Abstract
We investigated the effects of exogenous ghrelin on energy levels and tissue histology in skeletal muscle in experimentally lipopolysaccharide (LPS) induced septic rats. Male Wistar albino rats 200–250 g were separated into four groups; Control, LPS (5 mg/kg), Ghrelin (10 nmol/kg i.v.), and ghrelin+LPS. Gastrocnemius muscle tissue was taken and stained using modified Gomori trichrome (MGT), succinic dehydrogenase (SDH), and cytochrome oxidase (COX) and hematoxylin and eosin. In stained sections, histological score value was calculated according to the intensity and the distribution for MGT, SDH and COX stainings. Creatine, creatine phosphate, adenosine triphosphate (ATP), adenosine monophosphate (AMP) levels, and the ratios of AMP/ATP and CreaP/ATP were investigated using high performance liquid chromatography (HPLC) in muscle tissue. Significances between experimental groups were calculated with an analysis of variance (ANOVA) followed by Tukey’s tests. Myopathic changes were seen in the 50% of rats in the LPS group as rounding of muscle fibers and fiber size variation. In the ghrelin+LPS group, ghrelin treatment was reduced damage in skeletal muscle structure. There was no change in creatine or AMP levels between the groups. Ghrelin treatment significantly increased ATP values (P < 0.01) and improved tissue histology in septic rats. Ratios of both AMP/ATP and CreaP/ATP were found increased in the septic group, but there were decreaments in both the ghrelin and ghrelin-treated septic groups. Ghrelin could play an important role in energy balance and muscle morphology in skeletal muscle during sepsis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ghrelin is peptide hormone, a known endogenous ligand of the growth hormone (GH) secretagogue receptor, principally secreted from the stomach, has anti-inflammatory activities (Takiguchi et al. 2015). It is known that ghrelin modifies the neuropeptide Y expressed in the arcuate nucleus of the hypothalamus, and releases GH from the pituitary gland. For this reason, ghrelin has an important role in the regulation of energy balance. In organisms, ghrelin receptors has been demostrated commonly in peripheral tissues (Albarrán-Zeckler et al. 2013).
Sepsis is a serious complication after injury and surgical operations and remains a leading cause of death in surgical intensive care units. Sepsis is a critical state that is recognized by the release of inflammatory cytokines into the blood stream against bacterial endotoxin. Sepsis causes weakness and fatigue in muscle tissue due to increased loss of myofibrillary proteins (Giannesini et al. 2009). In particular, there are rapid changes in mitochondrial energy production in skeletal and cardiac muscle due to impaired oxidative phosphorylation in skeletal muscles.(Vanasco et al. 2008).
In septic conditions, exogenous ghrelin has been shown to decrease the inflammatory response, improve blood flow, and reduce tissue damage and mortality (Chang et al. 2003).
Understanding the mechanisms of sepsis-induced muscle catabolism, therefore, has important clinical implications.
Severe sepsis elicits mitochondrial injury, dysfunction, and depressed biogenesis in skeletal muscles. During the initial phase of sepsis, these changes are manifested as rapid increases in mitochondrial adenosine triphosphate (ATP) production (Singer 2008). As a consequence of the augmented oxidative phosphorylation that occurs during this phase, significant increases in oxygen and nitrogen radical production take place, which in turn trigger extensive mitochondrial injury in skeletal and cardiac muscles (Vanasco et al. 2008).
The initial phase of sepsis is usually followed by a second phase where numbers of mitochondria and activities of various mitochondrial enzymes in skeletal muscles are significantly reduced, which renders cellular ATP production more dependent on glycolysis (Callahan and Supinski 2005; Trumbeckaite et al. 2001).
Ghrelin has also been shown to modulate energy lipid metabolism in nonfat tissues, including skeletal muscle (Barazzoni et al. 2007).
Ghrelin-induced changes in muscle energy and lipid metabolism were independent of changes in food intake, thereby showing the emerging independent in vivo role of ghrelin as a positive modulator of muscle mitochondrial function in wasting disease conditions as well (Barazzoni et al. 2005).
There are a few studies related with the effect of ghrelin on energy levels during sepsis. In this study, the impact of ghrelin on energy levels and tissue histology were evaulated in skeletal muscle in septic rats.
Materials and Methods
Experimental Groups
This study was conducted at the Istanbul University Experimental Research Center. The experimental protocol and procedures were approved by the Istanbul University Animal Care and Use Committee (Resolution No: 2013/123).
In this study, male adult Wistar albino rats (200–250 g) were divided into four groups, each composed of eight rats: (1) control group, (2) LPS group, (3) ghrelin group, and (4) ghrelin+LPS group. The animals were fed with a commercial diet and tap water ad libitum, and housed in cages kept at a controlled temperature (22 ± 2 °C), humidity (55–60%), with a 12-h light/dark cycle.
Experimental Procedures
The control group was injected with saline at Time 0. For LPS group, Time 0, Rats were injected with LPS (5 mg/kg in 1 mL of 0.9% NaCl) by i.v., Time 12 h LPS (5 mg/kg in 1 mL of 0.9% NaCl) injected by i.p. For ghrelin group, Time 0, ghrelin (10 nmol/ kg) was injected by i.v, For ghrelin+LPS group, Time 0, LPS (5 mg/kg in 1 mL of 0.9% NaCl) by i.v and ghrelin (10 nmol/ kg) was injected by i.v. Time 12 h, LPS (5 mg/kg in 1 mL of 0.9% NaCl) injected by i.p.
All experimentally groups rats were decapitated time 24 h.
The rats were anesthetized with sodium pentothal (i.p. 30 mg/kg). After rats were decapitated and the gastrocnemius muscle was extracted.
Neuropathologic Procedures
The gastrocnemius muscle was taken while the animals were under anesthesia. The muscle samples were rapidly frozen in isopentane cooled by liquid nitrogen. We took serial cryostat sections 8 μm thick and stained them using the modified Gomori’s trichrome (MGT) method, succinic dehydrogenase (SDH), and cytochrome oxidase (COX) using standard protocols. The stained sections were visualized and photographed using a Nikon microscope (ECLIPSE 80i Nikon Corporation, JAPAN) (Engel and Cunningham 1963; Tanji and Bonilla 2008). Both the intensity and the distribution of specific MGT, SDH and COX staining were scored. For each section, a histological score (HSCORE) value was calculated the percentages of cells that stained at each intensity, multiplied by the weighted intensity of the staining [HSCORE = S Pi (i + 1), where i is the intensity score (0–4) and Pi is the corresponding percentage of the cells]; staining was scored as 0 = no staining, 1 = weak staining, 2 = middle staining, 3 = dark staining, 4 = very dark staining.
Histologic Procedures
The gastrocnemius muscle was fixed in 10% buffered formalin and embedded in paraffin wax. Five-micrometer-thick sections were placed on polylysine-coated slides and stained using hematoxylin and eosin (H&E). The stained sections were visualized and photographed under a light microscope at 100× magnification (ECLIPSE 80i Nikon Corporation, JAPAN).
Measurement of Creatine, Creatine Phosphate, and Adenosine Triphosphate, Adenosine Monophosphate
The gastrocnemius muscle samples were rapidly frozen in isopentane cooled by liquid nitrogen and stored −80 °C until analysis.
Muscle tissue samples weighing 200 mg were homogenized in 2 mL 0.42 M HCLO4 using a homogenizer (Ultraturrax T25) for 30 s. A 1.0 mL supernatant was taken for adjusting pH with 1.0 M K2HPO4 after centrifugation at 3000 rpm for 5 min.
Creatine, creatine phosphate, ATP, Adenosine monophosphate were evaluated using C18 colon (5 µ, 250 mm × 4.6 mm, Nucleodur, USA) with isocratic elution using a KOH/KH2PO4 buffer (215 mM, pH 6.25), 3 mM tetrabutylammonium phosphate, and 5% acetonitrile ion-paired reverse-phase chromatography using high-pressure liquid chromatography (HPLC) (Agilent 1100, USA) at 214 nm. Creatine, creatine phosphate, and adenine nucleotides were calculated from their external standard curves from different concentrations (Shellvold et al. 1986).
Statistical Analyses
Data were expressed as mean ± standard deviation (SD). Overall statistical significance between the groups was tested with one-way ANOVA or the Kruskal–Wallis test, depending on normality of distribution. In all cases, P < 0.05 was set as the limit of significance.
Results
Neuropathologic Results
Fifty percent of rats that were treated with LPS showed myopathic changes, with rounding of muscle fibers and fiber size variation. Stainings of SDH and COX were observed to increased in LPS group (P < 0.01). In the ghrelin and ghrelin+LPS group there were no myopathic changes, and the result of H-Score for MGT, SDH and COX results were decreased in ghrelin+LPS group compared to LPS group (P < 0.05) (Fig. 1A–L; Table 1).
Muscle tissue sections were stained experimental groups with modified Gomori trichrome, cytochrome oxidase, succinatedehydrogenase(A–L). Sections of muscle tissue from experimental groups stained with H&E, ×100 magnification. In image N; white arrows indicate weak fibre boundaries and irregular—shaped nuclei in the LPS group (M–P)
Histologic Results
Using H&E staining, weak fiber boundaries and loss of normal shape were seen in five animals of eight rats in the muscle sections of LPS group. In the ghrelin-treated septic groups, the histologic appearance of the muscle fibers was normal with no sign of injury, as with the controls (Fig. 1M–P).
Energy Results
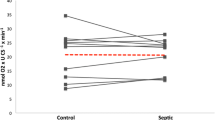
There were no changes in values of creatine and adenosine monophosphate (AMP) for the experimental groups (P > 0.05). Creatine phosphate levels were decreased in the ghrelin group compared with the other groups (P < 0.05). ATP values were found lower in the LPS group than in the other groups (P < 0.01), AMP/ATP and CreaP/ATP ratios were found increased in LPS group comparasion to other group. (p < 0.01) (Fig. 2A–F).
Discussion
Muscle dysfunction is a known critical feature of sepsis and also major cause of multiorgan failure in patients in intensive care units. In the literature, oxidative damage and imbalances of bioenergetic metabolism shown as important in sepsis. (Rocheteau et al. 2015).
Although skeletal muscle has marked regeneration in response to septic conditions, it is not clear how the function of myofibrillary structure is affected in skeletal muscle. Reduced oxidative phosphorylation and an increased rate of reactive oxygen species have been indicated in patients with sepsis and experimental septic models (Tiao et al. 1997). Degraded myofibrillar proteins are taken up by the ubiquitin–proteasome pathway in septic environments (Hobler et al. 1998; Tiao et al. 1994).
There are a few studies related with the effects of ghrelin on reducing muscle damage. We observed that treated with LPS showed myopathic changes as rounding of the muscle fibers and fiber size variation according to the results of histological damage score. In the sections of H&E stainings, it was observed loss of normal shape in the majority of animals in the LPS group. In the ghrelin-treated septic groups, the histologic appearance of the muscle fibers was normal with no sign of injury, as with the controls.
In literature, Ghrelin treatment reduced myopathic changes in muscle morphology and structure in patients and experimentally-induced sepsis in animals (Hauptmann et al. 1994; Chang et al. 2003).
In the present study, we studied the impact of ghrelin on oxidative phosphorylation parameters and COX, SDH, mitochondrial myopathy of muscle fibers in septic rats.
Sepsis results in impairments of glucose levels and mitochondrial oxidative phosphorylation, and subsequent synthesis of high-energy compounds such as creatine phosphate and ATP (Langhans et al. 2014).
Our previous study, glucose levels were found highly decreased in the LPS group, but glucose levels were found increased in the ghrelin-treated LPS group, the same as the controls (Ates et al. 2017). In parallel with our study, there are studies about improving of plasma glucose levels by altering mitochondrial oxidative phosphorylation in skeletal muscle in ghrelin-treated septic groups. According to this, grehlin behaved as a positive modulator during in vivo treatment (Barazzoni et al. 2007). Ghrelin has pleiotrophic effects on the regulation of energy metabolism through control of hypothalamic AMP-activated protein kinase (AMPK) (Kola 2008). In addition, it was shown that ghrelin inhibited muscle protein degradation in rats with induced thermal injury (Clark et al. 1984).
In our study, creatine phosphate levels were significantly decreased in the ghrelin group. There are scarce studies about the effect of ghrelin on creatine phosphate level. Ghrelin treatment may increase aerobic energy production.
In this study, there were no changes in creatine and AMP levels among the experimental groups. ATP values were significantly lower in the LPS group, whereas ghrelin treatment increased ATP levels. There were deficiencies in the electron transport chain via reduced ATP production in sepsis (Forget et al. 2000; Fink 2002; Chen et al. 2003; Brealey and Singer 2003). Chang et al. showed that ghrelin improved myocardial ATP levels in septic rats, and found that the mortality rate was decreased in sepsis (Chang et al. 2003).
Fukushima et al. demonstrated a burn-induced sepsis model with which there was an elevated survival rate with ghrelin treatment in BALB/C mice (Fukushima et al. 1999).
In cachexia or heart failure rats, plasma ghrelin’s effect maintained energy balance by inhibiting catabolism and increasing anabolism in pathophysiologic states. (Nagaya et al. 2001.)
In our study, both AMP/ATP and CreaP/ATP ratios were found increased in the septic group, but there were decrements in both the ghrelin and ghrelin-treated septic groups. Zheng et al. reported that the cause of the increment ratio of AMP/ATP was based on activated AMP protein kinase (AMPK) in patients with sepsis (Zheng et al. 2014). Our findings related with decreased ratios of AMP/ATP in the ghrelin and ghrelin-treated septic groups showed reductions in muscle protein degradation. In the early phase of sepsis, creatine phosphate degradation is increased for maintaining ATP levels to provide for cellular demand (Lara et al. 1998).
In conclusion, it was observed that improvements effects of ghrelin on muscle morphology and, high energy compounds in septic skeletal muscle.
References
Albarrán-Zeckler RG, Smith RG (2013) The ghrelin receptors (GHS-R1a and GHS-R1b). Endocr Dev 25:5–15
Ates G, Ozkok E, Yorulmaz H, Aydin I, Tamer S (2017) Effects of ghrelin on inflammation and oxidative stress parameters in sepsis-induced liver tissue of rat. Asian J Anim Vet Adv 12:17–23.
Barazzoni R, Bosutti A., Stebel M et al (2005) Ghrelin regulates mitochondriallipid metabolism gene expression and fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:228–235.
Barazzoni R, Zanetti M, Cattin MR et al (2007) Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rat. Obesity 15:2614–2623
Brealey D, Singer M (2003) Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep 5:365–371
Callahan LA, Supinski GS (2005) Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol 99:1120–1126
Chang L, Du JB, Gao LR, Pang YZ, Tang CS (2003) Effect of ghrelin on septic shock in rats. Acta Pharmacol Sin 24(1):45–49
Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC (2003) Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock 20:274–279
Clark AS, Kelly RA, Mitch WE (1984) Systemic response to thermal injury in rats. Accelerated protein degradation and altered glucose utilization in muscle. J Clin Invest 74:888–897
Engel WK, Cunningham GG (1963) Rapid examinaiıon of muscle tissue an improved trichrome method for fresh-frozen biopsy sections. Neurology 13:919–923
Fink MP (2002) Bench-to-bedside review: cytopathic hypoxia. Crit Care 6:491–499
Forget AP, Mangalaboyi J, Mordon S et al (2000) Escherichia coli endotoxin reduces cytochrome aa3 redox status in pig skeletal muscle. Crit Care Med 28:3491–3497
Fukushima R, Saito H, Inoue T et al (1999) Prophylactic treatment with growth hormone and insulin-like growth factor I improve systemic bacterial clearance and survival in a murine model of burn-induced gut derived sepsis. Burns 25:425–430
Giannesini B, Izquierdo M, Le Fur Y et al (2009) Beneficial effects of citrulline malate on skeletal muscle function in endotoxemic rat. Eur J Pharmacol 602(1):143–147
Hauptmann S, Klosterhalfen B, Weis J, Mittermeyer C, Kirkpatrick CJ (1994) Skeletal muscle oedema and muscle fibre necrosis during septic shock. Observations with a porcine septic shock model. Virchows Arch 424:653–659
Hobler SC, Tiao G, Fischer JE, Monaco J, Hasselgren PO (1998) The sepsis-induced increase in muscle proteolysis is blocked by specific proteasome inhibitors. Am J Physiol 274:30–37
Kola B (2008) Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol 20:942–951
Langhans C, Weber-Carstens S, Schmidt F et al (2014) Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PLoS ONE 9:92048
Lara TM, Wong MS, Rounds J, Robınson MK, Wilmore DW, Jacobs DO (1998) Skeletal muscle phosphocreatine depletion depresses myocellular energy status during sepsis. Arch Surg 133:1316–1321
Nagaya N, Uematsu M, Kojima M et al (2001) Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation 104:2034–2038
Rocheteau P, Chatre L, Briand D et al (2015) Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 6:10145
Shellvold OFM, Jnge P, Aarstad K (1986) High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds anda denine nucleotides in the myocardial tissues. J Mol Cell Cardiol 18:517–527
Singer M (2008) Cellular dysfunction in sepsis. Clin Chest Med 29:655–660
Takiguchi S, Murakami K, Yanagimoto Y et al (2015) Clinical application of ghrelin in the field of surgery. Surg Today 45(7):801–807
Tanji K, Bonilla E (2008) Light microscopic methods to visualize mitochondria on tissue sections. Methods 46:274–280
Tiao G, Fagan JM, Samuels N et al (1994) Sepsis stimulates nonlysosomal energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94:2255–2264
Tiao G, Hobler S, Wang JJ et al (1997) Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest 99:163–168
Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN (2001) Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem 268:1422–1429
Vanasco V, Cimolai MC, Evelson P, Alvarez S (2008) The oxidative stress and the mitochondrial dysfunction caused by endotoxemia are prevented by alphalipoic acid. Free Radic Res 42:815–823
Zheng X, Xu M, Fang Q. (2014) Role of AMPK α in skeletal muscle glycometabolism regulation and adaptation in relation to sepsis. Biomed Res Int 2014:390760
Acknowledgements
Our study was granted from Istanbul University Research Projects (Projects No: 42363). We would like also thank Mr. David F Chapman for editing the English in this article. Also we would like to the selection of muscle images to Dr Gokcen Unverengil and the stainings of muscle sections to Ms. Hatice Tasli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yorulmaz, H., Ozkok, E., Ates, G. et al. Ghrelin: Impact on Muscle Energy Metabolism in Sepsis. Int J Pept Res Ther 24, 259–264 (2018). https://doi.org/10.1007/s10989-017-9610-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9610-8