2 cells and HMCs were infected by HS. Chemotaxis assays further confirmed that the migration of Th22 cells induced by HK2 cells and HMCs was markedly increased under inactivated α-HS stimulation, and treatment with CD80, CD86, and CTLA-4 antibodies reduced the secretion of CCL20, CCL22, and CCL27, thus significantly inhibiting the recruitment of Th22 cells. As a result, we suggest that tubular epithelial cells and mesangial cells recruit Th22 cells into the kidney through the CCR4–CCL22, CCR6–CCL20, and/or CCR10–CCL27 axes, and that the increased numbers of Th22 cells in IgAN may be attributed to either Th22 cell differentiation or Th22 cell infiltration. Meng et al. observed an increase in CCL20 levels and Th17 cells in IgAN rats [1]. Ebefors et al. found that mesangial cells could secrete CCL5 in IgAN in response to stimulation by galactose-deficient IgA [24]. Therefore, CC chemokines, including but not limited to CCL20, CCL22, CCL27, and CCL5, may play an important role in IgAN.
Chronic fibrosis induced by chronic inflammation is a common pathological change in IgAN and is closely correlated with prognosis [25]. Chronic inflammation is characterized by recruitment of inflammatory cells, neutrophils, and macrophages. Moreover, almost all immune cell types including Th cell, regulatory T cells, and B lymphocytes are involved in this process. These immune cells, together with the injured intrinsic renal cells, release various cytokines, especially TGF-β1, which is the central driver of kidney fibrosis [8, 9]. In our study, as assessed by western blot, TGF-β1 levels in tubular epithelial cells and mesangial cells increased under inactivated α-HS stimulation. Moreover, the TGF-β1 levels were positively correlated with Th22 cell amounts. Anti-CD80, anti-CD86, and CTLA-4Ig individually or in combination significantly inhibited Th22 cell overrepresentation and TGF-β1 production. Although this has not been extensively studied so far, Th22 cells clearly contribute to fibrosis. Weathington et al. showed that IL-22 could bind to IL-22RA1, which is present in the kidneys, activate the JAKI–STAT1 and STAT3 signaling pathways, and act through the Akt, ERK, JNK, and p38 signaling pathways to regulate organ fibrosis [26]. Brembilla et al. showed that Th22 cells promote skin fibrosis by enabling fibroblasts to respond to TNF, thus promoting a proinflammatory fibroblast phenotype by favoring TNF-induced keratinocyte activation [27]. IL-1, IL-6, TNF-α, and CCL20 have also been shown to be profibrotic [28]. These findings collectively suggest that Th22 cells contribute to kidney fibrosis in infection-related IgAN.
Conclusions
This is the first study to suggest that Th22 cells are not only involved in but also accelerate renal fibrosis in HS infection-related IgAN. Moreover, this Th22 cell-mediated disorder was found to be caused by intrinsic renal cells that serve as non-hematopoietic professional antigen-presenting cells, secreting chemokines and proinflammatory cytokines and inducing Th22 cell infiltration and differentiation via the CCL–CCR and B7–CTLA-4 axes. However, this study did not address the other two types of intrinsic renal cells (vascular epithelial cells and podocytes), and the underlying molecular mechanism was not determined.
References
Meng T, Li X, Ao X, Zhong Y, Tang R, Peng W et al (2014) Hemolytic streptococcus may exacerbate kidney damage in IgA nephropathy through CCL20 response to the effect of Th17 cells. PLoS ONE 29:e108723
Yang L, Zhang X, Peng W, Wei M, Qin W (2017) MicroRNA-155-induced T lymphocyte subgroup drifting in IgA nephropathy. Int Urol Nephrol 49:353–361
Duan J, Liu D, Duan G, Liu Z (2017) Long-term efficacy of tonsillectomy as a treatment in patients with IgA nephropathy: a meta-analysis. Int Urol Nephrol 49:103–112
Huang H, Sun W, Liang Y, Peng Y, Long XD et al (2014) CD4(+)CD25(+)Treg cells and IgA nephropathy patients with tonsillectomy: a clinical and pathological study. Int Urol Nephrol 46:2361–2369
Jia L, Wu C (2014) The biology and functions of Th22 cells. Adv Exp Med Biol 841:209–230
Peng Z, Tian J, Cui X, Xian W, Sun H, Li E et al (2013) Increased number of Th22 cells and correlation with Th17 cells in peripheral blood of patients with IgA nephropathy. Hum Immunol 74:1586–1591
Huang H, Peng Y, Liu H, Yang X, Liu F (2010) Decreased CD4+ CD25+ cells and increased dimeric IgA-producing cells in tonsils in IgA nephropathy. J Nephrol 23:202–209
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Wang B, Komers R, Carew R, Winbanks CE, Xu B et al (2012) Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol 23:252–265
Floege J, Feehally J (2016) The mucosa–kidney axis in IgA nephropathy. Nat Rev Nephrol 12:147–156
Peng Z, Tian J, Cui X, Xian W, Sun H, Li E et al (2013) Increased number of Th22 cells and correlation with Th17 cells in peripheral blood of patients with IgA nephropathy. Hum Immunol 74:1586–1591
Nakayama M (2015) Antigen presentation by MHC-dressed cells. Front Immunol 5:672
Gluhovschi C, Gluhovschi G, Potencz E, Lazar E, Petrica L, Bozdog G et al (2012) What is the significance of HLA-DR antigen expression in the extraglomerular mesangium in glomerulonephritis? Hum Immunol 73:1098–1101
Schmitt R, Ståhl AL, Olin AI, Kristoffersson AC, Rebetz J, Novak J et al (2014) The combined role of galactose-deficient IgA1 and streptococcal IgA-binding M Protein in inducing IL-6 and C3 secretion from human mesangial cells: implications for IgA nephropathy. J Immunol 193:317–326
Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E et al (2008) TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19:704–713
Goldwich A, Burkard M, Olke M, Daniel C, Amann K et al (2013) Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol 24:906–916
Carreno BM, Collins M (2002) The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 20:29–53
Khoury SJ, Sayegh MH (2004) The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity 20:529–538
Keir ME, Sharpe AH (2005) The B7/CD28 costimulatory family in autoimmunity. Immunol Rev 204:128–143
Gorgi Y, Sfar I, Goucha R, Aouadi H, Amri M, Makhlouf M et al (2010) IL1/IL1 Ra, CTLA-4 and Apo1/Fas genes polymorphisms and susceptibility to IgA nephropathy in Tunisian patients. Tunis Med 88:789–793
Wang H, Sui W, Xue W, Wu J, Chen J, Dai Y (2014) Univariate and multiple linear regression analyses for 23 single nucleotide polymorphisms in 14 genes predisposing to chronic glomerular diseases and IgA nephropathy in Han Chinese. Saudi J Kidney Dis Transpl 25:992–997
Kim HJ, Chung JH, Kang S, Kim SK, Cho BS, Kim SD et al (2011) Association of CTLA4, CD28 and ICOS gene polymorphisms with clinicopathologic characteristics of childhood IgA nephropathy in Korean population. J Genet 90:151–155
Maruyama S, Gohda T, Suzuki Y, Suzuki H, Sonoda Y et al (2016) Beneficial effects of tonsillectomy plus steroid pulse therapy on inflammatory and tubular markers in patients with IgA nephropathy. Kidney Res Clin Pract 35:233–236
Ebefors K, Liu P, Lassén E, Elvin J, Candemark E et al (2016) Mesangial cells from patients with IgA nephropathy have increased susceptibility to galactose-deficient IgA1. BMC Nephrol 17:40
Mariani LH, Maarini S, Barisoni L, Canetta PA, Trosst JP, Hodgin JB, et al. (2017) Interstitial fibrosis scoredon whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transpl
Weathington NM, Snavely CA, Chen BB, Zhao J, Zhao Y et al (2014) Glycogen synthase kinase-3β stabilizes the interleukin (IL)-22 receptor from proteasomal degradation in murine lung epithelia. J Biol Chem 289:17610–17619
Brembilla NC, Dufour AM, Alvarez M, Hugues S, Montanari E et al (2016) IL-22 capacitates dermal fibroblast responses to TNF in scleroderma. Ann Rheum Dis 75:1697–1705
Sziksz E, Pap D, Lippai R, Béres NJ, Fekete A et al (2015) Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediat Inflamm 2015:764641
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81470933 and 81270786) (http://www.nsfc.gov.cn/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study protocol was approved by the Medical Ethics Committee of the Xiangya Hospital of Central South University for Human Studies (Approval Number 201403270).
Informed consent
All subjects provided signed informed consent.
Rights and permissions
About this article
Cite this article
Gan, L., Zhou, Q., Li, X. et al. Intrinsic renal cells induce lymphocytosis of Th22 cells from IgA nephropathy patients through B7–CTLA-4 and CCL-CCR pathways. Mol Cell Biochem 441, 191–199 (2018). https://doi.org/10.1007/s11010-017-3185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3185-8