Abstract
Parkinson’s disease (PD) can degenerate dopaminergic (DA) neurons in midbrain, substantia-nigra pars compacta. Alleviation of its symptoms and protection of normal neurons against degeneration are the main aspects of researches to establish novel therapeutic strategies. PPARγ as a member of PPARs have shown neuroprotection in a number of neurodegenerative disorders such as Alzheimer’s disease and PD. Nuclear receptor related 1 protein (Nurr1) is, respectively, member of NR4A family and has received great attentions as potential target for development, maintenance, and survival of DA neurons. Based on neuroprotective effects of PPARγ and dual role of Nurr1 in anti-inflammatory pathways and development of DA neurons, we hypothesize that PPARγ and Nurr1 agonists alone and in combined form can be targets for neuroprotective therapeutic development for PD in vitro model. 1-Methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in PC12 cells as an in vitro model for PD studies. Treatment/cotreatment with PPARγ and Nurr1 agonists 24 h prior to MPP+ induction enhanced the viability of PC12 cell. The viability of PC12 cells was determined by MTS test. Mitochondrial membrane potential (MMP) and intracellular reactive oxygen species (ROS) were detected by flow cytometry. In addition, the relative expression of four genes including TH (the marker of DA neurons), Ephrin A1, Nurr1, and Ferritin light chain were assessed by RT-qPCR. In the MPP+-pretreated PC12 cells, PPARγ and Nurr1 agonists and their combined form resulted in a decrease in the cell death rate. Moreover, production of intracellular ROS and MMP modulated by MPP+ was decreased by PPARγ and Nurr1 agonists’ treatment alone and in the combined form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second prevalent neurodegenerative disorder. It is characterized by the loss of dopaminergic neurons in substantia-nigra pars compacta (SNpc) [1]. Aging, environmental toxins, neuroinflammation, mitochondrial dysfunction, and oxidative stress have been shown to play crucial roles in the progression of PD [2, 3].
Neurotoxicity mechanisms of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) and its active metabolite 1-methyl-4-phenylpyridiniumion (MPP+) including mitochondrial complex I inhibition, inflammation, and generation of oxidative stress that finally cause cell death [4].
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated transcription factor that controls lipid metabolism, development of adipose tissue, and regulation of inflammation [5, 6]. PPARγ agonists have been shown to have neuroprotective roles in vivo and in vitro based on their anti-inflammatory characters [7, 8]. PPARγ synthetic agonist, GW1929 has the ability to rescue primary neocortical cells against treatment with tetrabromobisphenol A (2, 2-bis (4-hydroxy-3,5-dibromophenyl) propane (TBBPA), a neurotoxin which induces cell apoptosis and degeneration in neurons [9]. On the other hand, ameliorating effects of this agonist on the neurological damage in global cerebral ischemic–reperfusion injury through reduction of inflammation and DNA fragmentation have been proved [10]. This is necessary to notify that PPAR thiazolidinedione agonists, including rosiglitazone and pioglitazone, have also shown to have therapeutic effects on the neurodegenerative disorders, especially PD [11]. However, their administration should be carried out with special care as they could operate via nongenomic and PPARγ-independent pathways [12]. Moreover, the presence of Nurr1, as a member of the steroid/thyroid nuclear superfamily, is essential for development, survival, and maintenance of dopaminergic neurons, since its mutations contribute to the onset of PD [13]. Nurr1 was characterized as a regulator of the tyrosine hydroxylase (TH) expression in rat [14] and some recent studies indicate that Nurr1 can modulate neuroinflammatory pathways [15, 16]. In addition, any disruption in expression and function of the Nurr1 can cause neurodegeneration of DA neurons and development of PD [17]. Kim et al. [13] indicated that Nurr1 agonist improves behavioral deficits in an animal model of PD. 6-MP is a potent agonist of Nurr1 and activates Nurr1 transcription activity with excellent bioavailability and has a beneficial effects on the dopaminergic neurons in animal models of PD [18]. PC12 cells are rat adrenal pheochromocytoma cells line has been previously used as a cellular screening platform for assessing neuroprotection efficacy of tested agents [19].
Transcriptional activating of PPARγ and Nurr1 by their agonists, change expression of the several target genes such as Ferritin light chain (FTL) [20], Ephrin A [21], Thyrosine hydroxylase (TH), and Nurr1 [22]. These genes involve in anti-inflammatory signaling pathways, and they were also selected to be tested whether their expression could be upregulated by PPARγ, Nurr1 activation.
Iron as the most abundant metal in organism plays important roles in mitochondrial function, cell division, and oxygen transport. Deregulation in iron hemostasis can make it toxin to neurons which cause lipid peroxidation, DNA damage. In patients with AD and PD, misbalancing in iron hemostasis accelerates neurodegeneration and cause neuroinflammation [23, 24]. FTL is a member of cytosolic ferritin complex and has the ability to detoxify cell from free iron [25]. FTL is expressed at high level in the microglia, astrocytes, and SNpc [26] and mutation in this gene showed neurodegenerative pathology [27]. Ephrin A belongs to the Ephrin family proteins that can bind to their membrane-bound tyrosine kinase receptors, and exerts a substantial role in axon guidance, cell migration, synaptic plasticity, and neurite outgrowth in the adult mammalian nervous system [28, 29]. Ephrin A activation causes neural stem cell differentiation to the dopaminergic neurons, and also controls migration of dopaminergic neurons from subventricular zone (SVZ) [30, 31]. CNS injuries in vertebrates alter Ephrin mRNA and protein expression [32].
Expression of TH as the rate-limiting enzyme in brain catecholamine biosynthesis is controlled by Nurr1 [33]. In addition, Nurr1 has a critical role in dopaminergic neurogenesis, and its overexpression in stem cells leads to dopaminergic neurons generation [34]. Hence, TH is the marker of the dopaminergic neurons and TH+ neurons in PD patients are less than control [35].
Based on strong evidences showing that neuroinflammation triggers progression of neurological disorders such as PD pathology, it will be imperative to find out whether Nurr1 could be a therapeutic candidate to alleviate the progression of PD development. Given the critical function of PPARγ as a new therapeutic agent in neurological disorders, we used GW1929 as a high potential agonist of PPARγ in order to increase the survival of PC12 cells subject to the treatment by MPP+. We selected GW1929 and 6-MP as the specific agonists for PPARγ and Nurr1. This study is designed in order to investigate whether if these two transcription factors can act together for controlling the progress of neuroinflammation and cell death. To the best of our knowledge, both PPARγ and Nurr1 have their own signaling pathways. Thus, it would be interesting to examine whether respective agonists act synergistically or additively for ameliorating the inflammatory effects of MPTP. Therefore, a combinatorial effect of PPARγ and Nurr1 agonists was also investigated in this work. Here, we report that PPARγ and Nurr1 have similar effects on PC12 cell viability, but they presumably exert their effects through different molecular pathways.
Materials and methods
All the chemicals used in this study were supplied by Life Technologies (USA) unless indicated otherwise.
Cell culture
PC12 cells (Pasteur Institute, Iran) were grown on poly-l-ornithine (Sigma, USA)- and laminin (Sigma)-coated dishes in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10 % horse serum (PAA, USA) and 5 % fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37 °C in a humidified atmosphere containing 5 % CO2 and 95 % air. Cells were seeded in 96-well plate dishes at a density of 104 cells/well (TPP, USA). All the experiments were carried out 24 h after the seeding of cells in a serum-free medium. GW1929 (Sigma) (the PPARγ agonist) was dissolved in the dimethyl sulfoxide (DMSO) in order to make a 20 mM solution that was diluted with DMEM and supplemented with 1 % serum for the experimental usage. Meanwhile, 6-mercaptopurine (6-MP; Sigma) (the Nurr1 agonist) was dissolved in NaOH 1 N to reach the final concentration of 200 mM. Then the cells were plated at an appropriate density for a period of 24 h and later treated with MPP+ (Sigma) for another 24 h. In order to investigate the effects of PPARγ and Nurr1 agonists, the cells were preincubated with various concentrations of these agonists for one overnight period prior to the MPP+ treatment as already described in previous work [15–36].
Measurement of the cell viability
The cell viability was determined by the conversion of the 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) to formazan as an insoluble crystal. In the presence of phenazine methosulfate (PMS), MTS is converted to formazan crystals in the living cells by the mitochondrial dehydrogenase activity which is a member of mitochondrial electron transfer system (ETS) complex. Subsequently, 20 μL of MTS/PMS solution was added to each well of 96-well plates and incubated for 3 h at 37 °C. The absorbance of solution was measured at 490 nm by a microplate spectrophotometer (Awareness model, USA).
Cell death assay
The apoptosis PC12 cells was assessed through the annexin V staining by the flow cytometry on the PC12 cells under treatment with MPP+. To do so, ~6 × 105 cells were plated in a 6-well dish and preincubated with the effective concentrations of GW1929 (10 µM) and 6-MP (0.5 µM) overnight. Then the cells were treated with 400 µM of MPP+ at 37 °C for 24 h. Later, the cells were washed with PBS and stained with FITC-coupled antiannexin V antibody (Abcam, UK) on ice at 4 °C for 20 min. The evaluation was carried out with a FACSCalibur flow cytometer (Becton–Dickinson, USA). The stained cells were considered as the apoptotic cells and 104 events were recorded for each analysis.
PPARγ and Nurr1 activation assay
To ensure that the activation of PPARγ and Nurr1 has happened by the respective agonists, the subcellular distribution of PPARγ and Nurr1 was investigated under the treatment with agonists, as previously described for PPARγ [37, 38]. Hence, the cells were plated on coverslip and then treated with an effective concentration of GW1929 (10 µM) and 6-MP (0.5 µM) overnight. Subsequently, the treated cells were fixed with 4 % paraformaldehyde/PBS buffer and permeabilized with 0.2 % triton X-100 (Sigma) in 37 °C for 30 min. Several different fields (at least ten) were randomly selected and the whole cells in each field were examined for the intracellular distribution of PPARγ and Nurr1 through an indirect immunofluorescence staining procedure. The staining was performed using mouse antibody against PPARγ and rabbit antibody against Nurr1 (both at 1:100 dilutions, Santa Cruz Biotechnology). The secondary antibodies used were tetramethyl rhodamine-isothiocyanate (TRITC)-conjugated goat antimouse IgG (1:50 dilution, Chemicon) and fluoroisothiocyanate (FITC)-labeled goat antirabbit IgG (1:50 dilution, Millipore).
Immunostaining
The cells were cultured on glass coverslips. On the next day, the cells were washed with PBS and treated as described above. Then, the cells were incubated for 1 h with mouse anti-TH antibody (1:200 dilution, Sigma). Next, the cells were incubated for 1 h with FITC-labeled rabbit antimouse IgG as the secondary antibody (1:50 dilution, Milipore). For the nuclei staining, the cells were incubated for 3 min with 10 µg/mL 4′, 6-diamidino-2-phenylindole (DAPI; Sigma) in BSA. After washing the cells, the coverslips were mounted on the glass slides and the cells were observed under a fluorescent microscope (Olympus, Japan). Cell Images were acquired with an Olympus DP70 camera (Olympus), and the intracellular TH intensity was quantified according to the fluorescence intensity of the treated cells on the captured images of at least ten different fields with an Olympus DP70 camera (Olympus, Japan).
RNA extraction and RT-qPCR
The total cell RNA was isolated with RNeasy mini kit (Qiagen, Germany) and it was reverse transcribed to cDNA in a total volume of 20 μL using a random hexamer and the Revert Aid TM H Minus First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The cDNA (2 μL) was then amplified in a total volume of 10 μL containing SYBR Green Master Mix (TaKaRa, Japan). To perform the RT-qPCR, the forward (F) and reverse (R) primers for the specific amplification of TH were, respectively, F: 5′CAGCAGCAGCAGCGGTAG3′ and R: 5′CAGGGAGAAGAGCAGGTTGAG3′. The primer pairs for Nurr1 were F: 5′ TGGCTATGGTCACAGAGA3′ and R: 5′GTAGTTGGGTCGGTTCAA3′. The primer pairs for Ferritin light chain (FTL) were F: 5′GGAGAAGAACCTGAACCA3′ and R: 5′TTCCAAGAAGTCACAGAGG3′. The primer pairs for Ephrin A1 were F: 5′AAGAGACTCCAAGCAGATGA3′ and R: 5′CCGTTCCAATCCGTAAGC3′, and the primer pairs for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were F: 5′TGCCGCCTGGAGAAACC3′ and R: 5′TGAAGTCGCAGGAGACAACC3′. All primers are designed by Beacon Designer software (Version 7.2, USA) and were obtained from the Metabion Company (Germany). RT-qPCR was carried out in a thermal cycler Rotor gene 6000 (Corbett, Australia). Relative assessments of the gene expression vs. the expression of housekeeping GAPDH gene were performed using ΔΔCt method. The results were expressed as an average of the triplicate samples of three independent experiments for both control and treated cells.
Measurement of the intracellular reactive oxygen species (ROS) formation by flow cytometry
The intracellular ROS was detected using a dye DCFH2-DA (6-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate) probe. This probe will be oxidized and turned into fluorescent dye 2′, 7′-dichlorofluorescin (DCF) when come into contact with the intracellular ROS. After the MPP+ treatment, the PC12 cells were incubated by 50 μM of DCFH2-DA for 15 min and their fluorescence intensity was measured by FACSCalibur flow cytometer (excitation wavelength: 485 nm, emission wavelength: 530 nm; Becton–Dickinson, USA). The results were expressed as a percentage of the control value (MPP+-treated sample).
Measurement of mitochondrial membrane potential (MMP)
The MMP was measured using the J-aggregate-forming fluorescent dye, 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolocarbocyanine (JC-1) probe. The treated cells were washed by PBS and incubated by JC-1 (2.5 μg/mL) for 10 min. The fluorescent emission signals at 590 and 535 nm, in response to the excitation at 555 and 490 nm, were recorded by flow cytometry. The fluorescent signal intensity at 590 nm (red) was divided by the signal intensity value at 535 nm (green), and this red/green ratio was used to estimate the value of MMP (ΔΨm). The results were expressed as a percentage of the control value (untreated sample).
Western blot analysis
The cells were lysed with TRI reagent (Sigma) according to the kit manufacturer’s protocol. A solubilized protein fraction of each sample (30 µg) was loaded on the SDS–PAGE. Then the proteins were transferred to a PVDF membrane (Biorad, USA), and blocked with 5 % (w/v) nonfat dried milk (Merck, Germany) in PBS. The membrane was labeled with the rabbit anti-NF-κB p65 antibody (1:500; Abcam, UK) or an anti-GAPDH (Dako Cytomation, Denmark). The secondary antibody was the goat antirabbit IgG-HRP (1:16,000; Santa Cruz). The immunoreactive bands were visualized by Amersham enhanced chemiluminescence (ECL) using the advance western blotting detection kit (GE Healthcare, Germany).
Statistical analysis
The data in this study are expressed by their mean ± the standard error of the mean (SEM), estimated from at least three independent experiments. The statistical differences between the treatments were determined using one-way analysis of variance (ANOVA), where the Tukey post hoc analysis was implemented for comparisons between multiple means. Statistical differences between treatments were considered to be significant at p < 0.05.
Results
The effects of PPARγ and Nurr1 agonists on the MPP+ induced death in PC12 cells
In order to obtain the appropriate levels of PPARγ and Nurr1 agonists beyond the cytotoxicity induction, various concentrations of GW1929 and 6-MP were examined, in which, a concentration range of GW1929 (5–80 µM) and 6-MP (0.1–200 µM) were used for assessing cell viability (Supplementary Fig. 1). Therefore, nontoxic concentrations of aforementioned components were identified as 10 μM for GW1929 and 0.5 μM for 6-MP. Interestingly, a drastic reduction of cell viability at 5 µM GW1929 was observed, while higher concentrations did not show this effect. Consequently, the cell viability response to the application of 5 µM GW1929 remains obscure, as it was not confirmed statistically in the carried out duplicate.
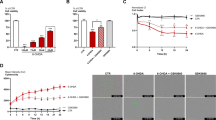
In the next step, PC12 cells were treated with a different concentration (50, 200, 400 μM) of MPP+ for 24 h and then the cell viability was evaluated by the MTS test for every cell (Fig. 1a). The cell viability was decreased by the MPP+ treatment compared to the control group (untreated cells) and the experiments confirmed that the MPP+ had a dose dependency on the reduction of the cell viability. The highest decrease in the cell viability occurred at 400 μM concentration of MPP+. Then, PC12 cells were pretreated with various amounts of GW1929 and/or 6-MP and incubated with MPP+ (400 μM) for 24 h. The cell viability reduction under the MPP+ treatment was significantly reversed by treating the cell with GW1929 (10 μM) or 6-MP (0.5 µM) (Fig. 1b, c). Hence, both components (GW1929, 6-MP) were used at effective concentrations against MPP+ cytotoxicity to examine their neuroprotective characteristics (Fig. 1d), and the results confirmed that the PPARγ and Nurr1 agonists do not have synergistic effects on the MPP+-treated PC12 cells. On the other hand, measurement of apoptosis induced by MPP+ treatment in PC12 cells indicated that a single or simultaneous pretreatment of cells with GW1929 and 6-MP significantly reduced the rate of apoptosis (Supplementary Fig. 2). This phenomenon implies that despite the induction of apoptosis by MPP+, GW1929 and 6-MP were able to reverse this condition.
GW1929 and 6-MP prevented the cell viability decrease induced by MPP+. PC12 cells were pretreated with agonists for 24 h followed by incubation with MPP+ for 24 h. a MPP+ decreased cell viability after 24 h. As shown, 400 µM of MPP+ significantly caused a decrease in cell viability (asterisk represents statistical significance of p < 0.05). b PPARγ agonist (GW1929) protected PC12 cells against MPP+-induced toxicity. PC12 cells were treated with MPP+ (400 μM) for 24 h and GW1929 in 10 μM reversed the viability of cells. c Nurr1 agonist (6-MP) protected PC12 cells against MPP+. Incubation of PC12 cells with MPP+ (400 μM) induced a decrease in cell viability, while both 0.5 and 1 μM of Nurr1 agonist had protective effect. d A combination of GW1929 (10 μM) and 6-MP (0.5 μM) did not show a more protective effect on cell viability than individual application of each compound. The letter of “a” indicates the significant difference between MPP+-treated sample and control (untreated sample) at p < 0.05. The letter of “b” indicates the significant difference between MPP+-treated sample and agonist plus MPP+-treated samples at p < 0.05. Error bars represent the mean of triplicate independent experiments ±SEM
The change in the intracellular distribution of PPARγ and Nurr1 after the activation by respective agonist at the effective concentrations
Upon activation of PPARγ and Nurr1, it would be expect to see a change in intracellular disturbance of these factors from the cytosol to the nucleus. To ensure that the effective concentrations of GW1929 (10 μM) or 6-MP (0.5 µM) were not below the threshold required for the activation of PPARγ and Nurr1, the intracellular localization of PPARγ and Nurr1 was measured following performing the treatment with the aforementioned agonists. As shown in Fig. 2 and Supplementary Fig. 3, in the presence of 6-MP (0.5 µM), Nurr1 entered the nucleus (85 % nuclear vs. 15 % cytosolic distribution) predominantly compared with the untreated sample (−6-MP; 23 % nuclear and 77 % cytosolic distribution). Furthermore, treatment with PPARγ agonist (10 μM) resulted in the nuclear localization of PPARγ (72 % nuclear distribution vs. 28 % cytosolic distribution) compared with the untreated sample (−GW1929, 14 % nuclear and 86 % cytosolic distribution). Therefore, these data ruled out the off target effects of the ligands at defined concentrations. Hence, the ligands were effectively used to prevent apoptosis.
PPARγ and Nurr1 activation changed their intracellular distributions. Intracellular localization of PPARγ and Nurr1 in cells indicated predominantly nuclear-sorting after activation by GW1929 (10 μM) and 6-MP (0.5 µM), respectively, as compared with the untreated samples (−GW1929 and −6-MP). Nuclei were counterstained with DAPI. Scale bar is 100 nm
GW1929 and 6-MP reversed MPP+ reduced tyrosine hydroxylase expression
To explore the protective effects of GW1929, 6-MP, and their combination, PC12 cells were immunostained for the tyrosine hydroxylase as a dopaminergic marker (Fig. 3a) and the intensity of TH expression was analyzed by flow cytometry (Fig. 3b). In the normal state, the PC12 cells expressed tyrosine hydroxylase, while in the presence of MPP+ (400 μM) this expression was reduced. To observe the dose-responsiveness of the ligands used in this study, the PC12 cells were pretreated with a range of concentrations of GW1929 (5–20 μM) and 6-MP (0.1–1 μM) and their combination which alleviated the MPP+ effect on the TH expression level (Fig. 4). This additional result confirmed that those concentrations for GW1929 (10 μM) and 6-MP (0.5 µM) were mostly effective. Thus, we concluded that the PPARγ agonist (GW1929) and Nurr1 agonist (6-MP) have enhanced the TH expression and blocked the MPP+ toxicity when they were used alone or combined together.
Treatment with GW1929, 6-MP, and their combinations modulated TH expression levels. a PC12 cells were pretreated by GW1929 (10 μM), 6-MP (0.5 µM), and their combination for 24 h before MPP+ treatment. Cells were stained with antibody against TH. Nuclei were counterstained with DAPI. Scale Bar is 200 µm. b Fluorescence intensity of TH was obtained and quantified according to the fluorescence intensity of treated cells on the captured images of at least ten different fields with an Olympus DP70 camera (Olympus, Japan) as described in Materials and methods. The letter of “a” indicates the significant difference between MPP+-treated sample and control (untreated sample) at p < 0.05. The letter of “b” indicates the significant difference between MPP+-treated sample and agonist plus MPP+-treated samples at p < 0.05
The effects of GW1929, 6-MP, and their combination on a TH and b Ferritin Light Chain (FTL), c Nurr1 and d Ephrin A1 transcript levels. PC12 cells were pretreated by GW1929 (5, 10, 20 μM), 6-MP (0.1, 0.5, and 1 µM), and their combinations for 24 h. Gene expression was evaluated by RT-qPCR, one day after MPP+ treatment. Error bars represent the mean of triplicate independent experiments ±SEM. Similarly, alphabets indicate significant difference between the same agonist plus MPP+-treated samples and MPP+-treated sample at p < 0.05
PPARγ and Nurr1 modulate different target genes in PC12 Cells
To gain further insight into the action of Nurr1 and PPARγ agonists, which promoted the cell survival under the MPP+-induced neuropathological stress, the expression of several target genes for Nurr1 and PPARγ was assessed by RT-qPCR. These candidate genes are linked with specific pathways leading to the onset of clinical symptoms in the PD patients. 6-MP (0.1–1 μM) increased the FTL transcript level that has a critical role in iron homeostasis. Vidal et al. [39] have shown that the FTL mutation is associated with the PD onset by enhancing oxidative stress reactions. However, GW1929 (5–20 μM) did not have any significant effect on the FTL expression (Fig. 4). The data in this study suggest that Nurr1 agonist could control the iron density in the PD patient when was applied alone or in combination with GW1929. Nurr1, as a transcription factor, is controlled by cAMP response element binding (CREB) protein in the pathways that are not associated with PPARγ modulation [40]. We confirmed this hypothesis by measuring the Nurr1 mRNA levels in the treated groups and we found out that PPARγ agonist (GW1929) did not have a significant effect on the Nurr1 expression, whereas 6-MP enhanced Nurr1 mRNA level in a dose-responsive manner. Moreover, a combined application of 6-MP and GW1929 caused a similar effect of increasing the Nurr1 expression (Fig. 4). Ephrin A1 signaling plays a critical role in the cell migration, axon guidance, and differentiation. Jing et al. [31] showed that this receptor was involved in the dopaminergic neurogenesis and angiogenesis in rat PD models. In addition, Ephrin A1 modulates synaptic plasticity and improves cognition [41]. Therefore, the high expression level of Ephrin A1 enhances synaptogenesis and improves cognitive performance in patients who are suffering from dementia. Based on these concepts, the mRNA levels of Ephrin A1 were measured in this study under circumstances similar to those described above. Interestingly, increased transcript levels of Ephrin A1 were observed when GW1929 was used at concentrations of 10 and 20 µM. Moreover, a simultaneous treatment with GW1929 (10 µM) and 6-MP (0.5 µM) synergistically increased the transcript levels of Ephrin A1. These data suggest that acceleration in dopaminergic neurons differentiation could be achieved by a cotreatment with 6-MP and GW1929 at effective concentrations. Moreover, the relative expression level of Ephrin A1 was significantly highest in the cotreated samples compared with the samples which were treated with individual agonists (Fig. 4). Presumably, this indicates that the associated form of these agonists could be useful in preventive medicine studies for increasing the cognition and reducing dementia in neurological disorders such as PD and Lewy body dementia (LBD).
PPARγ and Nurr1 agonists decreased the MPP+-induced oxidative stress in PC12 cells
The generation of ROS was assessed in order to further explore the cellular protective mechanism of GW1929 and 6-MP. The MPP+-treated PC12 cells presented high levels of ROS which may account for the cells death. This increase was attenuated by treatments of GW1929, 6-MP, and their combined form (Fig. 5a).
The protective effects of GW1929, 6-MP, and their combination on intracellular ROS levels and loss of MMP. a PC12 cells were treated by 400 μM MPP+ for 24 h in the absence or presence of GW1929 (10 μM), 6-MP (0.5 µM), and their combining form, and intracellular ROS was measured using the dye DCFH2-DA. b The same condition was applied to measure the MMP change by means of flow cytometry using JC-1 dye. The number of independent repeats was three for each experiment (n = 3). Letters indicate the significant difference between the same samples at p < 0.05
PPARγ and Nurr1 agonists inhibited MPP+ effects on MMP (ΔΨm)
MPP+ as a toxic agent is able to block mitochondrial complex, and hence, it can increase ROS production and affect the MMP. The flow cytometry results revealed that ΔΨm diminished in the PC12 cells which were treated by MPP+ (400 μM). This reduction was detected after 24 h by JC-1 (2.5 μg/mL). However, in the presence of GW1929, 6-MP, or their combination, the MMP of PC12 cells did not change. These data suggest that these agonists have a potency to maintain the ΔΨm level in the presence of MPP+ and show their effects on the mitochondrial function (Fig. 5b).
PPARγ and Nurr1 agonists decreased the MPP+-increased level of NFκB
The anti-inflammatory effect of GW1929 and 6-MP was estimated by analysis of NFκB content. As shown in Fig. 6, GW1929 and 6-MP significantly reduced the level of NFκB protein in the MPP+-treated cells when compared with the control group (only treated with MPP+).
Discussion
Finding proper agonists provide an effective treatment for PD. PC12 cells are suitable in vitro model for Parkinson and DA neurons studies. In our model, we used MPP+ to induce oxidative stress and neurotoxicity. Here we confirmed that MPP+ caused dose-dependent reduction in cell viability and 400 μM of that neurotoxin was enough to ~50 % cell death. PPARγ agonists (Rosiglitazone, Pioglitazone and GW1929) have neuroprotective potentials to ameliorate many neurological disorders, especially those associated with PD [42]. On the other hand, neuroprotective and anti-inflammatory effects of Nurr1 agonist have been implicated in a PD lesion model [43]. In the present study, we used GW1929 as a specific PPARγ synthetic agonist [44] and 6-MP as a Nurr1 agonist [15]. Treatment with GW1929 and 6-MP attenuates the loss of TH+ ratio and showed protective effects on PC12 cells against MPP+ toxicity. Higher levels of GW1929 and 6-MP declined their neuroprotective potential. This may reflect the toxicity of these compounds at higher concentrations or very possibly indicates the receptor-independent mechanisms of these agonists at higher concentrations which in turn may interfere with their protective roles observed in our study. In current study, we examined the combined activation of PPARγ and Nurr1 as therapeutic approach for PD. Our experimental data indicated that Nurr1 agonist not only had protective effects on the PC12 cells alone but also exerted a similar outcome when applied in combination with PPARγ agonist despite having different molecular pathways. To finding out signaling pathways which are related to PPARγ and Nurr1 activity during their protective behaviors, we assessed the expressions of several genes including FTL, Nurr1, TH, and Ephrin A1, the intracellular amounts of ROS and mitochondrial membrane potential (MMP).
The expression of Nurr1 has crucial role in the differentiation of the DA neurons and their maintenance, and reduction in its level or mutation occurrence help PD development [45–47]. Our findings, along with that outlined above [48], indicate that Nurr1 activation and upregulation could serve as a new therapeutic approach to relief the symptoms of PD.
In PD, the most abundant deposit of iron is in ferric (Fe3+) form which causes lipid peroxidation, oxidative stress, inflammation, and apoptosis in neurons and also iron overloading is not related to high transferrin expression or activity. Transferrin as iron transporter delivers iron and circulates in blood to give iron to various tissues, but mutation in light polypeptide of the ferritin was reported in PD patients [49]. FTL stabilizes ferritin complex and promotes long-term storage of iron [50]. In addition, transcriptional modification in several gene expressions that play a role in iron hemostasis has cross talking with inflammation in neurodegenerative disorders. Iron chelating agents have potential to abate parkinsonian patients’ symptoms and indication of various iron chelators as neuroprotective agents in many neurological disorders, suggesting that iron chelation might be a promising therapeutics [51, 52]. We found that MPP+ decreased the expression of the FTL but 6-MP could reverse that destructive effect. Unlike 6-MP, GW1929 treatment did not significantly enhance the FTL expression. However, a combinatorial form of GW1929 and 6-MP was effective on the enhancement of FTL expression. This is an interesting feature which should be further investigated in order to understand whether PPARγ acts with Nurr1 on the modulation of FTL expression.
Reports showed that exposing of neurons to oxidative stress increased PPARγ coactivator 1α (PGC1-α) expression [53]. cAMP response element binding protein (CREB) is a transcription factor, which can respond to oxidative stress, and works as a neuroprotective agent [54]. Neuroprotective role of the CREB is related to its ability to distinct activating of PGC1-α and Nurr1. Activation of PGC1-α and Nurr1 in response to oxidative stress triggers almost independent set of target genes [40]. Our data showed that a treatment with 6-MP increased Nurr1 expression, while PPARγ agonist GW1929 did not have any effect on the expression of Nurr1. Therefore, activated Nurr1 may attribute to the induction of expression of Nurr1. However, additional experiments are required in order to understand whether increased expression of Nurr1 by activated Nurr1 is mediated by another factor or not. Our data were in concordance with previous studies elucidating that PPARγ and Nurr1 could act in distinct molecular pathways (Fig. 7).
Schematic representation of molecular protective mechanism by GW1929, 6-MP. As shown, GW1929 and 6-MP activate PPARγ and Nurr1, respectively. Activated PPARγ and Nurr1 could significantly decrease ROS levels and protects MMP against MPP+ toxicity via controlling various cell signaling pathways and gene expressions triggering ROS clearance and maintain mitochondrial stability. These factors significantly reduce NF-κB content in PC12 cells and exert an anti-inflammatory role in MPP+-treated cells
Eph/ephrin signals play important roles in cell migration, axon guidance, and neuronal development [28]. In addition, this protein is expressed in distinct cell types of the neurogenic niche, and also has differential functions on stem cell proliferation, survival, and differentiation [55]. Recent studies have shown that Ephrin A1 mediated dopaminergic neurogenesis and angiogenesis in a rat model of PD [20]. Our results indicated that cotreating of GW1929 with 6-MP enhances the Ephrin A1 expression which highlighted a combinational therapy approach for PD treatment. It is important to note that such effect was not observed when these agonists were applied distinctly. On the other hand, altered cellular oxidation and impairment in cellular functions occur in neurodegenerative disorders including PD and AD.
ROS production and MMP disturbance are the main reasons for neuronal apoptosis and death. ROS as a second messenger plays a role in redox-sensitive signal transduction and can damage biomolecules in cells [56]. Oxygen-derived radicals participate in lipid oxidation and several protein degradations which contribute to neuron death [57]. A loss of mitochondria membrane potential acts as a potent mediator in cell apoptosis and plays an important role in ROS production [58]. In neurodegenerative conditions, mitochondria are damaged by numerous factors such as MPP+, which could block the activity of complex I, leading to MMP decrease and ROS generation [59]. Our results showed that GW1929, 6-MP, or their combinations caused a significant decrease in the ROS levels and an increase in MMP. PPARγ and Nurr1 may control various cell signaling pathways and gene expressions, hence playing a cumulative role in ROS clearance and maintaining mitochondrial stability in MPP+-treated cells (Fig. 7). Innate immune system is the first defense mechanism that organisms use to response to the exogenous and endogenous pathogens. In central nervous system (CNS), this responsibility against injuries and endogenous markers is related to supportive cells such as microglia and astrocytes [60]. They can trigger secondary responses to neurodegeneration in CNS by secreting various cytokines, chemokines, and initiation of the immune signaling pathways that are the basis of the neuroinflammation [61]. In neurodegenerative disorders such as AD and PD, inflammatory response and neuroinflammation could start during neuronal loss and increase the vulnerability of the normal neurons to degeneration [62]. Transcriptional levels of inflammatory cytokines such as tumor necrosis alpha (TNF-α) and interleukin 1beta (IL-1β) are controlled by the activity of NF-κB [63]. In PD patients, active form of NF-κB is detectable in SNpc, which is crucial for transcription of several proinflammatory genes [64, 65]. Our findings revealed that GW1929, 6-MP, or their combination significantly reduced active form of the NF-κB content in the PC12 cells, indicating that these two compounds play an anti-inflammatory role.
It is necessary to remind that in the present study, in order to avoid the interfering effects of nerve growth factor (NGF), the experiments were performed in the absence of this factor. To our knowledge, NGF is a required factor to induce signaling pathways for neural differentiation of PC12 cells as PI3K pathway, which leads to the cell survival [66]. Treating PC12 cells with the NGF may complicate or influence the ameliorating data obtained by combinatorial effects of PPARγ and Nurr1 agonists, and therefore, we decided to exclude it from our experiments. However, further experiments are required to verify the outcomes of this study on NGF-treated PC12 cells. Specially, these agonists should be tested after co- and posttreatment administration of the toxins for induction of inflammation.
Conclusion
Taken together, GW1929 and 6-MP may act as the neuroprotective agents controlling specific gene expressions in MPP+-treated PC12 cells. The ligands of orphan nuclear receptors are supposed to be potential therapeutic factors for a variety of human diseases especially the neurological disorders. In the present study, we applied GW1929 and 6-MP as the specific agonists for PPARγ and Nurr1, respectively, and showed their therapeutic abilities for PD treatments useful for ameliorating PD symptoms. It is necessary to notify that these data should be tested and extended in a primary cultured dopaminergic cells or in vivo models of PD. On the other hand, various concentrations of these agonists should be examined in vivo in order to obtain the effective concentrations of these compounds.
Abbreviations
- 6-MP:
-
6-Mercaptopurine
- AD:
-
Alzheimer’s disease
- CREB:
-
cAMP response element binding
- DA:
-
Dopaminergic
- DAPI:
-
4′, 6-Diamidino-2-phenylindole
- DCF:
-
2′, 7′-Dichlorofluorescin
- DCFH2-DA:
-
6-Carboxy-2′, 7′-dichlorodihydrofluorescein diacetate
- DMSO:
-
Dimethyl sulfoxide
- DMEM:
-
Dulbecco’s modified eagle medium
- ETS:
-
Electron transfer system
- FITC:
-
Fluoroisothiocyanate
- FTL:
-
Ferritin light chain
- JC-1:
-
J-aggregate-forming fluorescent dye, 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolocarbocyanine
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- IL-1β:
-
Interleukin 1 beta
- LBD:
-
Lewy body dementia
- MMP:
-
Mitochondrial membrane potential
- MPP+ :
-
1-Methyl-4-phenylpyridinium ion
- MPTP:
-
1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- NGF:
-
Nerve growth factor
- PD:
-
Parkinson’s disease
- PGC1-α:
-
PPARγ coactivator 1α
- PMS:
-
Phenazine methosulfate
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- ROS:
-
Reactive oxygen species
- SNpc:
-
Substantia-nigra pars compacta
- SVZ:
-
Subventricular zone
- TBBPA:
-
Tetrabromobisphenol A (2,2-bis(4-hydroxy-3,5-dibromophenyl)propane
- TH:
-
Tyrosine hydroxylase
- TNF-α:
-
Tumor necrosis alpha
- TRITC:
-
Tetramethyl rhodamine-isothiocyanate
References
De Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet neurol 5:525–535
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53:S26–S36
Jodeiri Farshbaf M, Ghaedi K, Megraw TL, Curtiss J, Shirani Faradonbeh M, Vaziri P et al (2016) Does PGC1α/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? Neuromol Med 18(1):1–15
Schulz JB, Falkenburger BH (2004) Neuronal pathology in Parkinson’s disease. Cell Tissue Res 318(1):135–147
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20(5):649–688
Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169(3):453–459
Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE (2000) Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci 20:558–567
Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P et al (2008) Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28(37):9287–9296
Wojtowicz AK, Szychowski KA, Kajta M (2014) PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotox Res 5:311–322
Kaundal RK, Sharma SS (2011) Ameliorative effects of GW1929, a nonthiazolidinedione PPARγ agonist, on inflammation and apoptosis in focal cerebral ischemic-reperfusion injury. Curr Neurovasc Res 8:236–245
Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88:494–501
Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V et al (2005) Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol 70:177–188
Kim CH, Han BS, Moon J, Kim DJ, Shin J, Rajan S et al (2015) Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci USA 112:8756–8761
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons in Parkinson’s disease. Prog Neurobiol 77:128–138
Ordentlich P, Yan Y, Zhou S, Heyman R (2003) An identification of the antineoplastic agent 6-mercaptopurine as an activator of the orphan nuclear hormone receptor Nurr1. J Biol Chem 278:24791–24799
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG et al (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Shi Y (2007) Orphan nuclear receptors in drug discovery. Drug Discov Today 12(11–12):440–445
Yan J, Sun J, Huang L, Fu Q, Du G (2014) Simvastatin prevents neuroinflammation by inhibiting N-methyl-d-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res 92:634–640
Jing X, Miwa H, Sawada T, Nakanishi I, Kondo T, Miyajima M et al (2012) Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One 7:e32019
Men T, Piao SH, Teng CB (2013) Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP. Yi Chuan 35:1283–1290
Parkinson GM, Dayas CV, Smith DW (2015) Age-related gene expression changes in substantia nigra dopamine neurons of the rat. Mech Ageing Dev 149:41–49
Praticó D, Pasin M, Barry OP, Ghiselli A, Sabatino G, Iuliano L et al (1999) Iron-dependent human platelet activation and hydroxyl radical formation: involvement of protein kinase C. Circulation 99(24):3118–3124
Dusek P, Schneider SA, Aaseth J (2016) Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol. doi:10.1016/j.jtemb.2016.03.010
Arosio P, Levi S (2010) Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta 1800(8):783–792
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203
Vidal R, Ghetti B, Takao M, Brefel-Courbon C, Uro-Coste E, Glazier BS et al (2004) Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol 63(4):363–380
Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L et al (2001) EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105:233–244
Gerlai R (2001) Eph receptors and neural plasticity. Nat Rev Neurosci 2(3):205–209
Aoki M, Yamashita T, Tohyama M (2004) EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J Biol Chem 279:32643–32650
Jing X, Miwa H, Sawada T, Nakanishi I, Kondo T, Miyajima M et al (2012) Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One 7(2):e32019
Wang AG, Chen CH, Yang CW, Yen MY, Hsu WM, Liu JH et al (2002) Change of gene expression profiles in the retina following optic nerve injury. Brain Res Mol Brain Res 101(1–2):82–92
Zhang Z, Li X, Xie WJ, Tuo H, Hintermann S, Jankovic J et al (2012) Anti-parkinsonian effects of Nurr1 activator in ubiquitin–proteasome system impairment induced animal model of Parkinson’s disease. CNS Neurol Disord Drug Targets 11:768–773
Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ et al (2003) Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem 85:622–634
Huot P, Lévesque M, Morissette M, Calon F, Dridi M, Di Paolo T et al (2008) L-Dopa treatment abolishes the numerical increase in striatal dopaminergic neurons in parkinsonian monkeys. J Chem Neuroanat 35(1):77–84
Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S (2010) A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci 30:963–972
Ghoochani A, Shabani K, Peymani M, Ghaedi K, Karamali F, Karbalaei K et al (2012) The influence of peroxisome proliferator-activated receptor γ1 during differentiation of mouse embryonic stem cells to neural cells. Differentiation 83:60–67
Peymani M, Ghoochani A, Ghaedi K, Karamali F, Karbalaie K, Kiani-Esfahani A et al (2013) Dual effects of peroxisome proliferator-activated receptor γ on embryonic stem cell self-renewal in presence and absence of leukemia inhibitory factor. Eur J Cell Biol 92:160–168
Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK et al (2008) Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 28:60–67
Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J et al (2010) NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci USA 107:12317–12322
Fu AK, Ip NY (2007) Cyclin-dependent kinase 5 links extracellular cues to actin cytoskeleton during dendritic spine development. Cell Adhes Migr 1:110–112
Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N et al (2008) The PPARγ agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br J Pharmacol 154:226–233
Smith GA, Rocha EM, Rooney T, Barneoud P, McLean JR, Beagan J et al (2015) A Nurr1 agonist causes neuroprotection in a Parkinson’s disease lesion model primed with the toll-like receptor 3 dsRNA inflammatory stimulant poly(I:C). PLoS One 10:e0121072
Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW et al (2009) PPARγ inhibits NF-κB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metabol 297:174–183
Dubois C, Hengerer B, Mattes H (2006) Identification of a potent agonist of the orphan nuclear receptor Nurr1. Chem Med Chem 1:955–958
De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, Tjalkens RB (2015) Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci 143:360–373
Lou X, Liao W (2012) Association of Nurr1 gene mutations with Parkinson’s disease in the Han population living in the Hubei province of China. Neural Regen Res 7(23):1791–1796
Esteves M, Cristóvão AC, Saraiva T, Rocha SM, Baltazar G, Ferreira L et al (2015) Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front Aging Neurosci 7:20
Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF et al (2001) Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 28(4):350–354
Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J (2011) Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord 17:423–430
Mounsey RB, Teismann P (2012) Chelators in the treatment of iron accumulation in Parkinson’s disease. Int J Cell Biol 2012:983245
Li X, Jankovic J, Le W (2011) Iron chelation and neuroprotection in neurodegenerative diseases. J Neural Transm (Vienna) 118(3):473–477
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Lonze BE, Riccio A, Cohen S, Ginty DD (2002) Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34:371–385
Wilkinson DG (2001) Multiple roles of Eph receptors and ephrins in neural development. Nat Rev Neurosci 2:155–164
Irani K, Goldschmidt-Clermont PJ (1998) Ras, superoxide and signal transduction. Biochem Pharmacol 55:1339–1346
Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ et al (1988) Terada and LS Xanthine oxidase derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Invest 81:1556–1562
Susin SA, Zamzami N, Kroemer G (1996) The cell biology of apoptosis: evidence for the implication of mitochondria. Apoptosis 1:231–242
Troy CM, Salvesen GS (2002) Caspases on the brain. J Neurosci Res 69:145–150
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm 1(1):14
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediat Inflamm 2013:342931
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47
Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S et al (2000) Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm (Vienna) 107(3):335–341
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM et al (2007) Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 104(47):18754–18759
Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-κB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol 7(3):544–556
Klesse LJ, Meyers KA, Marshall CJ, Parada LF (1999) Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene 18:2055–2068
Acknowledgments
This study was funded by a grant-in-aid of research from Royan Institute awarded to Kamran Ghaedi, Ph.D. as the Principal Investigator (P.I.), and in support of Mohammad Jodeiri Farshbaf for obtaining his M.Sc. degree from the University of Isfahan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interest to disclose and all authors support submission to this journal.
Ethical improvement statement
Approval for this study was obtained from the Institutional Review Board of Royan Institute (Tehran, Iran).
Additional information
Mohammad Jodeiri Farshbaf and Mahboobeh Forouzanfar contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2764_MOESM1_ESM.tif
Supplementary Figure 1: Cell proliferation modulation by GW1929, 6-MP. PC12 cells were cultured in the presence different amounts of GW1929, 6-MP. Results were expressed as percentages of viable cells in relation to the control samples (Vehicle only). Represented values are the mean of duplicate independent experiments. (TIFF 155 kb)
11010_2016_2764_MOESM2_ESM.tif
Supplementary Figure 2: Apoptosis rate was decreased by pretreatment of the cells with GW1929, 6-MP. The number of annexin V positive cells was significantly decreased when GW1929, 6-MP and their combination were used. Results were expressed as the percentage of cell number after treating with MPP+. Represented values are the mean of triplicate independent experiments ±SEM. Similar alphabets indicate significant difference between same samples at p<0.05. (TIFF 151 kb)
11010_2016_2764_MOESM3_ESM.tif
Supplementary Figure 3: Quantification of PPARγ and Nurr1 intracellular distribution upon activation. Intracellular localization of PPARγ and Nurr1 in cells indicated predominantly nuclear-sorting [72% nuclear distribution vs. 28% cytosolic distribution for PPARγ and 85% nuclear distribution vs. 15% cytosolic distribution for Nurr1] after activation by GW1929 (10 μM) and 6-MP (0.5 µM), respectively, as compared with the untreated samples (-GW1929 and -6-MP) [14% nuclear distribution vs. 86% cytosolic distribution for PPARγ and 23% nuclear distribution vs. 77% cytosolic distribution for Nurr1]. (TIFF 151 kb)
Rights and permissions
About this article
Cite this article
Jodeiri Farshbaf, M., Forouzanfar, M., Ghaedi, K. et al. Nurr1 and PPARγ protect PC12 cells against MPP+ toxicity: involvement of selective genes, anti-inflammatory, ROS generation, and antimitochondrial impairment. Mol Cell Biochem 420, 29–42 (2016). https://doi.org/10.1007/s11010-016-2764-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2764-4