Abstract
Context
Whilst the composition and arrangement of habitats within landscape mosaics are known to be important determinants of biodiversity patterns, the influence of seascape patterning and connectivity on temperate reef fish assemblages remains largely unknown.
Objectives
We examined how habitat patterns at multiple spatial scales (100–1000 m) explained the abundance and diversity of temperate reef fish in a reef-seagrass dominated seascape.
Methods
Fish assemblages were surveyed using remote underwater videos deployed on 22 reefs in Jervis Bay, NSW, Australia. Using full-subset GAMMs, we investigated if habitat area, edge, structural connectivity and a metric for habitat diversity (Shannon’s diversity index) of reef and seagrass can predict variations in a temperate reef fish assemblage.
Results
A key finding of the study was that temperate reefs close (< 55 m) to large (> 6.25 ha) seagrass meadows contained greater abundance and diversity of fish. A consistent negative correlation was also found between reef area (> 0.01 ha) and the fish assemblage. The influence of seascape metrics on the abundance of fishes varied with functional traits (trophic groups, mobility and habitat associations). Fish-seascape relationships occurred at a range of spatial scales with no single scale being solely important for structuring the fish assemblage.
Conclusions
We demonstrate that it is important not to view reef habitats in isolation, rather consider a reefs context to adjacent seagrass when predicting the distribution of temperate reef fish. This finding improves current understanding of the multi-scale factors structuring temperate reef fish assemblages and highlights the importance of reef-seagrass connectivty for the management of temperate marine ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the factors governing the distributions of organisms is fundamental in ecology (Andrewartha and Birch 1954). In terrestrial systems, landscape ecology has established that the composition (number and area) and spatial configuration of habitats within landscape mosaics (i.e. landscape structure) can profoundly affect patterns of biodiversity and ecological processes (Forman and Godron 1986; Turner 2005; Wu 2006). Although these concepts have been well documented for a range of terrestrial taxa (Turner 2005), the influence of landscape structure on marine species is less understood. The techniques and concepts developed in landscape analyses are, however, being increasingly applied in the marine environment (Grober-Dunsmore et al. 2009; Boström et al. 2011). This has led to the establishment of the burgeoning field of seascape ecology that focuses on understanding the ecological consequences of spatial patterning in seascapes across multiple spatiotemporal scales (Pittman 2017).
There is a growing appreciation that seascape structure at a range of scales influences the distribution of fishes. The characteristics of focal habitats, such as habitat area and edge environment have been highlighted as predictors for the observed variation in the structure of fish assemblages (Jackson et al. 2006; Smith et al. 2008; Boström et al. 2011). For instance, positive relationships have been reported between habitat area and fish diversity and abundance on both coral and rocky reefs (Sale and Douglas 1984; Acosta and Robertson 2002; Chittaro 2002; Parsons et al. 2016), however this relationship appears to be more ambiguous in seagrass habitats (Boström et al. 2011). Recently, the structural connectivity (i.e. the area and isolation; Calabrese and Fagan 2004) of habitats has been identified as a key predictor for fish communities in tropical seascapes (Grober-Dunsmore et al. 2008; Olds et al. 2012). For example, coral reefs in tropical seascapes with large areas of adjacent seagrass habitats at scales between 100 and 1000 m have been reported to contain a higher abundance and diversity of reef fish (Kendall et al. 2005; Grober-Dunsmore et al. 2007; Olds et al. 2012; Berkström et al. 2013). There is also evidence that the structural connectivity of reefs to seagrass may be of more importance for structuring reef fish assemblages than the area of reef habitat (Grober-Dunsmore et al. 2007).
In coastal marine ecosystems, reefs are often embedded within heterogeneous seascapes dominated by seagrass, mangrove and soft sediment habitats. Many reef fish species connect multiple habitat types as they move across the seascape to access resources throughout their life-histories (Pittman and McApline 2003; Green et al. 2015; Sambrook et al. 2019). These movements occur over a range of spatial scales, from 10 to 100’s of metres for foraging, diel or tidal migrations (Beets et al. 2003; Unsworth et al. 2007; Hitt et al. 2011). Movements may also spand over large scales (1–100’s of kilometres) for broader-scale dispersal such as ontogenetic shifts or spawning (Gillanders et al. 2003; Nagelkerken et al. 2015). Seagrass meadows are particularly important habitats for reef fishes, with many species using them as nursery areas or foraging locations (Gillanders 2006; Heck et al. 2008; Nagelkerken 2009). Consequently, the spatial context of reefs to adjacent seagrass meadows can have a major influence on resource availability and the structure of fish assemblages (Grober-Dunsmore et al. 2009).
Research into the effect of seascape patterns on reef fish has largely been focused in tropical regions containing coral reefs, mangroves and seagrass habitats (Dorenbosch et al. 2005; Grober-Dunsmore et al. 2007; Olds et al. 2012). Despite rocky reefs and seagrass being large components of temperate seascapes, few studies have explored how reef-seagrass connectivity influences temperate reef fish distributions and ecological processes (but see Ricart et al. 2018; Rees et al. 2018; Perry et al. 2018). The temperate seascape literature has instead investigated fish-habitat relationships in predominantly reef and soft sediment seascapes (Moore et al. 2011; Schultz et al. 2012; Rees et al. 2014; Ortodossi et al. 2018) or fish assemblages in vegetated habitats (Jackson et al. 2006; Green et al. 2012; Staveley et al. 2016). Furthermore, few studies in temperate seascapes have examined how the functional roles (e.g. feeding groups) and movement capabilities of fish species affect the response of reef fish to variations in seascape patterning. This information can elucidate relationships between ecological processes and seascape patterns, such as the link between trophic dynamics and the seascape (Berkström et al. 2012; Martin et al. 2018).
An initial investigation by Rees et al. (2018) suggested the area of seagrass meadows adjacent to reefs may be a key predictor for the distribution of demersal temperate reef fish. Research from tropical seascapes have reported that metrics other than seagrass area, such as the isolation of reefs from seagrass, reef area and edge can be useful for predicting variation in fish assemblages (Boström et al. 2011; Wedding et al. 2011). Here we sought to better understand how the distribution of temperate reef fish is associated to seascape structure and structural connectivity within a seascape dominated by seagrass (Posidonia australis) and rocky reefs; which are both habitats considered a high priority for management. This study builds on the work of Rees et al. (2018) but incorporates higher levels of replication and a greater number of seascape metrics. We predicted that greater abundance of reef fish, individuals in functional groups and diversity would be found on reefs with low isolation to large seagrass meadows in comparison to reefs further away. Our expectations were based on the likelihood of increased resource availability and immigration rates of recruits from nursery seagrass habitats to nearby reefs. We also predicted large reefs will contain a greater abundance and diversity of fish due to species-area relationships.
Methods
Study site
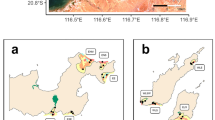
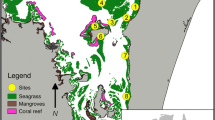
This study surveyed fish on 22 shallow-water temperate reefs in Jervis Bay, a large oceanic embayment in south eastern Australia (Fig. 1) that forms the central section of the Jervis Bay Marine Park. The Jervis Bay seascape is dominated by rocky intertidal and subtidal reefs, seagrass meadows (Posidonia australis) and soft sediments. These habitats are replicated across the seascape and vary spatially in their size and arrangement (Fig. 1). Therefore, Jervis Bay offers an excellent opportunity to examine how seascape patterns drive the abundance and diversity of temperate reef fishes.
Location of sites within Jervis Bay and types of benthic habitats; rocky reef (brown), seagrass (green), unconsolidated soft sediment (dark grey) and land (light grey). Black circles represent the 250-m scale around each of the twenty-two reef sites where four RUVs were deployed (n = 88). Circular insets illustrate the variability of the seascape at the 250-m scale between three sites; from top, small reef with extensive seagrass, reef with a small seagrass meadow and reef with no seagrass present (Note White areas in each inset represent land)
Fish surveys
Reef fish assemblages were surveyed using single camera Remote Underwater Video stations (RUVs) from March to May in 2016 within Jervis Bay (Fig. 1). RUVs consisted of a GoPro Hero 3 camera mounted to a brick fixed onto a metal wire base (Kiggins et al. 2018). Four RUVs were deployed 60 m apart on each of the 22 reef locations (n = 88). The reef locations were separated by a minimum linear distance of 500 m which allowed us to systematically sample nearly every reef in Jervis Bay (Fig. 1). The RUVs were deployed at a depth of 2 m on subtidal reefs for a minimum of 35-min to ensure a 30-min sample of footage and a 5 min settlement period post deployment. This set time has been reported to be appropriate for both baited and unbaited underwater videos sampling fishes on temperate reefs (Bernard and Götz 2012; Harasti et al. 2015). To minimize potential spatial and temporal confounding, reefs were sampled in a haphazard order around the Bay. Sampling was conducted in daylight hours (08:00–16:00) to mitigate potential effects of crepuscular feeding behaviours (Wraith et al. 2013) and restricted to three hours either side of high tide.
Fish diversity (species richness) and abundance (MaxN) were recorded from RUV deployments. MaxN was defined as the maximum number of individuals for a given species in a single frame during the 30-min footage (Harvey et al. 2007). Total MaxN for each deployment (n = 88) was calculated by summing the MaxN values for all species. Each fish species was then placed into functional groups based on their mobility, trophic status and habitat associations. The mobility groups included three categories; (i) resident species known to be site attached to focal reefs or sedentary (10–100’s of metres), (ii) mobile species which can migrate to adjacent habitat patches but show site fidelity to a focal reef or move over scales of 100’s of metres to kilometres, and (iii) transient species that are highly vagile and move over the scale of 1–100’s km (Grober-Dunsmore et al. 2007). If limited information was available for a species movement patterns, they were assigned a movement group based on their maximum length, as movement distances generally increase with increasing body size (Green et al. 2015). Trophic groupings were based on the predominance of prey items and included; algal invertebrate consumers, generalist carnivores, invertebrate carnivores, herbivores, macroinvertebrate carnivores and zooplanktivores (Wraith et al. 2013). Fish were also grouped on their associations to seagrass forming two categories; (1) seagrass associated taxa which have been observed on seagrass meadows as juvenile or adults, and (2) non-seagrass associated taxa not known to reside on seagrass. We used information from Fish Base (Froese and Pauly 2010), the Australian Museum Fish Database (McGrouther 2001), local identification guides (Hutchins and Swainston 1986; Kuiter 1993), Bell and Westoby (1986), Ferrel et al. (1993), Hannan and Williams (1998), Jelbart et al. (2007), Curley et al. (2013), Wraith et al. (2013) and personal observations to classify species into diet or habitat association groups. In circumstances where no data were available for a species diet or habitat associations, species were placed into the group most common for their genus. Definitions for each functional guild can be found in Online Appendix S1.
Habitat sampling
The seascape surrounding the 88 deployments were quantified from existing benthic habitat maps of Jervis Bay (source: NSW State Government, Office of Environment and Heritage). These data were derived from swath mapping with a spatial resolution of up to two meters, Laser Airborne depth sounders (LADS) and high-resolution aerial photographs (< 1 m pixels) and ground-truthing (Williams et al. 2007; Creese et al. 2009). Seagrass was classified by its presence/absence, meaning if there was any seagrass within the seagrass polygons delineated from aerial imagery it was classified as seagrass (Williams et al. 2007; Creese et al. 2009). Habitat patterns were depicted using the patch-matrix model (Boström et al. 2011) with reef and seagrass habitats embedded in an unconsolidated sediment matrix.
Seascape patterns surrounding each deployment were quantified using spatial pattern metrics at four spatial scales; 100 m, 250 m, 500 m and 1000 m (Rees et al. 2018). Each scale was measured as radii centred over each of the 88 deployments. These scales were selected to provide sufficient data on the response of fishes to seascape patterning and incorporate the known home ranges of common reef fish in JBMP (Curley et al. 2013). Each radii or scale represented a “seascape” and each seascape (n = 88) was analysed using spatial pattern metrics quantified in ArcGIS version 10 spatial analyst extension and FRAGSTATS 4.2 spatial analysis software. The metrics selected were based on previous research showing their potential to explain variations in demersal fish assemblages and included; (1) the total area of reef and seagrass (Kendall 2005; Grober-Dunsmore et al. 2008; Rees et al. 2018), (2) the length of reef and seagrass edge (Kendall et al. 2011; Moore et al. 2011), (3) the Shannon’s diversity index, a measure of habitat diversity where 0 means only one patch is present in the seascape (no diversity) and increases with a greater number of habitat types and as the proportion of different habitat types within the seascape becomes equal (Wedding et al. 2011; Staveley et al. 2016), and (4) the edge to edge distance of focal reefs to the nearest seagrass meadow (Olds et al. 2012). This metric was calculated irrespective of spatial scale, with the distance to seagrass measured even if seagrass was not present within the 1000 m spatial scale.
Statistical analyses
Correlations between the fish assemblage and seascape metrics were examined using generalized additive mixed models (GAMMs) (Hastie and Tibshirani 1987; Hastie 2017). GAMMs account for non-linear and non-monotonic relationships between the response and explanatory variables by the addition of a smoothed function (Guisan et al. 2002). Preliminary data exploration was performed to assess for potential outliers, homogeneity and collinearity of the explanatory variables using the methods outlined in (Zuur et al. 2009). Pearson’s correlations and Variance Inflation Factor (VIF) indicated the presence of strong collinearity between explanatory variables. Spatial autocorrelation in the data was assessed using spline correlograms (Bjornstad and Falck 2001) and Morans I. Spatial autocorrelation was present in the raw data and was accounted for by adding ‘site’ as a random effect in the GAMM models (Online Appendix S3–S8).
To overcome issues with collinearity among explanatory variables a full subsets approach was used to construct the GAMMs (Fisher et al. 2018). The approach constructs all possible combinations of models and excludes models with collinear variables specified by a Pearson’s correlation > 0.28 (Graham 2003). This technique has great utility for ecological applications exploring the influence of environmental factors on the distribution of biota (Bond et al. 2018; Wellington et al. 2018).
The GAMMs for reef fish diversity and the abundance of macroinvertebrate consumers were fitted using a Poisson distribution. A Tweedie error distribution was fitted for all other models due to the large number of zeroes (Tweedie 1984). The maximum number of explanatory variables for each model was limited to three and all continuous variables were fitted with smoothing splines with the number of knots k = 4. These parameters were set to prevent overfitting and create conservative, ecologically interpretable models (Wood 2006; Fisher et al. 2018). Models were compared using Akaike information criterion corrected for small sample sizes (AICc), with the best fitting model having the lowest AICc (Burnham and Anderson 2004). When multiple candidate models occurred within ± 2 AICc of the best model, the most parsimonious model(s), that is the model(s) containing fewest predictors, was selected. The relative importance of predictor variables for the model set was determined by summing the weight of all models containing each variable (Burnham and Anderson 2004). All statistical analyses and plots were developed using the statistical computing program R (R Core Development Team 2018) and the functions; FSSGAM 1.11 (Fisher et al. 2018), mgcv (Wood and Wood 2015), visreg (Breheny and Burchett 2013) and gamm4 (Wood and Scheipl 2014).
Results
In total, we observed 81 species comprising 40 families of fish on shallow water rocky reefs in Jervis Bay (Online Appendix S2). Reef area was the most common predictor explaining variations in the temperate reef fish assemblage (Fig. 2). Other important seascape variables included the distance to seagrass and the Shannon’s diversity index, whilst the length of seagrass edge was the poorest performing predictor (Table 1; Fig. 2). Fish responded to seascape predictors over a range of spatial scales and no specific scale appeared to be universally important (Fig. 2).
A heat-map highlighting the relative importance (calculated as the sum of the AIC weights/number of models) for each explanatory variable (x-axis) against each of the fish assemblage variables (y-axis). The X label indicates the explanatory variable selected in the most parsimonious model(s) for the given response variable. See Table 1 for the acronyms used for each explanatory variable. Numbers next to acronyms represent the spatial scale. Sqrt and log denote the variable has either been square root or log transformed
At the assemblage level, the abundance of reef fish was found to be greater on reefs close to large seagrass meadows, with declines in abundance from approximately 100 on reefs connected (i.e. 0 m) to seagrass meadows to 50 on reefs 55 m from seagrass (Table 2; Fig. 3a). Further, reef fish abundance increased from 20 to 75 as the area of seagrass adjacent reefs rose from 0 to 64 hectares (Table 2; Fig. 3a). Reef fish abundance and diversity decreased from 100 to 20 individuals and 13 to 5 species, respectively, with increasing reef area (Table 2; Fig. 3a, b). Increases in reef fish diversity from 7 to 14 species were found as seagrass area increased from 0 to 6.25 hectares before a sharp decrease (Table 2; Fig. 3b). It is notable, that the two key predictors for reef fish diversity exhibited the strongest correlations of any response variables (R2 = 0.59–0.6) (Table 2).
Relationships for the most parsimonious models found to predict a reef fish abundance (MaxN), b species diversity, c abundance of resident taxa and d abundance of mobile taxa. Solid lines illustrate the predictions of the model and shaded areas define the 95% confidence intervals around the fitted values. The summary of each model is provided in Table 2. Sqrt and log denote the variable has either been square root or log transformed
For the mobility groups, the abundance of resident taxa exhibited the strongest relationship with seascape patterns; revealing a negative relationship with reef edge until a threshold of 1500 m, where abundance gradually increased (R2 = 0.53; Table 2; Fig. 3c). Mobile taxa were found to be more abundant on reefs close to seagrass, with declines from approximately 60 to 20 individuals when a reefs distance from seagrass increased from 0 to 55 m (Table 2; Fig. 3d). Transient taxa exhibited no relationship to seascape patterns (Table 2).
The abundance of trophic groups showed differing responses to seascape metrics (Table 1; Fig. 2). The abundance of zooplanktivores and invertebrate consumers showed positive relationships with the Shannon’s diversity index, increasing from approximately 0 to 50 and 2 to 8 respectively (Table 2; Fig. 4a, c). The Shannon’s diversity index was also important for the abundance of generalist carnivores exhibiting a negative relationship, declining from 4 to 1 individual as the Shannon’s diversity index reached 1 (Table 2; Fig. 4c). Both algal invertebrate and macroinvertebrate carnivore abundance were found to have non-linear relationships to the Shannon’s diversity index (Table 2; Figs. 4d, 5). Algal invertebrate consumer abundance increased to 18 with a Shannon’s diversity index of 0.7 and then decreased (Fig. 5). Macroinvertebrate carnivore abundance decreased to 1.5 at a Shannon’s diversity index of 0.6 before increasing to 2.5 individuals at an index of 0.9 and then decreasing again (Fig. 4d). The abundance of invertebrate carnivores and generalist carnivores declined from approximately 8 to 2 and 4 to 2, respectively, as reef area increased from 0.01 to 0.1225 hectares (Table 2; Fig. 4b, c). Zooplanktivore abundance had a non-linear relationship to reef area, showing an increase from approximately 10 to 30 individuals as reef size increased from 0.01 to 0.06 hectares before declining on larger reefs (Table 2; Fig. 4a). Further, algal invertebrate consumer abundance declined with reef area, with abundance decreasing from approximately 35 to 5 individuals as reef area rose from 0.04 to 1.44 hectares (Table 2; Fig. 5). The abundance of algal invertebrate consumers also declined from 50 to 20 as the distance to seagrass from reefs increased to 10 m and from 40 to 10 individuals as the length of reef edge increased from 500 to 2000 m (Table 2; Fig. 5). Lastly, macroinvertebrate consumers abundance on reefs increased from 1.5 to 3 individuals as the length of seagrass edge habitat increased from 0 to 2000 m (Table 2; Fig. 4d). There were no relationships observed between the abundance of herbivorous fishes and seascape variables (Table 2).
Relationships for the most parsimonious models found to predict the abundance of a zooplanktivores, b invertebrate carnivores, c generalist carnivores and d macroinvertebrate carnivores. Solid lines illustrate the predictions of the model and shaded areas define the 95% confidence intervals around the fitted. The summary of each model is provided in Table 2. Sqrt and log denote the variable has been either square root or log transformed
Relationships for the most parsimonious models found to predict the abundance of algal invertebrate consumers. Solid lines illustrate the predictions of the model and shaded areas define the 95% confidence intervals around the fitted values. The summary of each model is provided in Table 2. Sqrt and log denote the variable has either been square root or log transformed
The abundance of both seagrass associated and non-seagrass associated taxa was found to correlate with seascape variables, although the explanatory power of models was greater for seagrass associated (R2 = 0.57–0.48) than non-seagrass associated taxa (R2 = 0.23–0.22) (Table 2). Reductions in the abundance of non-seagrass associated and seagrass associated taxa were found as reefs became larger, both decreasing from 40 to 10 individuals as reef size increased from 0.005 to 0.03 and 0.01 to 1.44 hectares respectively (Table 2; Fig. 6a, b). The abundance of non-seagrass associated taxa increased from 10 to 30 as the area of seagrass adjacent to reefs increased from 0 to 64 hectares (Table 2; Fig. 6a). Non-seagrass associated taxa also declined from 30 individuals on reefs with 500 m of reef edge to 10 on reefs with greater than 2000 m of edge environment (Table 2; Fig. 6a). Finally, the abundance of seagrass associated taxa decreased from 70 on reefs with seagrass directly adjacent to 20 on reefs 55 m from seagrass (Table 2; Fig. 6b).
Relationships for the most parsimonious models found to predict the abundance of a non-seagrass associated taxa and b seagrass associated taxa. Solid lines illustrate the predictions of the model and shaded areas define the 95% confidence intervals around the fitted values. The summary of each model is provided in Table 2. Sqrt and log denote the variable has either been square root or log transformed
Discussion
The spatial context of reefs to adjacent seagrass meadows has been reported to drive the distribution of tropical reef fish (Grober-Dunsmore et al. 2007; Olds et al. 2012; Berkström et al. 2013), but these relationships are largely unknown in temperate seascapes. A key finding from this study was that temperate reef fish assemblages had a greater (i) total abundance, (ii) abundance of mobile taxa, (iii) abundance of seagrass associated taxa, and (iv) diversity on reefs close to seagrass (< 55 m) or with large (> 6.25 ha) adjacent seagrass meadows. Additionally, reef area was found to have a consistent negative relationship with reef fish abundance, diversity and the abundance of several functional guilds. The importance of adjacent seagrass meadows structuring temperate reef fish assemblages found in the current study provides robust support for the findings of Rees et al. (2018) and the notion that temperate systems act in parallel fashion to seascape patterns as tropical systems (Kendall 2005; Grober-Dunsmore et al. 2007; Olds et al. 2012). Therefore, we suggest that generalities exist for the response of reef fish to the seascape between tropical and temperate systems and that seascape patterning should be considered more integral to the ecology of temperate marine systems.
The size of habitats has been commonly regarded as a key predictor for species diversity and abundance in a range of taxa, including reef fish (MacArthur and Wilson 1967; Simberloff 1976; Boström et al. 2011). The findings of the present study contradict studies exploring species-area relationships in both temperate (Parsons et al. 2016) and tropical marine biomes (Sale and Douglas 1984; Acosta and Robertson 2002; Chittaro 2002) with smaller reefs observed to have greater reef fish abundance, diversity and the abundance of several functional groups. The increased biodiversity observed on small reefs may be attributed to the smaller reefs in this study having large seagrass meadows in close proximity in comparison to the larger reefs which were surrounded by smaller areas of seagrass. Conversely, increased biodiversity on small reefs could be driven by edge effects, as smaller habitats contain large edge to area ratios (Smith et al. 2008). There have been contradictory findings for the response of reef fish assemblages to reef edge environments with both increases and decreases in abundance and diversity reported (Acosta and Robertson 2002; Moore et al. 2011; Rees et al. 2014). Importantly, these studies were conducted on reefs with soft sediment boundaries whereas reefs in this study often bordered seagrass habitats. In comparison to a boundary between reef and unconsolidated sediment, the reef-seagrass interface offers greater structural complexity and provide extra refugial opportunities for reef fish (Heck Jnr et al. 2003; Dorenbosch et al. 2005). Further, the reef-seagrass interface may promote greater abundance and diversity because seagrass meadows may subsidize nutrients to adjacent reefs leading to a higher holding capacity (Parrish 1989; Dorenbosch et al. 2005; Valentine et al. 2007; Heck Jr et al. 2008).
Reef fish were found to be more abundant on reefs close to large seagrass meadows, highlighting the importance of structural connectivity for the composition of reef fish assemblages. Increased structural connectivity improves the ability of fishes to move between habitats and access resources causing increased diversity and abundance (i.e. landscape complementation and supplementation; Dunning et al. 1992). For instance, tracking data has revealed routine movements of temperate reef fish, such as the generalist carnivore Acanthopagrus australis, to adjacent seagrass meadows presumably for foraging (Taylor et al. 2018). Additionally, many of the species observed in this study are known to recruit to seagrass as juveniles and perform ontogenetic migrations to reefs once a certain age or size class is reached (Gray et al. 1996; Smith and Sinerchia 2004; Curley et al. 2013). Increased structural connectivity between reefs and seagrass meadows likely enhances the number of recruits performing ontogenetic migrations. This is supported by research comparing the spatial partitioning of size classes in fish assemblages between habitats in tropical seascapes (Dorenbosch et al. 2005, Nagelkerken et al. 2017). For example, Nagelkerken et al. (2017) reported the biomass and density of reef fish that use seagrass meadows as nurseries declined once reefs were within 4 km from seagrass and were close to zero at a distance of 14 km. Overall, our findings suggest not all reefs are equal and it is important not to view reef habitats in isolation; rather the context of a reef within the seascape must be considered when predicting the abundance and diversity of temperate reef fish.
Mobility has been suggested to be an important functional trait determining how variation in the abundances of fishes relate to seascape patterning and our findings support this notion (Caldwell and Gergel 2013). Resident fishes were more abundant on reefs with low amounts of edge environments, that is those which contain greater core reef habitat. Resident taxa have limited mobility and small home ranges and have been reported to interact with the within-patch features of a reef (Hixon and Beets 1989; Sale 1998). Movement data of a resident species commonly observed in this study, the Eastern Blue Groper (Achoerodus viridis) supports our findings, with individuals avoiding the reef boundary in their daily home ranges (Lee et al. 2015). In contrast, mobile taxa were found to be more abundant on reefs close to seagrass, a pattern observed on coral reefs (Grober-Dunsmore et al. 2007). Mobile species migrating between seagrass and reefs have been reported to play an important role in transferring nutrients (Davis et al. 2014). Although speculative, the transfer of nutrients between habitats may be an indirect mechanism causing increased abundance of non-seagrass associated taxa on small reefs and reefs with large adjacent seagrass meadows. In contrast, the abundance of transient taxa was not influenced by seascape variables, a finding consistent with previous research (Grober-Dunsmore et al. 2007). Transient species, such as Pseudocaranx georgianus and Pomatomus saltatrix have large home ranges and are less likely to be associated with seascape patterns (Young et al. 1999; Fowler et al. 2018). Alternatively, it is possible that transient fish interact with seascape patterning at scales greater than those investigated in this study.
Seascape features were an important driver for the abundance of all trophic guilds excluding herbivores. Generally, fish consuming any invertebrates in their diets were more abundant in heterogeneous seascapes or on reefs close to seagrass. These fish likely forage on the substantial epibenthic and infaunal invertebrate assemblages found within seagrass meadows (Edgar and Shaw 1995; Bologna and Heck 2002; Bloomfield and Gillanders 2005). Generalist carnivores were found to be more abundant on small reefs in seascapes with less habitat diversity. This group consisted of larger, more transient species which can migrate between small habitat patches in lower diversity seascapes, comprised largely of sand. Zooplanktivores showed a small increase in abundance with reef size before decreasing in numbers on medium to large (> 0.063 hectares) reefs. Research has suggested that although smaller reefs deliver better access to zooplankton rich currents, they also have reduced refuge volumes and higher predation risk. Therefore, causing reduced numbers of zooplanktivores on both small and large reefs (Champion et al. 2015).
Our findings highlight the importance of employing a multi-scale design and considering the ecological traits of organisms to account for how fishes respond to seascape patterning at a hierarchy of scales (Kendall et al. 2011; Ricart et al. 2018). No distinct scale appeared to be universally important, although the 250 m spatial scale had the most explanatory value. Further, the abundance and diversity of reef fish was found to be greater on reefs within 55 m of seagrass. These scales are smaller than those from studies in tropical seascapes, where positive correlations between reef fish abundance and diversity with seagrass area peak at scales between 500 and 1000 m (Grober-Dunsmore et al. 2007; Kendall et al. 2011; Berkström et al. 2012). Different species will respond to similar seascapes in different ways due to variations in life-history traits. Therefore, future studies should investigate the influence of the seascape on temperate reef fish at the species level as this information can contribute to the management of harvested species or managing multi-species assemblages across a range of scales (Grober-Dunsmore et al. 2009). In addition, future research should examine how temperate reef fish respond to both within-habitat condition (e.g. structural complexity, canopy structure) and broad-scale seascape patterns (e.g. van Lier et al. 2018).
In conclusion, this research has established that seascape patterns, especially the context of reefs to adjacent seagrass greatly influence temperate reef fish assemblages. These findings have important implications for the management of temperate coastal seascapes, such as the design of marine reserves (Pittman 2017). Specifically, the placement of reserves in areas with high structural connectivity between reef and seagrass may ensure the protection of increased levels of reef fish abundance and diversity, which is often a goal of management. Further, the global decline of seagrass meadows may not only influence seagrass fishes but also have flow on effects for the abundance and diversity of adjacent reef fish communities (Waycott et al. 2009; Nakamura 2010). The challenge for future research is testing the actual mechanisms underpinning the patterns observed in this study. This can be achieved using a variety of techniques such as telemetry, genetics and diet analyses and will allow for seascape patterning and connectivity to be better integrated into spatial conservation and management (Pittman 2017).
References
Acosta CA, Robertson DN (2002) Diversity in coral reef fish communities: the effects of habitat patchiness revisited. Mar Ecol Prog Ser 227(1):87–96
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Beets J, Muehlstein L, Haught K, Schmitges H (2003) Habitat connectivity in coastal environments: patterns and movements of Caribbean coral reef fishes with emphasis on bluestriped grunt, Haemulon sciurus. Gulf Carrib Res 14(2):29–42
Bell JD, Westoby M (1986) Variation in seagrass height and density over a wide spatial scale: effects on common fish and decapods. J Exp Mar Biol Ecol 104(1–3):275–295
Berkström C, Gullström M, Lindborg R, Mwandya AW, Yahya SA, Kautsky N, Nyström M (2012) Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs. Estuar Coast Shelf Sci 107:1–21
Berkström C, Lindborg R, Thyresson M, Gullström M (2013) Assessing connectivity in a tropical embayment: fish migrations and seascape ecology. Biol Conserv 166:43–53
Bernard ATF, Götz A (2012) Bait increases the precision in count data from remote underwater video for most subtidal reef fish in the warm-temperate Agulhas bioregion. Mar Ecol Prog Ser 471:235–252
BjØrnstad ON, Falck W (2001) Nonparametric spatial covariance functions: estimation and testing. Environ Ecol Stat 8(1):53–70
Bloomfield A, Gillanders B (2005) Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28(1):63–77
Bologna PA, Heck KL (2002) Impact of habitat edges on density and secondary production of seagrass-associated fauna. Estuaries 25(5):1033–1044
Bond T, Langlois TJ, Partridge J, Birt M, Malseed B, Smith L, McLean D (2018) Diel shifts and habitat associations of fish assemblages on a subsea pipeline. Fish Res 206:220–234
Boström C, Pittman SJ, Simenstad C, Kneib RT (2011) Seascape ecology of coastal biogenic habitats: advances, gaps, and challenges. Mar Ecol Prog Ser 427:191–217
Breheny P, Burchett W (2013) Visualization of regression models using visreg. R package pp 1–5
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Soc Method Res 33(2):261–304
Calabrese JM, Fagan WF (2004) A comparison-shopper’s guide to connectivity metrics. Front Ecol Environ 2(10):529–536
Caldwell IR, Gergel SE (2013) Thresholds in seascape connectivity: influence of mobility, habitat distribution, and current strength on fish movement. Landscape Ecol 28(10):1937–1948
Champion C, Suthers I, Smith J (2015) Zooplanktivory is a key process for fish production on a coastal artificial reef. Mar Ecol Prog Ser 541:1–14
Chittaro BM (2002) Species-area relationships for coral reef fish assemblages of St. Croix, US Virgin Islands. Mar Ecol Prog Ser 233:251–261
Creese R, Glasby T, West G, Gallen C (2009) Mapping the habitats of NSW estuaries. Port Stephens Fisheries Institute, Sydney
Curley BG, Jordan AR, Figueira WF, Valenzuela VC (2013) A review of the biology and ecology of key fishes targeted by coastal fisheries in south-east Australia: identifying critical knowledge gaps required to improve spatial management. Rev Fish Biol Fish 23(4):435–458
Davis JP, Pitt KA, Fry B, Olds AD, Connolly RM (2014) Seascape-scale trophic links for fish on inshore coral reefs. Coral Reefs 33(4):897–907
Dorenbosch M, Grol MGG, Christianen MJA, Nagelkerken I, Van Der Velde G (2005) Indo-Pacific seagrass beds and mangroves contribute to fish density and diversity on adjacent coral reefs. Mar Ecol Prog Ser 302:63–76
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Edgar GJ, Shaw C (1995) The production and trophic ecology of shallow-water fish assemblages in southern Australia I: Species richness, size-structure and production of fishes in Western Port, Victoria. J Exp Mar Biol Ecol 194(1):53–81
Ferrell DJ, McNeill SE, Worthington DG, Bell JD (1993) Temporal and spatial variation in the abundance of fish associated with the seagrass Posidonia australis in south-eastern Australia. Mar Freshwater Res 44(6):881–899
Fisher R, Wilson SK, Sin TM, Lee AC, Langlois TJ (2018) A simple function for full-subsets multiple regression in ecology with R. Ecol Evol 8(12):6104–6113
Forman RTT, Godron M (1986) Landscape ecology. Wiley, New York
Fowler AM, Chick RC, Stewart J (2018) Patterns and drivers of movement for a coastal benthopelagic fish, Pseudocaranx georgianus, on Australia’s southeast coast. Sci Rep 8(1):16738
Froese R, Pauly D (2010) FishBase. World Wide Web Electronic publication. www.fishbase.org
Gillanders BM (2006) Seagrasses, Fish, and Fisheries. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Netherlands, pp 503–505
Gillanders BM, Able KW, Brown JA, Eggleston DB, Sheridan PF (2003) Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar Ecol Prog Ser 247:281–295
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84(11):2809–2815
Gray CA, McElligott DJ, Chick RC (1996) Intra- and inter-estuary differences in assemblages of fishes associated with shallow seagrass and bare sand. Mar Freshwater Res 47(5):723–735
Green AL, Maypa AP, Almany GR, Rhodes KL, Weeks R, Abesamis RA, Gleason MG, Mumby PJ, White AT (2015) Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol Rev 90(4):1215–1247
Green BC, Smith DJ, Underwood GJ (2012) Habitat connectivity and spatial complexity differentially affect mangrove and salt marsh fish assemblages. Mar Ecol Prog Ser 466:177–192
Grober-Dunsmore R, Frazer TK, Beets JP, Lindberg WJ, Zwick P, Funicelli NA (2008) Influence of landscape structure on reef fish assemblages. Landscape Ecol 23(S1):37–53
Grober-Dunsmore R, Frazer TK, Lindberg WJ, Beets J (2007) Reef fish and habitat relationships in a Caribbean seascape: the importance of reef context. Coral Reefs 26(1):201–216
Grober-Dunsmore R, Pittman SJ, Caldow C, Kendall MS, Frazer TK (2009) A landscape ecology approach for the study of ecological connectivity across tropical marine seascapes. In: Nagelkerken I (ed) Ecological connectivity among tropical coastal ecosystems. Springer, Dordrecht Heidelberg, pp 493–530
Guisan A, Edwards TC, Hastie TJ (2002) Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Modell 157(2):89–100
Hannan JC, Williams RJ (1998) Recruitment of juvenile marine fishes to seagrass habitat in a temperature Australian estuary. Estuaries 21(1):29–51
Harasti D, Malcom H, Gallen C, Coleman MA, Jordan A, Knott NA (2015) Appropriate set times to represent patterns of rocky reef fishes using baited video. J Exp Mar Biol Ecol 463:173–180
Harvey ES, Cappo M, Butler JJ, Hall N, Kendirck GA (2007) Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar Ecol Prog Ser 350:245–254
Hastie TJ (2017) Generalized additive models. Statistical models in S. Routledge, Abingdon, pp 249–307
Hastie TJ, Tibshirani R (1987) Generalized additive models: some applications. J Am Stat Assoc 82(398):371–386
Heck Jnr K, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136
Heck Jnr K, Hays G, Orth RJ (2008) Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 253:1198–1210
Hitt S, Pittman SJ, Nemeth RS (2011) Diel movements of fishes linked to benthic seascape structure in a Caribbean coral reef ecosystem. Mar Ecol Prog Ser 427:275–291
Hixon MA, Beets JP (1989) Shelter characteristics and Caribbean fish assemblages: experiments with artificial reefs. Bull Mar Sci 44(2):666–680
Hutchins B, Swainston R (1986) Sea fishes of southern Australia: complete field guide for anglers and divers. Swainston Publication, Perth
Jackson EL, Attrill MJ, Rowden AA, Jones MB (2006) Seagrass complexity hierarchies: influence on fish groups around the coast of Jersey (English Channel). J Exp Mar Biol Ecol 330(1):38–54
Jelbart JE, Ross PM, Connolly RM (2007) Patterns of small fish distributions in seagrass beds in a temperate Australian estuary. J Mar Biol Assoc UK 87(5):1297–1307
Kendall MS (2005) A method for investigating seascape ecology of reef fish. Proc Gulf Carib Fish Inst 56:1–11
Kendall MS, Miller TJ, Pittman SJ (2011) Patterns of scale-dependency and the influence of map resolution on the seascape ecology of reef fish. Mar Ecol Prog Ser 427:259–274
Kiggins RS, Knott NA, Davis AR (2018) Miniature baited remote underwater video (mini-BRUV) reveals the response of cryptic fishes to seagrass cover. Environ Biol Fishes 101(12):1717–1722
Kuiter RH (1993) Coastal fishes of South-Eastern Australia. Crawford House Press, Honolulu
Lee KA, Huveneers C, Macdonald T, Harcourt RG (2015) Size isn’t everything: movements, home range, and habitat preferences of eastern blue gropers (Achoerodus viridis) demonstrate the efficacy of a small marine reserve. Aquat Conserv 25(2):174–186
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Martin TSH, Olds AD, Olalde ABH, Berkström C, Gilby BL, Schlacher TA, Butler IR, Yabsley NA, Zann M, Connolly RM (2018) Habitat proximity exerts opposing effects on key ecological functions. Landscape Ecol 33(8):1273–1286
McGrouther MA (2001) The Australian Museum Fish Database. World Wide Web Electronic publication. https://australianmuseum.net.au/learn/animals/fishes/
Moore CH, Van Niel K, Harvey ES (2011) The effect of landscape composition and configuration on the spatial distribution of temperate demersal fish. Ecography 34(3):425–435
Nagelkerken I (2009) Evaluation of nursery function of mangroves and seagrass beds for tropical decapods and reef fishes: patterns and underlying mechanisms. Ecological connectivity among tropical coastal ecosystems. Springer, Dordrecht, pp 357–399
Nagelkerken I, Huebert KB, Serafy JE, Grol MG, Dorenbosch M, Bradshaw CJ (2017) Highly localized replenishment of coral reef fish populations near nursery habitats. Mar Ecol Prog Ser 568:137–150
Nagelkerken I, Sheaves M, Baker R, Connolly RM (2015) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish 16(2):362–371
Nakamura Y (2010) Patterns in fish response to seagrass bed loss at the southern Ryukyu Islands, Japan. Mar Biol 157(11):2397–2406
Olds AD, Connolly RM, Pitt KA, Maxwell PS (2012) Primacy of seascape connectivity effects in structuring coral reef fish assemblages. Mar Ecol Prog Ser 462:191–203
Ortodossi NL, Gilby BL, Schlacher TA, Connolly RM, Yabsley NA, Henderson CJ, Olds AD (2018) Effects of seascape connectivity on reserve performance along exposed coastlines. Conserv Biol 0:1–10
Parrish JD (1989) Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar Ecol Prog Ser Oldendorf 58(1):143–160
Parsons DF, Suthers IM, Cruz DO, Smith JA (2016) Effects of habitat on fish abundance and species composition on temperate rocky reefs. Mar Ecol Prog Ser 561:155–171
Perry D, Staveley TAB, Gullström M (2018) Habitat connectivity of fish in temperate shallow-water seascapes. Front Mar Sci 4:001–012
Pittman SJ (ed) (2017) Seascape ecology. Wiley Blackwell, Oxford
Pittman SJ, McAlpine CA (2003) Movements of marine fish and decapod crustaceans: process, theory and application. Adv Mar Biol 44(1):205–294
R Core Development Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rees MJ, Jordan A, Price OF, Coleman MA, Davis AR (2014) Abiotic surrogates for temperate rocky reef biodiversity: implications for marine protected areas. Divers Distrib 20(3):284–296
Rees MJ, Knott NA, Davis AR (2018) Habitat and seascape patterns drive spatial variability in temperate fish assemblages: implications for marine protected areas. Mar Ecol Prog Ser 607:171–186
Ricart AM, Sanmartí N, Pérez M, Romero J (2018) Multilevel assessments reveal spatially scaled landscape patterns driving coastal fish assemblages. Mar Environ Res 140:210–220
Sale PF (1998) Appropriate spatial scales for studies of reef-fish ecology. Aust J Ecol 23(3):202–208
Sale PF, Douglas WA (1984) Temporal variability in the community structure of fish on coral patch reefs and the relation of community structure to reef structure. Ecology 65(2):409–422
Sambrook K, Hoey AS, Andréfouët S, Cumming GS, Duce S, Bonin MC (2019) Beyond the reef: the widespread use of non-reef habitats by coral reef fishes. Fish Fish 00:1–18
Schultz AL, Malcolm HA, Bucher DJ, Smith SDA (2012) Effects of reef proximity on the structure of fish assemblages of unconsolidated substrata. PLoS ONE 7(11):1–10
Simberloff D (1976) Experimental zoogeography of islands: effects of island size. Ecology 57(4):629–648
Smith TM, Hindell JS, Jenkins GP, Connolly RM (2008) Edge effects on fish associated with seagrass and sand patches. Mar Ecol Prog Ser 359(1):203–213
Smith KA, Sinerchia M (2004) Timing of recruitment events, residence periods and post-settlement growth of juvenile fish in a seagrass Nursery Area, South-Eastern Australia. Environ Biol Fishes 71(1):73–84
Staveley TAB, Perry D, Lindborg R, Gullström M (2016) Seascape structure and complexity influence temperate seagrass fish assemblage composition. Ecography 39:001–011
Taylor MD, Becker A, Lowry MB (2018) Investigating the functional role of an artificial reef within an estuarine seascape: a case study of yellowfin bream (Acanthopagrus australis). Estuaries Coasts 41(6):1782–1792
Turner MG (2005) Landscape ecology: what is the state of the science? Ann Rev Ecol Evol Syst 36:319–344
Tweedie MC (1984) An index which distinguishes between some important exponential families. Statistics 579:579–604
Unsworth RK, Bell JJ, Smith DJ (2007) Tidal fish connectivity of reef and seagrass habitats in the Indo-Pacific. J Mar Biol Assoc UK 87(5):1287–1296
Valentine JF, Heck KL Jr, Blackmon D, Goecker ME, Christian J, Kroutil RM, Kirsch KD, Peterson BJ, Beck M, Vanderklift MA (2007) Food web interactions along seagrass–coral reef boundaries: effects of piscivore reductions on cross-habitat energy exchange. Mar Ecol Prog Ser 333:37–50
van Lier JR, Wilson SK, Depczynski M, Wenger LN, Fulton CJ (2018) Habitat connectivity and complexity underpin fish community structure across a seascape of tropical macroalgae meadows. Landscape Ecol 33(8):1287–1300
Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS 106(30):12377–12381
Wedding LM, Christopher LA, Pittman SJ, Friedlander AM, Jorgensen S (2011) Quantifying seascape structure: extending terrestrial spatial pattern metrics to the marine realm. Mar Ecol Prog Ser 427:219–232
Wellington CM, Harvey ES, Wakefield CB, Langlois TJ, Williams A, White WT, Newman SJ (2018) Peak in biomass driven by larger-bodied meso-predators in demersal fish communities between shelf and slope habitats at the head of a submarine canyon in the south-eastern Indian Ocean. Cont Shelf Res 167:55–64
Williams R, West G, Morrison D, Creese R (2007) Estuarine resources of NSW. The NSW comprehensive coastal assessment toolkit. CD ROM. ISBN: 0.7347:7
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC
Wood SN, Scheipl F (2014) gamm4: Generalized additive mixed models using mgcv and lme4. R package version 0.2–3
Wood SN, Wood MS (2015) Package ‘mgcv’. R package version 1:29
Wraith J, Lynch T, Minchinton TE, Broad A, Davis AR (2013) Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar Ecol Prog Ser 477:189–199
Wu J (2006) Landscape ecology, cross-disciplinarity, and sustainability science. Landscape Ecol 21:1–4
Young GC, Wise BS, Ayvazian SG (1999) A tagging study on tailor (Pomatomus saltatrix) in Western Australian waters: their movement, exploitation, growth and mortality. Mar Freshw Res 50(7):633–642
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank E. Messer, P. Gordon, J. Lester, C. Virtue, C. Evans-Turner, K. Gilles and K. Swadling for fieldwork assistance. We would also like to thank H. Brown for assisting with the spatial analysis. We acknowledge support from the staff of Jervis Bay Marine Park and Booderee National Park. This research was conducted in accordance to the methods approved by the University of Wollongong’s animal ethics committee (AE 12/07r15), NSW DPI scientific collection permit P01/0059(A)-2.0 and Booderee National Park scientific research permit BDR16/00002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swadling, D.S., Knott, N.A., Rees, M.J. et al. Temperate zone coastal seascapes: seascape patterning and adjacent seagrass habitat shape the distribution of rocky reef fish assemblages. Landscape Ecol 34, 2337–2352 (2019). https://doi.org/10.1007/s10980-019-00892-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00892-x