Abstract
To study pathological changes of fibromuscular system and the role of TGF-β1/Smad pathway in the urethra of a parturition-induced stress urinary incontinence (SUI) rat model. Twenty-eight 8-week-old Sprague-Dawley female rats at gestational day 16 were used and randomized into two groups: sham group and SUI group. After delivery, rats in the SUI group underwent postpartum vaginal balloon dilation and bilateral ovariectomy. 1 month after ovariectomy, urodynamics was assessed. Histological examination (Masson’s trichrome stain, picrosirius red stain, Hart’s elastin stain, Gordon & Sweet’s stain, and immunohistochemical stain) and Western blot were performed on urethral tissues. Both leak point pressure and maximal bladder capacity were significantly decreased in the balloon-injured ovariectomized rats, compared with the sham rats. Muscle was significantly decreased in the urethra of SUI rats compare with sham rats. Collagen I/III and reticular fibers from SUI group were also significantly lower than sham group. Meanwhile, elastic fibers and reticular fibers showed fragmentation and disorganization indicating impairment in the fibromuscular system in SUI rats. TGF-β1, MMP-9, and phosphorylated Smad2 (p-Smad2) were expressed significantly higher in SUI than in sham rats. Simulated birth trauma and menopause induced an upregulation of the TGF-β1/Smad pathway and impairment of the fibromuscular system in the urethra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is a prevalent urological problem that is common in women. Up to 50% of women older than 60-year-old have symptoms of stress-induced urinary incontinence [1]. It had been reported that damages on the urethra striated muscle and smooth muscle are the key component in this pathogenesis [2, 3]. The inherent weakness of the urethral sphincter mechanism in women and the stress of vaginal delivery have been suggested as important factors in the pathogenesis of SUI [4]. Recently, there are more researches indicated that urethral extracellular matrix (ECM) including collagen fiber and elastic fiber may also play a critical role. Chen et al. [5] reported that incontinence have associated with a decreased percentage of area of type I-like collagen fibers and an increased percentage of area of type III-like collagen fibers. Lin et al. [6] reported that the urethras of SUI rats had shorter elastic fiber compared with normal rats. However, there is no well accepted conclusion on this point regarding the SUI.
An abnormal ECM regulation has been implicated as a direct causal factor in a number of important disease processes, such as pelvic organ prolapsed [7], atherosclerotic plaque rupture [8], and congestive heart failure development [9]. Matrix metalloproteinases (MMPs) are a family of zinc dependant extracellular endoproteinases that are collectively capable of hydrolyzing essentially all components of the ECM and basement membrane components. Recently, Zhang et al. [10] reported that MMP-9 cause collagen breakdown in vaginal wall, which may play an important role in the onset and development of SUI. Because of the difficulty in obtaining tissues from human female, studies on SUI mechanism about MMP-9 are lacking. In this study, we used a parturition-induced SUI model in which female rats were subjected to intravaginal balloon dilation after pregnancy and vaginal delivery of pups, followed by ovariectomy 1 week later to simulate menopause [11]. This animal model provides us an opportunity to study the mechanism of SUI and the possible therapy methods.
TGF-β1 is known to play a vital role in tissue remodeling during inflammatory responses. TGF-β1 has been found to stimulate the expression of MMP-9 in endothelial cells, fibroblasts, and epithelial cells [12, 13]. After binding to the receptors, TGF-β1 activates both receptor-associated Smads (Smad2 and Smad3). Activated Smad2 and Smad3 heteroligomerize with the common partner Smad4 (Co-Smad) to form the complexes and translocate into the nucleus to regulate target gene expression [14].
Lin et al. [15] reported a significant increase of TGF-β1/Smad signaling pathway in the urethras of SUI rats by Microarray analysis and TGF-β1 could activate Smad2 in urethral smooth muscle cells in vitro. In the present study, we quantified the key structural components of the urethra, including muscle cell, collagen fiber, elastic fiber, and reticular fiber and their effects in the pathology of SUI, also explored the distribution and activity of the TGF-β1/Smad signaling pathway and MMP-9 in the urethras of SUI rats.
Materials and methods
Animals and overview
All experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. Twenty-eight 8-week-old primiparous Sprague-Dawley rats at gestational day 16 were obtained. They were randomized into two groups: sham group and SUI group. SUI rats were subjected to the development of voiding dysfunction by a previously described procedure [11]. In brief, immediately after delivery, the rats underwent vaginal balloon dilation for 4 h to simulate prolonged labor. 1 week later, the rats were anesthetized, a midline incision made in the abdomen, and both ovaries were excised. The sham rats were not treated with vaginal balloon dilation but underwent sham surgery (midline abdominal incision with no ovariectomy). Four more weeks later, urinary function was assessed and their urethras were harvested for histology and western blot.
Urodynamics examination
Under ketamine and xylazine anesthesia, a midline longitudinal abdominal incision (1-cm length) was made in the upper part of the bladder. One polyethylene-90 tube was inserted into the bladder dome to measure vesical pressure. The bladder catheter was connected to both a syringe pump and a pressure transducer. Then, LPP was measured in the following way by MP150 System (Biopac Systens, Inc.) [16]. The average bladder capacity of each rat was determined after 3–5 voiding cycles. When half bladder capacity was reached, gentle pressure with one finger was applied to the animal’s abdomen. Pressure was gently increased until urine leaked, at which time the externally applied pressure was rapidly removed. The peak bladder pressure was taken as the LPP. At least five LPPs were obtained on each animal and the mean LPP was calculated. Upon completion of cystometry the animals were killed by bilateral thoracotomy.
Histological examination, immunohistochemical stain, and morphometric analysis
The urethrovaginal tissues were harvested (cross-section of the mid-urethra and anterior vagina), and the tissues were immersed in neutral buffered formalin containing 4% formaldehyde for a period of 4 h, embedded in paraffin. Sections of 5 μm thickness were cut using a rotor microtome. The sections were stained with the standard procedures of Masson’s trichrome stain [11], picrosirius red stain [17], Hart’s elastin [6] stain, and Gordon & Sweet’s stain [18].
For immunohistochemistry (N = 10 per group), sections were incubated with antibodies to TGF-β1 (Abcam, Cambridge, MA, USA), Phospho-Smad2 (P-Smad2) (Cell Signaling, Beverly, MA, USA), overnight at 4°C. Slides were rinsed in PBS, followed by the second antibody using the MaxVision™ HRP-Polymer anti-Rabbit IHC Kit, (Maxim Co. China). Semiquantitative analysis was performed to evaluate the intensity by the use of Image pro plus software (Bethesda, MD, USA).
Western blot
Urethral tissue protein samples were prepared by homogenizing in RIPA lysis buffer. Equal amounts of protein (20 μg/lane) were electrophoresed on 10% SDS-PAGE and then transferred to polyvinylidene fluoride membrane (MilliporeCorp, Bedford, MA, USA). Western blotting was performed with antibodies against α-SMA (Abcam, Cambridge, MA, USA), TGF-β1, MMP-9 (Abcam, Cambridge, MA, USA), Smad2 (Cell Signaling, Beverly, MA, USA), P-Smad2, and β-Actin (Santa Cruz Biotechnology, CA, USA). Results were quantified by densitometry (N = 4 per group).
Statistical analysis
Results are expressed as means ± SD. Mann–Whitney U tests were used to evaluate whether differences between groups were significant. P values <0.05 were considered significant.
Results
Development of SUI
LPP and bladder capacity were measured by anesthetized cystometry. In the sham group, LPP was 48.92 ± 1.73 cm H2O compared with 34.90 ± 3.22 cm H2O after balloon-injured ovariectomy in SUI group (Fig. 1). Bladder capacity was also higher in the sham group (1.18 ± 0.18 ml) compared with the SUI group (0.81 ± 0.15 ml).
ECM and muscular damage in the SUI model
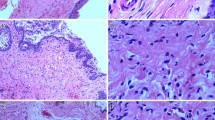
To well define the pathological changes of fibromuscular system related to the SUI, several histological methods were applied. The trichrome stain revealed that striated muscle of the urethras from sham rats was compact and nearly circumferential. However, after balloon-injured ovariectomy SUI rats resulted in mild focal disruption of striated muscle fibers (Fig. 2). Meanwhile, the overall urethral content of smooth muscle in the SUI group was lower than what was observed in the sham group (Fig. 2). To further quantify the amount of urethra muscle content, western blot of α-SMA was applied. The results showed that SUI group had significantly lower muscle content than sham group (Fig. 2).
Assessment of muscle in the urethra. a Representative photographs of cross-sections of mid-urethra with trichrome staining. a and c: striated muscle, b and d: smooth muscle. The total muscle content is decreased and the striated muscle is fragmented and disorganized in SUI rats. b Decrease in α-SMA expression in SUI rats by western blot. c Data are presented as the relative density of α-SMA compared with that of β-Actin. Each bar depicts the mean value ± SD from four experiments per group
Picrosirius red stain showed that the ratio of collagen I/III in the urethra was decreased in the SUI group relative to sham animals (Fig. 3). Elastic fibers and reticular fibers were long, well organized and tightly connected to the muscle bundles in sham group. However, the SUI group showed fragmentation and disorganization (Fig. 4). Reticular fibers were significantly decreased in SUI group relative to sham group. However, there were no statistical differences in volumetric density of elastic fibers (Fig. 4).
Assessment of collagen I/III ratio in the urethra. a Representative photographs of cross-sections of mid-urethra with picrosirius red stain. b Quantification of collagen I/III was assessed using Image Pro software. Each bar depicts the mean value ± SD from 10 animals per group. *P < 0.05 compared with sham group. (Color figure online)
Assessment of elastic fiber and reticular fiber in the urethra. a Representative photographs of cross-sections of mid-urethra with Hart’s elastin stain. Elastic fiber is black, connect tissue is pink and other tissue elements is yellow. b Representative photographs of cross-sections of mid-urethra with Gordon & Sweet’s stain. Reticular fiber is black and nuclei are red. c Maximum elastic fiber and reticular fiber length analysis results. d Quantification of elastic fiber and reticular fiber were assessed using Image Pro software and expressed as a percentage of the imaged area. Each bar depicts the mean value ± SD from 10 animals per group. *P < 0.05 compared with sham group. (Color figure online)
TGF-β1/Smad pathway and MMP-9 in the urethra of SUI
We first performed TGF-β1 immunohistochemical staining in the urethra. Results revealed that TGF-β1 was not only located in muscle cells, but also appearanced in epithelium cells (Fig. 5). Both immunohistochemical and western blot results indicated that SUI group had significantly greater TGF-β1 expression than sham group in the urethra (Fig. 5).
Assessment of TGF-β1 expression and Smad2 phosphorylation in the urethra. a Representative TGF-β1 staining of cross-sections of mid-urethra for sham group and SUI group. b Representative P-Smad2 staining of cross-sections of mid-urethra for sham group and SUI group. c Western blot analysis showing the expression of MMP-9, TGF-β1, Smad2 and P-Smad2. d Quantitative analysis of western blot data. Each bar depicts the mean value ± SD from 4 experiments per group. *P < 0.05 compared with sham group
To localize Smad2 protein expression in the urethra, we performed immunohistochemical staining with antibody to P-Smad2. The results showed that P-Smad2 was mainly located in the nuclei of muscle cells and epithelial cells (Fig. 5). We also quantified the ratio of P-Smad2 to total Smad2 to determine the activity of Smad2. Similar to the results of the immunohistochemistry, the ratio of P-Smad2 to total Smad2 was significantly higher in SUI group compared with sham group (Fig. 5). Finally, we found that MMP-9 was upregulated in SUI group (Fig. 5).
Discussion
Previous studies reported that a reduction in muscle content may negatively affect structural and functional integrity of the urethra [19–21]. Our result showed that not only the total muscle is less, but also the striated muscle is fragmented and disorganized in SUI rats. These changes possible extremely impaired the urethral closing pressure.
ECM contains collagen, elastic fibres, reticular fibers, proteoglycans, and other glycoproteins [22]. Previous study showed that the fibroelastic elements (collagen and elastin) combine to form key structures in compliant tissues [23]. Collagen I forms the largest strongest fibers and Collagen III forms smaller fibers. Even small variations in the ratios of the fibrillar collagen subtypes can significantly alter the tensile strength of a tissue. For this reason, the relative ratio of the collagen I to collagen III is used as an indicator of tensile strength [24]. Our results show that the ratio of collagen I/III was lower in SUI group. Besides, elastic fibres are found in different organs, for which they provide elasticity and some biomechanical resistance. Lin et al. [11] reported that ADSC-treated groups had significantly higher elastin content than SUI group. However, there was no statistical difference in elastin content between normal voiding and abnormal voiding rats within the control group. In agreement with their result, there have no different on elastic fiber content between two groups, but elastic fibers were fragmented, disorganized and far away from the muscle bundles in SUI group.
Reticular fibers are fine fibers that contain primarily collagen III. They are the supportive elements in bone marrow, lymph nodes, spleen, kidney, liver, thymus, and testis [18, 25]. There is no report that the urethra contains reticular fiber. Our current report was the first to demonstrate that the urethra had abundant reticular fibers that provide supportive frame. Meanwhile, this result showed that reticular fibers were significantly decreased in SUI group. We have also found that the reticular fibers were fragmented and disorganized which result the urethra losing the normal structure and function.
It has been reported that TGF-β1 inhibits smooth muscle cell proliferation via extension of the G2 phase or arrest in the late G1 phase of the cell cycle [26, 27]. In agreement with the result of previous studies [19, 21], quantitative damage to the muscle cells of the urethra was seen in the SUI rats. Therefore, we performed immunohistochemical staining to identify which subpopulation of urethra cells expressed P-Smad2, the crucial step in the initiation of TGF-β1 signal transduction. Our results suggested that P-Smad2 expression both striated muscle and smooth muscle highly express which may play an important role in the parturition-induced changes, loss of muscle cell content in the urethra.
Previous studies showed that MMP-9 was important to ECM breakdown in the vagina and periurethral tissue of SUI patients [10, 28]. In agreement with the result of these studies, MMP-9 was also a higher expression in the urethra of SUI rats than in sham rats, which might be the reason of low ratio of collagen I/III and less reticular fibers and disorganized elastic fibers and reticular fibers.
Previous studies reported that TGF-β1 increases MMP-9 expression through activation of both Smad and MAPK signaling pathways in corneal epithelial cell and meningeal cells [13, 29]. Our results showed that TGF-β1 and P-Smad2 was unregulated not only in muscle cells, but also in epithelium cells. We think that the urethra epithelium cell and muscle cell might also play an important role in the secretion of MMP-9 through TGF-β1/Smad pathway.
Our study does have some limitations. First, although this finding provides direct clues concerning the role of TGF-β1/Smad in SUI. The question remains whether the upregulation of TGF-β1/Smad in smooth muscle and epithelium cells has different activation and function which needed to investigate in vitro. Second, further studies are needed to determine whether the inhibition of Smad2—using transgenic mice or antisense genes for Smad2—or inhibition of TGF-b type I receptor (ALK5) can restore parturition-induced structural and functional derangements in the urethra to clarify the precise roles and specific functions of TGF/Smad pathway.
Conclusions
The fibromuscular system of urethra was impaired in SUI rat. TGF-β1/Smad pathway might play an important role in regulation of pathological changes in SUI.
References
Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF (1999) Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & estrogen/progestin replacement study (HERS) research group. Obstet Gynecol 94(1):66–70
Bakircioglu ME, Sievert KD, Lau A, Lin CS, Lue TF (2000) The effect of pregnancy and delivery on the function and ultrastructure of the rat bladder and urethra. BJU Int 85(3):350–361
Lin AS, Carrier S, Morgan DM, Lue TF (1998) Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 52(1):143–151
van Geelen JM, Lemmens WA, Eskes TK, Martin CB Jr (1982) The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obstet Gynecol 144(6):636–649
Chen CC, Hijaz A, Drazba JA, Damaser MS, Daneshgari F (2009) Collagen remodeling and suburethral inflammation might account for preserved anti-incontinence effects of cut polypropylene sling in rat model. Urology 73(2):415–420. doi:10.1016/j.urology.2008.07.033
Lin G, Ning H, Wang G, Banie L, Lue TF, Lin CS (2010) Effects of birth trauma and estrogen on urethral elastic fibers and elastin expression. Urology 76(4):1018.e8–1018.e13. doi:10.3109/14653240903350265
Rahn DD, Acevedo JF, Word RA (2008) Effect of vaginal distention on elastic fiber synthesis and matrix degradation in the vaginal wall: potential role in the pathogenesis of pelvic organ prolapse. Am J Physiol 295(4):R1351–R1358. doi:10.1152/ajpregu.90447.2008
Lijnen HR (2003) Metalloproteinases in development and progression of vascular disease. Pathophysiol Haemost Thromb 33(5–6):275–281 10.1159/000083814
Peterson JT, Hallak H, Johnson L, Li H, O’Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG (2001) Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 103(18):2303–2309
Zhang QF, Song YF, Zhu ZY (2006) Expression of matrix metalloproteinases-9 and tissue inhibitors of matrix metalloproteinases-1 in connective tissue of vaginal wall of women with stress urinary incontinence. Zhonghua Fu Chan Ke Za Zhi 41(12):810–813
Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin CS (2010) Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy 12(1):88–95. doi:10.3109/14653240903350265
Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB (2001) TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 42(3):853–859
Kim HS, Luo L, Pflugfelder SC, Li DQ (2005) Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci 46(3):840–848. doi:10.1167/iovs.04-0929
ten Dijke P, Hill CS (2004) New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29(5):265–273. doi:10.1016/j.tibs.2004.03.008
Lin G, Shindel AW, Banie L, Deng D, Wang G, Hayashi N, Lin CS, Lue TF (2009) Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol 55(5):1213–1222. doi:10.1016/j.eururo.2008.02.027
Fu Q, Song XF, Liao GL, Deng CL, Cui L (2010) Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology 75(3):718–723. doi:10.1016/j.urology.2009.10.003
Breyer BN, Wang G, Lin G, Shindel AW, Yang R, Lin CS, Lue TF (2010) The effect of long-term hormonal treatment on voiding patterns during filling cystometry and on urethral histology in a postpartum, ovariectomized female rat. BJU Int 106(11):1775–1781. doi:10.1111/j.1464-410X.2010.09268.x
Fakoya FA (2002) Reticulin fibres in the tunica albuginea and peritubular tissue of seminiferous tubules of adult male Wistar rats. Acta Histochem 104(3):279–283
Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF (2001) The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol 166(1):311–317
Dorschner W, Stolzenburg JU, Neuhaus J (2001) Structure and function of the bladder neck. Adv Anat Embryol Cell Biol 159(III–XII):1–109
Lin YH, Liu G, Daneshgari F (2008) A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn 27(4):353–358. doi:10.1002/nau.20509
Da-Silva EA, Sampaio FJ, Dornas MC, Damiao R, Cardoso LE (2002) Extracellular matrix changes in urethral stricture disease. J Urol 168(2):805–807
Goepel C, Thomssen C (2006) Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem 108(6):441–445. doi:10.1016/j.acthis.2006.07.001
Wieslander CK, Rahn DD, McIntire DD, Acevedo JF, Drewes PG, Yanagisawa H, Word RA (2009) Quantification of pelvic organ prolapse in mice: vaginal protease activity precedes increased MOPQ scores in fibulin 5 knockout mice. Biol Reprod 80(3):407–414. doi:10.1095/biolreprod.108.072900
Yu E, Lee I (1993) Reticular network of the human thymus. J Korean Med Sci 8(6):431–436
Reddy KB, Howe PH (1993) Transforming growth factor beta 1-mediated inhibition of smooth muscle cell proliferation is associated with a late G1 cell cycle arrest. J Cell Physiol 156(1):48–55. doi:10.1002/jcp.1041560108
Grainger DJ, Kemp PR, Witchell CM, Weissberg PL, Metcalfe JC (1994) Transforming growth factor beta decreases the rate of proliferation of rat vascular smooth muscle cells by extending the G2 phase of the cell cycle and delays the rise in cyclic AMP before entry into M phase. Biochem J 299(1):227–235
Wieslander CK, Marinis SI, Drewes PG, Keller PW, Acevedo JF, Word RA (2008) Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biol Reprod 78(3):521–528. doi:10.1095/biolreprod.107.063024
Okamoto T, Takahashi S, Nakamura E, Nagaya K, Hayashi T, Fujieda K (2009) Transforming growth factor-beta1 induces matrix metalloproteinase-9 expression in human meningeal cells via ERK and Smad pathways. Biochem Biophys Res Commun 383(4):475–479. doi:10.1016/j.bbrc.2009.04.038
Acknowledgments
This study was supported by the Peking Natural Science Foundation Grant No. Z080507030808011 and the National Natural Science Foundation of China Grant No. 30972993.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, GY., Cui, WS., Zhou, F. et al. Pathology of urethral fibromuscular system related to parturition-induced stress urinary incontinence and TGF-β1/Smad pathway. Mol Cell Biochem 364, 329–335 (2012). https://doi.org/10.1007/s11010-012-1234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1234-x