Abstract
Expression of MACC1 (metastasis-associated in colon cancer-1) protein is associated with metastasis of various human cancers. This study analyzed MACC1 protein expression in hepatocellular carcinoma (HCC) tissue specimens and then investigated the effects of MACC1 knockdown on HCC cell migration and invasion, and gene expression levels. Sixty pairs of HCC and adjacent normal liver tissues from HCC patients were analyzed for MACC1 expression immunohistochemically. The HCC cell lines Hep3B, Huh7, MHCC97H, SMMC-7721, Bel-7402, and HepG2 and the normal liver cell line LO2 were used to assess expressions of MACC1 mRNA and MACC1 protein using qRT-PCR and western blot, respectively. MACC1 short hairpin RNA (shRNA) was used to knockdown MACC1 protein expression in Huh7 cells. Changes in the tumor phenotype of these cells were analyzed with wound healing assay and invasion assays, and differences in gene expression were evaluated via western blot. Immunofluorescence was used to locate MACC1 protein in the above cell lines. MACC1 was highly expressed in HCC tissues and the nuclear expression of MACC1 protein was associated with poor tumor differentiation and intrahepatic metastasis or portal invasion. Moreover, MACC1 mRNA and MACC1 protein was also expressed in HCC cell lines. Immunostaining showed that MACC1 protein was localized in both nuclei and cytoplasm of HCC cell lines and the nuclear localization of MACC1 protein was associated with increased aggressiveness of HCC in cell lines. Knockdown of MACC1 expression using MACC1-shRNA reduced Huh7 cell migration and invasion abilities, which was associated with downregulation of MMP2, MMP9, and c-Met proteins in Huh7 cells. Localization of MACC1 protein to the nucleus may predict HCC progression. Knockdown of MACC1 expression using MACC1 shRNA warrants further evaluation as a novel therapeutic strategy for control of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for ~750,000 new cases and more than 670,000 cancer-related deaths annually in the world. Approximately, half of new cases and deaths due to HCC occur in China [1]. HCC is usually diagnosed at advanced clinical stages, and advanced stage, tumor recurrence, and metastasis contribute to poor survival of the patients.
Tumor metastasis is a complex multistep process in which tumor cells leave the original site, migrate into the lymphatic system or bloodstream, and then form secondary tumors in other parts of the body. Tumor metastasis is very common in the late stages of cancer and is responsible for the majority of cancer deaths. Molecularly, a number of genes participate in the process of tumor metastasis, including the gene metastasis-associated in colon cancer-1 (MACC1). The gene’s protein product MACC1 was first identified through analysis of normal colon mucosae and primary and secondary colorectal cancer tissues, using differential-display reverse transcription PCR [2, 3]. A subsequent study found that MACC1 protein in vitro promoted colon cancer cell migration and invasion, and hepatocyte growth factor (HGF)-induced tumor cell scattering. In xenograft models, MACC1 induced tumor growth, and liver and lung metastases [3–5]. Furthermore, MACC1 levels were significantly higher in primary and metastatic tumor tissues compared with normal colon and liver tissues; it appears that the induction of MACC1 occurs at the crucial step of transition from a benign to a malignant phenotype and can be allocated to the adenoma-carcinoma sequence for colon cancer [6]. When restricted to cell nuclei, MACC1 protein was associated with development of distant tumor metastases, whereas cytoplasmic expression was almost exclusively found in non-metastasizing tumors [3, 6].
An analysis of tumor tissues specifically from HCC patients showed that MACC1 expression was significantly associated with HCC tumor cell vascular invasion and α-fetoprotein levels (a biomarker of HCC and its metastases) [7]. Another study also found that MACC1 levels were associated with high α-fetoprotein levels, overall survival, and disease-free survival of HCC patients [8]. According to these results, MACC1 is involved in HCC progression and metastasis.
Structural analysis of MACC1 has linked this protein that regulates cell apoptosis and HGF receptor (c-Met) [9]. As a proto-oncogene, c-Met is mainly expressed on the membrane of epithelia-originated tumor cells, and while essential for embryonic development and wound healing, the tyrosine kinase c-Met is also the only known membrane receptor of HGF (also referred to as HGF/scatter factor [HGF/SF]) [10–12]. As such, the c-Met receptor may be either anti- or pro-apoptotic depending on cell type and in response to diverse stress conditions. When activated by its ligand, it can induce cell survival [9]. C-Met and HGF play a role in development of various cancers.
MACC1 protein can bind to the promoter of the c-Met gene to regulate its expression [13, 14]. Since dysregulation of c-Met expression and activity frequently occur in HCC [15–18], we hypothesized that further study of MACC1 in HCC may yield a prognostic marker of HCC progression and metastasis. In this study, we therefore analyzed MACC1 expression and the protein’s cellular localization in HCC cell lines. We then knocked down production of MACC1 protein in an HCC cell line using MACC1 short hairpin RNA (shRNA) to investigate changes in tumor cell phenotype and gene expression levels.
Results
Differential expression of MACC1 mRNA and protein in normal and HCC tissues and cells
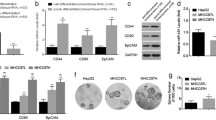
In this study, we performed RT-PCR and qRT-PCR analysis in six HCC cell lines (HepG2, Hep3B, SMMC-7721, Bel-7402, Huh7, and MHCC97H), each of which have different biological characteristics, and found that all six HCC cell lines expressed significantly higher levels of MACC1 and c-Met mRNA than the normal liver LO2 cell line (P < 0.05). Among these HCC cell lines, MHCC97H and Huh7 expressed the highest levels of MACC1 and c-Met mRNA, and western blot results confirmed the RT-PCR data (Fig. 1).
Expression of MACC1 and c-Met mRNA and MACC1 and c-Met protein. The cells of six hepatoma cell lines and a normal liver cell line were subjected to RT-PCR (a, b), qRT-PCR (c, d), western blot (e, f), and then summarized (g, h) as MACC1 protein expression compared to beta-actin. Analysis of determined expression levels of MACC1 (a, c), MACC1 (e, g), c-Met (b, d), and c-Met (f, h). *P < 0.01. ACTB was the internal control
We also analyzed MACC1 expression in 60 cases of paired HCC and distant normal liver tissue specimens and found that many more of the HCC tissues were positive for MACC1 than the matched normal liver tissues. Fifty-six cases (93.3 %) of HCC expressed MACC1 protein, while only 11 (18.3 %) of the normal tissues did so (Fig. 2a, b).
Localization of MACC1 proteins in HCC tissue samples. a IHC staining of MACC1 in hepatocarcinoma tissue (100× for upper line and 400× for lower line). The brown color indicates positive staining for MACC1. b Expression of MACC1 in 60 pairs of HCC by Edmondson classification, and normal (negative control) liver tissues (*P < 0.05). c Subcellular localization of MACC1 in MACC1-positive HCC tissues by Edmondson classification, and normal (negative control) liver tissues (*P < 0.05)
Cellular localization of MACC1 protein in HCC cells
We performed immunohistochemistry to detect the localization of MACC1 protein in cells of HCC tissues and paired normal liver tissues. The results showed positive staining of MACC1 protein in the nuclei of HCC cells, which was associated with Edmondson III–IV classification and intrahepatic metastasis or portal invasion in HCC patients. By contrast, in HCC patients classified as Edmondson I–II and no intrahepatic metastasis or portal invasion, MACC1 protein was localized in both the cytosol and nuclei of HCC cells. Corresponding distant normal liver tissues only expressed very low levels of MACC1 protein, in both nuclei and cytosol (Fig. 2a, c).
We then visualized the cellular location of MACC1 and c-Met proteins in these 6 HCC cell lines using single-labeled and double-labeled immunofluorescence. Consistent with the immunohistochemical data from the HCC tissues, the single cell-labeling revealed that positive staining for MACC1 protein was equally intense in both the nuclei and cytosol of cells of all the tested HCC cell lines except Huh7, where staining for MACC1 was strong in the nuclei but weak in the cytosol (Fig. 3). In contrast, the expression of MACC1 protein was very weak in both the nuclei and cytosol of normal liver LO2 cells (Fig. 3).
Using double-labeled immunofluorescence, we visualized the expressions of both MACC1 and c-Met simultaneously in Huh7 cells. MACC1-positive staining was localized to the nuclei, while c-Met-positive staining was found mostly in the cytosol of these cells (Fig. 4).
Knockdown of MACC1 expression suppressed HCC cell migration and invasion
We investigated whether knockdown of MACC1 expression could inhibit HCC cell migration and invasion, via tumor cell migration and Matrigel invasion assays of Huh7 cells transfected with an MACC1 shRNA vector. Tests confirmed that the transfection of these cells successfully silenced expression of MACC1 (Fig. 5).
We then performed a wound healing assay and found that the migratory potential of MACC1-silenced Huh7 cells was significantly decreased compared with the control Huh7 cells transfected with either the empty plasmid or control shRNA (Fig. 6).
Effects of MACC1 knockdown on inhibition of HCC cell migration. HCC Huh7 cells were grown and transfected with MACC1 shRNA, control shRNA, or empty vector for 48 h and then subjected to the wound healing assay at 0, 12, and 24 h. The data were then averaged and summarized as width ratio of migratory inhibition (at the bottom of the figure), *P < 0.01
The Matrigel invasion assay showed that Huh7 cells transfected with MACC1-shRNA, the control shRNA, and the empty plasmid each differed in their invasion capabilities 24 h after transfection. In Huh7 cells that had been transfected with the MACC1 shRNA vector, 40 ± 2.6 cells/field crossed the Matrigel basement membrane, whereas of those cells transfected with the empty plasmid or control shRNA, 93.7 ± 4.5 and 83.3 ± 3.2 cells/field, respectively, had successfully invaded (P < 0.01, both; Fig. 7).
MACC1 knockdown in HCC Huh7 cells inhibited Matrigel invasion. HCC Huh7 cells were grown and transfected with MACC1 shRNA, control shRNA, or empty vector for 48 h and then subjected to Matrigel invasion assay (top). The data are then summarized (bottom) as % of the control reference. *P < 0.01, compared with the empty plasmid and negative control groups
Effects of MACC1 silencing on MMP2 and MMP9 protein expression
Since MMP2 and MMP9 are members of the matrix metalloproteinase (MMP) family, which have important roles in tumor cell migration and invasion [19–23], we assessed whether MACC1 knockdown in Huh7 cells had an effect on the protein expressions of MMP2 and MMP9.
We performed a western blot for MMP2 and MMP9 levels in MACC1 shRNA-, control shRNA-, and empty plasmid-transfected Huh7 cells and found that silence of MACC1 significantly reduced the expressions of MMP2 and MMP9 (P < 0.01; Fig. 8).
Expressions of MMP2 and MMP9 in MACC1-silenced HCC cells. HCC Huh7 cells were grown and transfected with MACC1 shRNA, control shRNA, or empty vector for 48 or 72 h. Resolution of extracted proteins via western blot showed that knockdown of MACC1 significantly reduced MMP2 and MMP9 levels compared with cells transfected with either the empty plasmid or the control shRNA (*P < 0.01, compared with the empty plasmid, both). The data are then summarized (at the bottom of the figure) as MMP2 and MMP9 protein expression
Discussion
In this study, we evaluated the in vitro expression levels and cellular locations of MACC1 and c-Met in 6 HCC cell lines and compared them with that of a normal liver cell line. Consistent with the high expression and nuclei location of MACC1 in HCC tissue, we found that levels of MACC1 and c-Met mRNA, and that of their respective protein products, were higher in the HCC cells than in the normal liver cells. MACC1 was expressed in both the cytosol and nuclei in these six HCC cell lines, but Huh7 cells expressed MACC1 protein predominantly in the nuclei. After silencing the MACC1 protein expression in Huh7 cells, it was found that tumor cell migration and invasion were also reduced significantly, as well as the levels of MMP2 and MMP9. These data suggest that the expression and cellular location of MACC1 may be useful in predicting HCC progression and metastasis. Further study should determine the underlying mechanism by which MACC1 affects MMP2 and MMP9 expression in HCC cells.
Although many efforts have been devoted to better understanding the molecular pathogenesis and mechanisms of HCC metastasis, this and the high rates for recurrence mean a poor prognosis for HCC patients. This study found higher levels of MACC1 mRNA and MACC1 protein in the six tested HCC cell lines, and MACC1 protein was highly expressed in HCC tissue samples. Previous studies have shown that MACC1 and c-Met levels were correlated, and c-Met is involved in multiple cellular responses including stimulation of cell morphogenesis, promotion of tumor cell migration and invasion, wound healing, and tumor survival, but suppresses apoptosis and the breakdown of the extracellular matrix [15–18]. Qu et al. [24] also showed recently that intratumoral MACC1 expression was closely associated with tumor progression in HBV-induced HCC. Thus, we speculate that MACC1 protein may also affect HCC progression.
In this study, we also detected the cellular localization of MACC1 protein in HCC and normal cell lines, and in HCC tissues. Similar to colorectal cancer [3], we found that in HCC patients classified as Edmondson I–II but without intrahepatic metastasis or portal invasion, MACC1 protein was expressed in both the nuclei and cytoplasm of HCC cells. However, in patients at Edmondson III–IV and with intrahepatic metastasis or portal invasion, MACC1 protein was concentrated in the nuclei of these more severely afflicted liver cancer tissues. It was very interesting that the well-differentiated but invasive Huh7 cells expressed the highest levels of nuclear MACC1 protein. This may be because nuclear localization of MACC1 protein is associated with certain characteristics of HCC cells, both known (such as the degree of differentiation and invasion ability) and as yet unclear (including susceptibility to hepatitis virus infection [25]). It seems to us that the higher concentration of MACC1 protein in the nuclei signifies a promotion of HCC progression. In this study, we also found that the normal liver LO2 cells expressed low levels of MACC1 protein, which is not consistent with a previous study [8]. This may be because our LO2 cells were an immortalized cell line that may have altered in gene expression during immortalization, or over the course of many passages in vitro the gene profile might have changed compared to the normal primary cells. It is true that culture of LO2 cells at different passages showed some slight discrepancies in gene expressions (our preliminary observation).
This study also showed that knockdown of MACC1 protein expression in vitro was associated with changes in tumor cell migration and invasion properties. This is consistent with data reported for colorectal and gastric carcinomas, lung adenocarcinoma, and other tumors [26–28], in which MACC1 was implicated in the regulation of tumor cell migration and invasion. Mechanistically, it is well known that degradation of the extracellular matrix surrounding tumors is the most common feature of tumor cell invasion into surrounding tissues and early metastasis, and members of the MMP family secreted by invading tumor cells can degrade all essential components of the extracellular matrix. Thus, expression levels of these MMPs effectively reflect the aggressiveness of tumor cells and are associated with poor prognosis in various cancers. Such MMPs include MMP2 and MMP9, which are pivotal in degrading the basement membrane and have been proven to facilitate tumor invasion and metastasis in many types of cancer cells [19, 29, 30]. It has also been shown that the invasive and metastatic capabilities of HCC were restrained by suppression of MMP2 and MMP9 [31–33]. Indeed, in this study, we found that inhibition of MMP2 and MMP9 expression was associated with the suppression of HCC cell migration and invasion in vitro after knockdown of MACC1. However, it is unknown how MACC1 regulates MMP2 and MMP9 expression in Huh7 cells. Future study will investigate this underlying mechanism.
Materials and methods
Cell lines and culture
The human liver cancer cell lines Hep3B and Huh7 were obtained from Shanghai Institute of Cell Biology at the Chinese Academy of Sciences (Shanghai, China), while MHCC97H was a kind gift from Professor Dou Ke-feng of the Fourth Military Medical University (Xi’an, China). These three cell lines were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA). The human hepatoma cell lines SMMC-7721, Bel-7402, and HepG2 and the normal liver cell line LO2 were obtained from the American Type Culture Collection and cultured in RPMI-1640 medium (Invitrogen). All media were supplemented with 10 % fetal bovine serum, 50 U/ml ampicillin, and 50 μg/ml streptomycin. Cells were incubated in a 37 °C incubator with 5 % CO2 and 95 % air.
RNA isolation, RT-PCR, and quantitative RT-PCR
Total cellular RNA was isolated from the cultured cells using a Fastgen200 RNA isolation System (Fastgen, Shanghai, China) in accordance with the manufacturer’s protocol. RNA samples were then reverse transcribed into cDNA with a RevertAid Premium First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). PCR amplification of the cDNA was performed at an initial 94 °C for 5 min and then 30 cycles of 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s, and a final extension of 72 °C for 7 min using 2× Taq mix (Kangwei, Beijing, China). The PCR products were then loaded into a 2 % agarose gel (Gene, Spain) for electrophoresis. Ethidium bromide was from Invitrogen and the molecular weight marker DS500 was from Dongsheng (Beijing, China).
In addition, we also performed quantitative real-time RT-PCR (qRT-PCR) using SYBR Green PCR master mix (TakaRa, Dalian, China) with an IQ5 System. The primers to detect mRNA were: MACC1, 5′-CTTGCGGAGGTCACCATAGC-3′ and 5′-GATTTCCAACAACGGGCTCA-3′; c-MET, 5′-CTCTTAACATCTATATCCACCTTC-3′ and 5′-TGCTCCACAATCACTTCTG-3′; and β-actin (ACTB) were 5′-GGGAAATCGTGCGTGACAT-3′ and 5′-CTGGAAGGTGGACAGCGAG-3′. All samples were normalized to internal controls and fold changes were calculated through relative quantification (2−ΔΔCt).
Protein extraction and western blot
Total protein was extracted from the cultured cells using radioimmunoprecipitation lysis buffer (Beyotime, Beijing, China). Protein lysates were quantified with a BioRad kit (Hercules, CA, USA). Fifty microgram protein samples of each were separated via 10 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore, MA, USA). Membranes were incubated in 5 % bovine serum albumin in phosphate buffered saline (PBS) for 2 h, and then serially with rabbit polyclonal antibodies against human MACC1 (1:750, Abcam, Cambridge, UK), mouse monoclonal antibody against human c-Met (1:500, Cell signaling, MA, USA), MMP2 (1:600, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a goat polyclonal antibody against human MMP9 (1:500, Santa Cruz Biotechnology), overnight at 4 °C. After each primary antibody, membranes were incubated in a horseradish peroxidase-labeled goat anti-rabbit/mouse or rabbit anti-goat IgG (Santa Cruz Biotechnology) and detected by chemiluminescence (Millipore). ACTB (1:1000, Cell signaling, MA, USA) was used as a protein-loading control. The intensity of protein bands was quantified using Quantity One software 4.5.0 basic (Bio-Rad).
Immunofluorescence
Cells were grown on 13-mm diameter coverslips for 3 days and then fixed with 4 % paraformaldehyde in PBS and permeabilized in 0.2 % Triton X-100 in PBS. Unspecific binding was blocked by incubating cells with 10 % normal rabbit serum (Sigma, St Louis, MO, USA) for 30 min at room temperature. Subsequently, the cells were incubated with the primary antibody against MACC1 or c-Met (1:200) overnight at 4 °C. These antibodies comprised anti-goat MACC1 (Santa Cruz Biotechnology) for single-label immunofluorescence and rabbit anti-MACC1 (Abcam) or mouse anti-c-Met (Cell signaling, USA) for double-label immunofluorescence. The next day, the cells were rinsed with PBS and incubated with the secondary antibodies (1:500; Santa Cruz Biotechnology) for 1 h at room temperature.
For the single-label immunofluorescence, the red fluorescence secondary antibody was used for detection of MACC1 protein, while the green fluorescence secondary antibody was used to detect MACC1 protein. The red fluorescence antibody was used to detect c-Met protein in the double-label immunofluorescence. These secondary antibodies were guaranteed by the manufacturer for multiple labeling procedures, since they were purified against any cross-reactivity to other species. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Finally, the cells were washed in PBS before being mounted with 95 % glycerin (Sigma-Aldrich).
Immunohistochemistry (IHC)
The Research Ethics Committee of First Affiliated Hospital of Medicine College of Xi’an Jiaotong University approved this study. We collected 60 pairs of HCC and adjacent normal liver tissues from HCC patients, consisting of 38 cases of Edmondson [34, 35] I–II without intrahepatic metastasis and portal invasion (as group 1) and 22 cases of Edmondson III–IV liver cancer with intrahepatic metastasis or portal invasion (as group 2). Specimens were fixed in 4 % paraformaldehyde, embedded in paraffin, and sectioned in accordance with standard protocol, after which IHC was performed.
For IHC, the sections were dewaxed and dehydrated and then incubated with a methanol solution containing 0.3 % hydrogen peroxide for 30 min to block any endogenous peroxidase activities. After antigen retrieval in a citrate buffer with a microwave for 15 min, the sections were incubated with normal serum at 37 °C for 30 min and then with the primary antibody against MACC1 (Santa Cruz Biotechnology) or c-Met (Cell Signaling) at 4 °C overnight. The next day, the sections were washed with PBS and incubated with biotinylated secondary antibodies (Zhongshan Goldenbridge Biotechnology, Beijing, China) and avidin–biotin–peroxidase complex in accordance with the manufacturer’s recommendations. The positive signal was visualized by incubating the sections with a diaminobenzidine solution and counterstaining with hematoxylin. The sections were then dehydrated in an alcohol series, cleared in xylene, and mounted onto glass slides.
We inspected the sections under an inverted microscope. Results were considered positive if >25 % of total cells were stained in 5–10 random high power fields. Staining was rated positive, if cells showed enough brown dye to appear much stronger than the background, whether in the cytoplasm or nucleus. We first rated each group for positive staining (Fig. 2b), and then focused on the location of the dye (cytoplasm or nucleus) among the groups that had already been considered positive. The 67 positive cases consisted of 35 positive cases in group 1, 21 positive cases in group 2, and 11 positive cases in the negative control group (Fig. 2c).
MACC1 shRNA vector and gene transfection
The double-stranded shRNA oligonucleotide against MACC1 in a plasmid vector with U6 promoter and green fluorescent protein was purchased from Shanghai GenePharma (Shanghai, China). Two sets of MACC1 shRNA vectors, designated MACC1-1 and MACC1-2, were also tested in HCC cells. In brief, HCC Huh7 cells were grown overnight and transfected with 1 μg MACC1 shRNA, negative control shRNA, an empty vector, 6 μg MACC1 shRNA, or negative control shRNA, using a transfection reagent from Fermentas for 24, 48, or 72 h, in accordance with the manufacturer’s protocol. The cells were inspected 24 h after gene transfection for transfection rate under a fluorescence microscope, which could reach to >80 %.
The DNA sequences of the double-stranded MACC1 shRNAs were 5′-CACCGAAGATTGGACTTGTACACTGCTTCAAGAGAGCAGTGTACAAGTCCAATCTTTTTTTTG-3′ and 5′-GATCCAAAAAAAAGATTGGACTTGTACACTGCTCTCTTGAAGCAGTGTACAAGTCCAATCTTC-3′. The negative control shRNAs were 5′-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3′ and 5′-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3′. MACC1-1 siRNA sequences were 5′-AAGAUUGGACUUGUACACUGC-3′ (sense) and 5′-GCAGUGUACAAGUCCAAUCUU-3′ (antisense). MACC1-2 siRNA sequences were 5′-AAGCUUGGAAAAGGCUGGAGG-3′ (sense) and 5′-CCUCCAGCCUUUUCCAAGCUU-3′ (antisense). The negative control siRNA sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT -3′ (antisense).
Tumor cell wound healing assay
Tumor cell migration was assessed using an in vitro wound healing assay. Briefly, the hepatoma HepG2, SMMC-7721, and Huh7 cells were grown and transfected with empty vector, control shRNA, or MACC1 shRNA vector for 48 h. The cells were then collected after trypsinization and inoculated into a 6-well plate at 5 × 10 [5] cells per well. The plates were cultured in a conventional way with DMEM for 24 h to ~100 % confluency in a monolayer. The growth medium was then removed and the wells were cross-scratched with a 200-μL pipette tip and washed twice with sterile PBS to remove the cell debris. The cells were then cultured for 0, 12, or 24 h and inspected under an inverted microscope (AE31, Motic, China). The distance from one side of the scratch to the other was measured at different intervals using Image Pro-Plus 6.0 software (Media Cybernetics, USA). For each experiment, [5] visual fields and two repeated wells were measured, with three replications.
Matrigel invasion assay
To assess the invasion ability of HCC cells, an invasion assay was performed with a Millicell invasion chamber (8-μm pore size, Millipore, MA, USA). Hepatoma Huh7 cells were grown and transfected with empty vector, control shRNA, or MACC1 shRNA vector for 48 h. The cells were collected after trypsinization, resuspended in DMEM medium supplemented with 1 % fetal bovine serum, and added (100 μL/well) into the upper chamber that was pre-coated with basement membrane Matrigel (Millipore). Simultaneously, 600 μL DMEM medium supplemented with 20 % fetal bovine serum was placed into each bottom chamber, and these chambers were cultured at 37 °C for over 24 h. At the end of the experiments, the top surface of the microporous membranes was carefully swabbed with cotton swabs and the membranes were fixed with 4 % paraformaldehyde for 10 min, stained with a crystal violet solution for 10 min, and then inspected under an inverted microscope (AE31). The upper, lower, left, right, and central visional fields of each membrane were chosen, and the cells were counted under a light microscope (40×). The average values were calculated and compared.
Statistical analyses
Statistical analysis was performed with SPSS software (version 16.0, SPSS, Chicago, USA). All data are represented as mean ± standard deviation. Multiple group comparisons were achieved by one-way analysis of variance followed by the Bonferroni post-hoc test. P < 0.0 [5] was considered statistically significant. All experiments were repeated independently ≥3 times.
Abbreviations
- c-Met:
-
Hepatocyte growth factor receptor
- HCC:
-
Hepatocellular carcinoma
- MACC1:
-
Metastasis-associated colon cancer 1
- MMP2/9:
-
Matrix metalloproteinase 2/9
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Liang P, Pardee AB (1992) Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257(5072):967–971
Stein U, Walther W, Arlt F et al (2009) MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 15(1):59–67
Chundong G, Uramoto H, Onitsuka T et al (2011) Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res 31(4):1141–1145
Stein U, Dahlmann M, Walther W (2010) MACC1—more than metastasis? Facts and predictions about a novel gene. J Mol Med (Berl) 88(1):11–18
Arlt F, Stein U (2009) Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol 41(12):2356–2359
Shirahata A, Fan W, Sakuraba K et al (2011) MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res 31(3):777–780
Qiu J, Huang P, Liu Q et al (2011) Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma. J Transl Med 9:166
Kokoszynska K, Krynski J, Rychlewski L et al (2009) Unexpected domain composition of MACC1 links MET signaling and apoptosis. Acta Biochim Pol 56(2):317–323
Weidner KM, Arakaki N, Hartmann G et al (1991) Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA 88(16):7001–7005
Bardelli A, Comoglio PM (1997) Scatter factor receptors are key players in a unique multistep program leading to invasive growth. Ciba Found Symp 212:133–144 (discussion 144–137)
Baldus SE, Kort EJ, Schirmacher P et al (2007) Quantification of MET and hepatocyte growth factor/scatter factor expression in colorectal adenomas, carcinomas and non-neoplastic epithelia by quantitative laser scanning microscopy. Int J Oncol 31(1):199–204
Birchmeier C, Birchmeier W, Gherardi E et al (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12):915–925
Stein U, Smith J, Walther W et al (2009) MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle 8(15):2467–2469
Sattler M, Salgia R (2007) c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep 9(2):102–108
Bottaro DP, Rubin JS, Faletto DL et al (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251(4995):802–804
Chmielowiec J, Borowiak M, Morkel M et al (2007) c-Met is essential for wound healing in the skin. J Cell Biol 177(1):151–162
Kaposi-Novak P, Lee JS, Gomez-Quiroz L et al (2006) Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest 116(6):1582–1595
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Bjorklund M, Koivunen E (2005) Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755(1):37–69
Canel M, Secades P, Garzon-Arango M et al (2008) Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. Br J Cancer 98(7):1274–1284
Lamar JM, Pumiglia KM, DiPersio CM (2008) An immortalization-dependent switch in integrin function up-regulates MMP-9 to enhance tumor cell invasion. Cancer Res 68(18):7371–7379
Zheng H, Takahashi H, Murai Y et al (2006) Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res 26(5A):3579–3583
Qu JH, Chang XJ, Lu YY et al (2012) Overexpression of metastasis-associated in colon cancer 1 predicts a poor outcome of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 18(23):2995–3003
Sainz B Jr, Barretto N, Uprichard SL (2009) Hepatitis C virus infection in phenotypically distinct Huh7 cell lines. PLoS ONE 4(8):e6561
Shirahata A, Shinmura K, Kitamura Y et al (2010) MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res 30(7):2689–2692
Shirahata A, Sakata M, Kitamura Y et al (2010) MACC 1 as a marker for peritoneal-disseminated gastric carcinoma. Anticancer Res 30(9):3441–3444
Shimokawa H, Uramoto H, Onitsuka T et al (2011) Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg 141(4):895–898
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Fingleton B (2006) Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci 11:479–491
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67
Bu W, Huang X, Tang Z (1997) The role of MMP-2 in the invasion and metastasis of hepatocellular carcinoma (HCC). Zhonghua Yi Xue Za Zhi 77(9):661–664
Yang P, Yuan W, He J et al (2009) Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: implications for tumor progression and prognosis. Hepatol Res 39(12):1169–1177
Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7(3):462–503
Zhou L, Rui JA, Ye DX et al (2008) Edmondson-Steiner grading increases the predictive efficiency of TNM staging for long-term survival of patients with hepatocellular carcinoma after curative resection. World J Surg 32(8):1748–1756
Acknowledgments
We thank Medjaden Bioscience Limited, Hong Kong, China, for assisting in the preparation of this manuscript. This study was supported in part by grants from the National Natural Scientific Foundation of China (No. 81071897 to YY).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2012_1545_MOESM1_ESM.tif
Supplement Figure 1: Knockdown of MACC1 mRNA expression by MACC1-1 and MACC1-2 shRNA transfection. The HCC cells were grown and transiently transfected with MACC1-1 and MACC1-2 siRNA for 24 h or 48 h and total cellular RNA was isolated and subjected to RT-PCR analysis (TIFF 88 kb)
Rights and permissions
About this article
Cite this article
Gao, J., Ding, F., Liu, Q. et al. Knockdown of MACC1 expression suppressed hepatocellular carcinoma cell migration and invasion and inhibited expression of MMP2 and MMP9. Mol Cell Biochem 376, 21–32 (2013). https://doi.org/10.1007/s11010-012-1545-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1545-y