Abstract
Context
Habitat loss and habitat fragmentation negatively affect amphibian populations. Roads impact amphibian species through barrier effects and traffic mortality. The landscape variable ‘accessible habitat’ considers the combined effects of habitat loss and roads on populations.
Objectives
The aim was to test whether accessible habitat was a better predictor of amphibian species richness than separate measures of road effects and habitat loss. I assessed how accessible habitat and local habitat variables determine species richness and community composition.
Methods
Frog and tadpole surveys were conducted at 52 wetlands in a peri-urban area of eastern Australia. Accessible habitat was delineated using a highway. Regressions were used to examine relationships between species richness and eleven landscape and local habitat variables. Redundancy analysis was used to examine relationships between community composition and accessible habitat and local habitat variables.
Results
Best-ranked models of species richness included both landscape and local habitat variables. There were positive relationships between species richness and accessible habitat and distance to the highway, and uncertain relationships with proportion cover of native vegetation and road density. There were negative relationships between species richness and concreted wetlands and wetland electrical conductivity. Four species were positively associated with accessible habitat, whereas all species were negatively associated with wetland type.
Conclusions
Barrier effects caused by the highway and habitat loss have negatively affected the amphibian community. Local habitat variables had strong relationships with species richness and community composition, highlighting the importance of both availability and quality of habitat for amphibian conservation near major roads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation, and broad-scale landscape change, are among the largest threats to biodiversity (Fischer and Lindenmayer 2007). Habitat fragmentation is a landscape-scale process that involves the breaking apart of habitat independent of habitat loss (Fahrig 2003). Roads contribute greatly to both the loss of habitat for wildlife, and to the fragmentation of landscapes due to barrier effects that restrict the ability of individuals to disperse among habitat patches (Forman and Alexander 1998), affecting both terrestrial and aquatic ecosystems (Trombulak and Frissell 2000). In some cases, roads do not entirely prohibit movement, but act as selective filters according to species behaviour, movement patterns and body size (Fahrig and Rytwinski 2009; Rytwinski and Fahrig 2012). Both the physical characteristics of a road such as its width and traffic volume affect rates of road mortality, with amphibians being particularly susceptible to being killed by passing vehicles while attempting to cross a road (Forman and Alexander 1998). A challenge for landscape ecologists is to measure the effects of habitat loss and roads not separately, but in combination where the position of a linear barrier such as a road may restrict a species’ ability to access habitat resources on the other side of the road.
Amphibian populations world-wide are currently threatened by three key processes of anthropogenic landscape change: habitat loss, habitat fragmentation and isolation, and habitat degradation (Cushman 2006; Hamer and McDonnell 2008). Furthermore, because aquatic-breeding amphibians have a biphasic life cycle, disconnection between the aquatic and terrestrial habitats required by species to satisfy this complex life history can result in ‘habitat split’, which has been shown to negatively affect the richness of species with aquatic larvae in human-altered landscapes (Becker et al. 2007). Linear barriers such as roads can restrict the movement of species, preventing individuals from accessing critical habitats between life stages (e.g., post-metamorphic dispersal) and during the terrestrial phase (e.g., overwintering and breeding sites), thereby disrupting the metapopulation dynamics of many pond-breeding species (Beebee 2013; Hamer et al. 2015; Jackson et al. 2015). High mortality rates from traffic can occur when individuals attempt to cross busy roads (Hels and Buchwald 2001; Glista et al. 2008; Sutherland et al. 2010) leading to reduced population sizes (Marsh and Jaeger 2015; Rytwinski and Fahrig 2015). Both road mortality and road-barrier effects can negatively affect amphibians through decreased species richness, abundance and occurrence patterns (Fahrig et al. 1995; Carr and Fahrig 2001; Pellet et al. 2004; Parris 2006; Hartel et al. 2010; Cosentino et al. 2014).
The extent of terrestrial upland habitat (e.g., forests and woodlands) in which roads and road networks are embedded can influence the persistence of many amphibian species with aquatic-breeding larvae; studies conducted in human-altered landscapes have shown that species richness, abundance and occurrence patterns generally increase with increases in forest cover at distances up to 1500 m from breeding sites (Gibbs 1998; Knutson et al. 1999; Guerry and Hunter 2002; Gagné and Fahrig 2007). Terrestrial non-breeding habitat provides essential resources for amphibians such as dispersal, foraging, sheltering and overwintering sites, and this habitat needs to be linked with breeding habitat that allows movement to complete the life cycles of many aquatic-breeding amphibians (Pope et al. 2000). In areas with dense road networks (e.g., urban centres), terrestrial habitat can be a critical component in ensuring successful dispersal and recolonisation within regional metapopulations, provided there is functional connectivity among habitat patches (Hamer and McDonnell 2008). In these fragmented landscapes, species with the greatest dispersal requirements are often more impacted than sedentary species (Gibbs 1998).
Using uncorrelated measures of forest cover and traffic density, Eigenbrod et al. (2008a) found a negative association between amphibian species richness and traffic that was stronger than the positive association with forests. Eigenbrod et al. (2008b) further explored these relationships by introducing a landscape variable known as ‘accessible habitat’ to measure the combined effects of habitat loss and roads which accounts for the location of habitat in relation to roads. For instance, a road that needs to be crossed by an individual to access a habitat patch is likely to have a far greater negative effect on the population than one that does not impede movement (Eigenbrod et al. 2008b). Furthermore, Eigenbrod et al. (2008b) hypothesised that accessible habitat will be more strongly related to species persistence than total habitat cover (e.g., forest), road density or distance to a road when populations are negatively affected both by habitat loss and roads. Landscape studies examining road effects on amphibian distribution need to include biologically-realistic variables such as accessible habitat because measuring habitat loss and roads separately can underestimate the effects of roads on populations.
Here I sought to test whether accessible habitat was a better predictor of amphibian species richness than landscape variables such as road density, total remnant habitat and distance to a highway, and local habitat variables that are known to influence amphibian occurrence. I also explored the relative importance of accessible habitat and local habitat variables in determining amphibian community structure. Important relationships between amphibian distributions and community composition with both landscape and local habitat variables have been previously demonstrated (Van Buskirk 2005; Hamer and Parris 2011; Kruger et al. 2015).
Methods
Study area
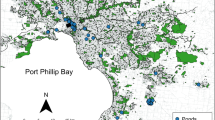
The study was conducted south of the township of Nowra on the south coast of New South Wales, Australia (Fig. 1). Nowra is a rapidly urbanising town with a population of around 19,000 people (Australian Bureau of Statistics 2015). The study area is situated on a coastal lowland floodplain grading into forest on undulating hills that occur generally to the south and west. Mixed land uses dominate the study area; residential areas including recent suburban developments predominantly occur towards Nowra, whereas agricultural lands towards the south accommodate livestock grazing. As such, the study area can be considered peri-urban. There are two conservation reserves and two state forests in the study area, situated to the south and east. The road network includes a four-lane divided highway (Princes Highway, herein referred to as ‘the highway’) and two-lane roads that intersect the highway, as well as residential streets; all are mostly sealed roads. The highway forms part of the main north–south road route along the east coast of Australia and connects the cities of Sydney and Melbourne. The study area has a temperate maritime climate, with a mean annual maximum temperature of 21.3 °C and an annual mean precipitation of 1112 mm, peaking in February and March (Bureau of Meteorology 2015). Daly (2014) detected ten aquatic-breeding frog species throughout the study area from 1996 to 2014.
Map of the study area, showing 52 wetlands distributed throughout the South Nowra area of New South Wales, Australia. Inset map depicts location of study area in south-eastern Australia. The road network is shown relative to the Princes Highway. Greyed areas indicate the extent of native vegetation mapped by Tozer et al. (2010)
From 2011 to 2014 a 6.3 km section of the highway was upgraded from a two to a four-lane road. Because the highway upgrade was likely to impact a population of the endangered green and golden bell frog (Litoria aurea) due to direct mortality, habitat loss and fragmentation, several mitigation measures were implemented to minimise any impacts on this species. During construction, frog exclusion fencing was installed along sections of the highway to prevent amphibian mortality from machinery. To facilitate frog movement under the highway once the upgrade was operational, two box-cell culverts (underpasses) were installed specifically designed for amphibian passage, in addition to other drainage culverts positioned along the highway. Eight ponds were constructed in road reserves, three east and five west of the highway, between 21 and 182 m from the highway to function as replacement habitat for L. aurea.
Wetland site selection
Initial examination of aerial photographs (Google Earth) revealed over 150 potential amphibian-breeding sites in the study area. Reconnaissance surveys were conducted in the field at up to 100 of these wetlands in March and September 2013. Of these, 52 wetlands were selected primarily on the basis of their proximity to a 10 km section of the highway (Fig. 1), and secondly, to maximise variation in wetland type. Because this study was undertaken in concert with a study investigating the distribution of L. aurea, some wetlands were selected on the basis of previous records of this species. Site selection was also restricted to those sites where permission for access was granted by landholders. There were 35 and 17 wetlands situated east and west of the highway, respectively, with distances to the highway ranging from 21 to 4278 m (mean distance = 1284 m). Wetland type ranged from stormwater retention ponds, replacement ponds for L. aurea that were constructed of concrete, freshwater marshes, drainage lines (creeks) and agricultural canals, golf course ponds and farm dams. At one site, a large marsh was sub-sampled to include an area where L. aurea had been previously recorded (Daly 2014). Drainage line and canal sections that were selected as sites consisted of pooled areas of non-flowing open water. Lentic wetlands and pools along creeks and canals are jointly referred to as ‘wetlands’ in this study following the methodology of Heard et al. (2012). There were seven creek and one canal sections, and 45 lentic wetlands included in this study. Site selection also aimed to include wetlands at varying distances from the nearest wetland (range 35–1440 m), and to include a range of hydroperiods, although selection was biased towards permanent (n = 41) rather than ephemeral wetlands (n = 11) based on availability. Mean wetland area was 2763 m2 (range 119–26532 m2).
Amphibian surveys
Six surveys were conducted over two breeding seasons in the Austral spring and summer (November, January and February): three surveys in 2013/2014 and three in 2014/2015. These survey periods coincided with the breeding season of most frog species recorded in the region (Anstis 2002), and also coincided with the final stages of construction of the highway upgrade. No ‘pre-construction’ data was collected in this study. The 52 wetlands were assigned into geographic clusters of between two and eight near-neighbour wetlands to facilitate sampling, surveying each cluster over six consecutive nights. The chronology of surveys between clusters was randomised. Surveys were conducted at 52 wetlands in 2013/2014 and at 50 of these in 2014/2015 because access was denied at two sites (one east and one west of the highway). Frog surveys commenced approximately 30 min after sunset (around 2100 h Australian Eastern Daylight Time, AEDT) and continued until 0200 h. Because this study also targeted L. aurea, call playback techniques were used. Surveys for frogs comprised a quiet listening period of 5 min followed by call imitation of L. aurea for 2 min, ending with 3 min of quiet listening. Two field workers then used headlights to spotlight for frogs in areas of vegetation, open water and shoreline, as well as the ground and vegetation within 10 m of the shoreline. The number of frog species heard and seen was recorded. The amount of time spent searching was proportional to the size and habitat complexity of the wetland. Surveys at large wetlands ceased after 60 min regardless of whether the entire site had been searched. Surveys at one wetland were confined to call playback techniques in November 2014 and January 2015 due to access restrictions.
Surveys for tadpoles were conducted when wetlands contained sufficient water levels using three techniques simultaneously at each wetland: (1) fish traps (45 cm long × 25 cm high × 25 cm wide, 15 cm long funnel at each end, 5 cm aperture) made of nylon mesh (mesh size 2 mm) and containing a yellow fluorescent glow-stick to attract larvae; (2) dip-netting using a triangular-framed dip-net (35 × 30 × 30 cm, mesh size 1.4 mm); and (3) direct observation of free-swimming larvae. The total number of fish traps deployed was proportional to a pond’s surface area (2 traps minimum, plus 1–4 additional traps for increases in area greater than 1000 m2; modified sampling protocol from Adams et al. 1997; Supplementary Material, Table S1). Dip-net surveys were time-constrained; a minimum 10 min was spent dip-netting, plus 5–40 additional minutes for increases in surface area greater than 500 m2; Table S2). Traps were distributed randomly within wetlands so that unbiased sampling occurred among microhabitat types (e.g., open water, emergent vegetation). This was done by placing a compass at the centre of a map diagram of the wetland and selecting which point of the wetland shoreline or creek bank to place a trap, using a table of random numbers between 0 and 360°. Tadpole surveys were restricted to direct observation if the water depth was <5 cm. Traps were placed into wetlands immediately before or during the frog surveys between 2000 and 0200 h AEDT, and retrieved the following morning (0700–1300 h). There was no bias in the time over which traps were set at a particular wetland because site selection within clusters was randomised. Floats were placed into the traps to ensure there was adequate air space to allow trapped organisms to breathe. Dip-net surveys commenced immediately following or during trap collection at a wetland. Two field workers vigorously dip-netted all available microhabitats at a wetland. Larvae were identified to species using a field guide (Anstis 2002) and then released. The count of all organisms captured in fish traps and dip-nets was recorded. Fish species were identified in the field using Allen et al. (2003). Where identification of specimens in the field was difficult, individuals were humanely anaesthetised and preserved in 70 % ethanol for later identification. Protocols to reduce the risk of spreading the amphibian chytrid fungus were followed when conducting fieldwork.
Landscape variables
Accessible habitat (ACC_HAB) was defined as the total area of native vegetation (i.e., amphibian habitat: wetland and terrestrial plant communities mapped by Tozer et al. 2010) within a 1000 m radius of a wetland (centroid) that could be accessed without crossing the highway, expressed as proportion cover (Data Source: ‘Native vegetation of southeast NSW: a revised classification and map for the coast and eastern tablelands. Version 1.0.’ Department of Environment and Conservation NSW, and Department of Natural Resources). There is a dense network of suburban roads within the study area. The carcasses of freshly-killed frogs have been observed on several of these roads on wet nights (A. J. Hamer pers. obs.) and so road mortality is occurring on roads other than the highway. Based on these observations, I assumed that frog species in the study area are attempting to cross most roads, but that the highway represents the greatest barrier and that smaller roads are having a weaker barrier effect (Eigenbrod et al. 2008b). For example, the highway’s physical footprint is much larger than that of other roads in the area (four lanes versus two lanes), the speed limit is higher and traffic volumes at night are high (A. J. Hamer pers. obs.). The highway was therefore used to delineate accessible habitat. Although culverts were installed under the highway within 6 months of fieldwork commencing, I assumed that there had not been sufficient time for potential frog movements through them to result in changes to frog communities either side of the highway. Therefore, I did not consider the location of the culverts in calculating accessible habitat. To assess the performance of accessible habitat in predicting the combined effect of habitat loss and roads on species richness, a variable was also included that described the total area of native vegetation (Tozer et al. 2010) within a 1000 m radius, using proportion cover, and that ignored the presence of the highway within a buffer (NATIVE_VEG). This variable therefore assessed habitat loss only. A 1000 m buffer was used to assess relationships because it likely encompassed the dispersal distances of the frog species previously found in the study area (Daly 2014), and it has also been used in other studies that assessed landscape predictors of amphibian distributions (e.g., Eigenbrod et al. 2008b; Hamer and Parris 2011). Positive associations between the amount of vegetation in a 1000-m radius around a wetland and the number of frog species have also been demonstrated in areas of south-eastern Australia (Hamer and Parris 2011; Smallbone et al. 2011). Road density (km of road/km2; ROAD) was calculated for each wetland site by summing the total length of roads in a 1000 m radius around the wetland (Data Source: ‘Road Network 1:25,000 [20 March 2013]’, NSW Roads and Maritime Services) and done to assess the effect of roads on species richness separately from habitat loss. The distance from the centroid of each wetland to the highway (HWY_DIST) was measured to also assess road effects separately. Lastly, the distance from a wetland to the nearest accessible wetland (centroid to centroid) was calculated as a measure of spatial proximity (DIST_WET), because some wetlands were clustered and therefore not demographically independent. ArcGIS 10.2.2 (Environmental Systems Research Institute Inc., Redlands, California, USA) was used to measure all landscape variables.
Local habitat variables
Previous studies of amphibian distributions have highlighted the importance of local habitat factors in addition to landscape variables (Pillsbury and Miller 2008; Hamer and Parris 2011). The following habitat variables were therefore measured at wetlands during January and November 2014, and February 2015: proportion of the pond surface area covered by aquatic vegetation (i.e., emergent, submerged vegetation and floating vegetation; AQVEG) and electrical conductivity of water (EC). These two parameters were measured as they have been shown in recent studies to positively and negatively affect amphibian species richness, respectively (Hamer and McDonnell 2008; Hamer and Parris 2011; Kruger et al. 2015). Emergent and submerged vegetation were defined as aquatic vegetation that extended above or below the water surface, respectively. Floating vegetation included surface algae and rooted macrophytes. Water conductivity (µS/cm) was measured in 200 ml water samples collected from 2 to 6 points around the wetland, ~1 m from the shoreline and at a depth of 5–10 cm using a handheld electronic meter (Tracer Pocketester, LaMotte Company, Chestertown, Maryland, USA). No water measurements were taken at dry wetlands. Measurements were taken during the tadpole surveys at each wetland. Hydroperiod and the presence of predatory fish species are often important determinants of amphibian community structure (Wellborn et al. 1996). The hydroperiod of each wetland (HYDRO) was assigned as either ephemeral (1) or permanent (0). Ephemeral wetlands were observed to dry down (i.e., <5 % of full water capacity) during at least one site visit, whereas permanent wetlands retained water throughout the study. The mean relative abundance of predatory fish (FISH) was calculated at each wetland using numbers of individuals captured in fish traps. Predatory fish were those species recorded in the study area that are known or suspected to eat frog eggs or tadpoles (Pyke and White 2000) and included short-finned eel (Anguilla australis), goldfish (Carassius auratus), mosquitofish (Gambusia holbrooki), striped gudgeon (Gobiomorphus australis), empire gudgeon (Hypseleotris compressa), catfish (Arius sp.), and perch (Perca sp.). Because wetland type may influence the suitability of a waterbody as amphibian habitat (Brand and Snodgrass 2010), each wetland in this study was assigned (WET_TYPE) as either a constructed concrete pond (i.e., the eight replacement ponds for L. aurea and two ornamental ponds; = 1), or a constructed or natural wetland composed of natural substrate (e.g., farm dam, marsh, creek section; = 0). The ten concrete ponds had vertical bank walls that may deter occupancy by ground-dwelling frogs that cannot climb vertical surfaces; such pond types support lower species richness in some instances (Parris 2006). The concrete ponds varied in their age of construction from about three months prior to fieldwork commencing (replacement ponds) to greater than 20 years (ornamental ponds). One ornamental pond was situated 2.7 km from the highway. The surface area of each wetland (AREA) was calculated from digitised polygons using ArcGIS.
Data analysis
Species richness was defined as the total number of species detected during surveys for frogs and tadpoles at each wetland over the two breeding seasons (2013/2014 and 2014/2015). The bleating tree frog (Litoria dentata) was excluded from the study due to the ambiguity in its detection: on occasions it could be heard calling up to 50 m from a wetland in surrounding vegetation; also L. dentata is an ephemeral pond-breeder that is not likely to reproduce at most of the wetlands included in this study.
I examined relationships between species richness and the five landscape and six local habitat variables at a wetland using Poisson regression modelling and multi-model inference within a set of 37 models. Firstly, I assessed six landscape models that each contained one or two of four landscape variables (ACC_HAB, NATIVE_VEG, ROAD and HWY_DIST) plus a constant and a spatial term. Because some wetlands were located close together (e.g., <100 m) and frog species may be moving between them, models accounted for spatial autocorrelation between wetlands by containing the variable DIST_WET. Calculation of Moran’s index in ArcGIS indicated positive spatial autocorrelation of species richness (Moran’s I = 0.38) thereby justifying inclusion of a spatial variable in each model. I then assessed ten local habitat models and 19 landscape + local habitat models that each contained three to five variables. One model included DIST_WET only, and a null model of ‘no effect’ was included that contained a constant term only.
I examined the landscape and local habitat variables for multicollinearity using Spearman rank correlation coefficients and excluded strongly correlated variables (|r s | ≥ 0.4) from the same model (Table S3). DIST_WET was not included in models containing the variable AREA due to a strong correlation between the two variables (r s = 0.52). A conservative correlation coefficient was used to discriminate between weak and strong correlations to reduce the likelihood of confounded explanatory variables biasing interpretation of the models (Graham 2003). The following variables were log10(x)-transformed: HWY_DIST, DIST_WET, EC and AREA. The variable FISH was log10(x + 1)-transformed. HYDRO and WET_TYPE were assessed as binary variables. Using Bayesian inference, I identified relationships between species richness and the variables in Poisson regression models that included uninformative priors (mean and precision [1/variance]) for the intercept term (a ~ dnorm[0, 1.0 × 10−6]) and the regression coefficients (beta[j] ~ dnorm[0, 1.0 × 10−6]) where j is an explanatory variable. Models were limited to a maximum of five explanatory variables given the recommendation to restrict regression models to include one variable for every ten data points (Wintle et al. 2005).
Models were run in OpenBUGS 3.2.2 (Spiegelhalter et al. 2007) to generate 100,000 samples from the posterior distribution of each model term, discarding the first 10,000 samples as a ‘burn-in'. All explanatory variables were centred by subtracting the mean from each variable in an effort to minimise autocorrelation between successive samples obtained from the Monte Carlo Markov Chain algorithm. Three replicate Monte Carlo Markov Chains were run for each model with a suitable number of iterations so that convergence was reached for all variables on the basis of the Brooks-Gelman-Rubin statistic (i.e., R < 1.05) and by visual inspection of the chain histories. I used Bayesian credible intervals (BCIs) from the 2.5th and 97.5th percentiles of the distribution of each model term as 95 % credible intervals. The relative fit of the models against model complexity was evaluated using the Deviance Information Criterion (DIC; Spiegelhalter et al. 2002). The best models were considered those with a ΔDIC <2 (ΔDIC = DIC − DICmin, where DICmin = lowest DIC value in the model set). Models with a ΔDIC of 2–7 were considered to have a moderate level of support and any models with a ΔDIC >7 were considered to be unsupported (McCarthy 2007).
Effect sizes were used to gauge the relative performance of each variable in predicting species richness at a wetland. Effect sizes enable an ecologically-meaningful comparison of confidence intervals rather than relying on statistical significance, and allow one to quantify the magnitude of effects and the precision of their estimates (McCarthy 2007). I calculated the multiplicative effect size (E i ) with 95 % BCIs of each variable on species richness across the range of the variable. In Poisson regression, the multiplicative effect is calculated as the exponent of the standardised coefficient:
where E i is the multiplicative effect of variable i, b i is the regression coefficient of variable i, and range i is the range of values for variable i. A multiplicative effect size of one corresponds to no change in species richness. Effect sizes >1 indicate a positive effect of the explanatory variable on species richness, whereas effect sizes <1 indicate negative effects. Uncertainty in a mean effect size is denoted by a 95 % BCI that overlaps one.
I assessed the dissimilarity of wetlands where frog species were detected by calculating the Bray–Curtis measure of compositional dissimilarity (Bray and Curtis 1957), because wetlands may have identical numbers of species but with different species composition. The Bray–Curtis measure produces a dissimilarity value (D) between 0 and 1 for each pair of wetlands; D = 0 when two wetlands have identical species, whereas D = 1 when two wetlands have no shared species. Primer 5 (Clarke and Gorley 2001) was used for calculations.
I conducted a redundancy analysis (RDA) to examine the relationship between the composition of species assemblages at wetlands and accessible habitat and local habitat variables. An initial Detrended Correspondence Analysis (DCA) was conducted that showed the species had a linear relationship with the explanatory variables, as the gradient lengths of four axes were all <4 standard deviations. RDA is a constrained ordination method that is used when variables have a linear relationship with environmental gradients (McCune and Grace 2002), producing axes that are linear combinations of environmental variables (ter Braak 2000). The RDA ordination diagram can be interpreted as a biplot where species arrows (or centroids) and environmental arrows jointly approximate the covariances between species and environmental variables; the cosine of the angle between the arrows of a species and a variable represents an approximation of the correlation coefficient between the two (ter Braak 2000). I included data on species detections at each of the 52 wetlands and seven explanatory variables: ACC_HAB, DIST_WET, AQVEG, EC, HYDRO, FISH and WET_TYPE. The analysis was run using CANOCO 4 (ter Braak and Šmilauer 1998) with a Monte Carlo permutation test to determine the eigenvalues of the first ordination axis and the sum of eigenvalues of all canonical axes together (9999 permutations). Axes with eigenvalues closer to one can be interpreted as having stronger relative importance than axes with smaller values. The variance inflation factors (VIF) of the explanatory variables were all less than two, so the seven variables were included in the same RDA. Three species were excluded from the analysis because they were each detected at only one wetland on a single survey.
I estimated detection probabilities (d) for species detected at more than one wetland based on a null model using logistic regression that accounted for imperfect detection, with uninformative priors for the constant (a ~ dnorm[0, 1.0 × 10−6]) and for detection probabilities when the species is present (d[1] ~ dunif[0, 1]) using OpenBUGS 3.2.2. The detection of a species at a wetland was ascertained using all survey methods over the two breeding seasons. Detection probabilities were then used to calculate the minimum number of visits (N min) necessary to be 95 % certain that a species is absent (Pellet and Schmidt 2005):
Results
A total of ten frog species (excluding Litoria dentata) were detected in the study area, with between one and seven frog species detected at a wetland (mean, BCI: 4.4, 3.9–4.9). The most frequently detected species were the striped marsh frog (Limnodynastes peronii) and the dwarf tree frog (Litoria fallax), occurring at 90 and 88 % of the 52 wetlands, respectively (Table S4). The remaining eight species were detected at 2–77 % of the wetlands surveyed. All species detected were pond-breeding with aquatic-developing larvae. Detection probabilities for seven species ranged from 0.15 to 0.68 (Table S4). Mean number of species detected in wetlands constructed of concrete was 2.8 (BCI: 1.6–4.0) compared with 4.8 species (BCI: 4.3–5.3) in non-concreted wetlands.
Model inference
Six models of species richness were the best-ranked (ΔDIC <2): in addition to distance to the nearest wetland, three models included one landscape variable each (accessible habitat, road density and cover of native vegetation) together with electrical conductivity, hydroperiod and wetland type (models 1, 4 and 5; Table 1). Model 6 contained two landscape variables (road density and distance to the highway). Models 2 and 3 contained local habitat variables only; wetland type was included in all models except model 6, while electrical conductivity was included in all but model 2.
The remaining 31 models had ΔDIC values ranging 2.7–9.4; however, this set of models included the null model of ‘no effect’ (ΔDIC = 4.4; model 12), which suggests that they were no better than random in predicting species richness. This set included models containing only a single landscape variable (models 14, 17, 23 and 25), in addition to models that included both cover of native vegetation and distance to the highway (models 7, 15 and 22). Therefore, Models 7–37 can be regarded as having no relative importance on species richness (i.e., no effect) based on multi-model inference.
Landscape relationships
There was a clear and positive relationship between species richness and distance to the highway, with a multiplicative effect size of 1.66 (model 6; Fig. 2). This finding predicts that, holding the other variables constant, the wetland furthest from the highway would have 1.66 times (i.e., nearly twice) the number of frog species than the wetland closest to the highway. There was also a positive relationship between species richness and accessible habitat (E i = 1.54), although the BCI overlapped one slightly (Fig. 2). There was a slightly smaller and more ambiguous relationship between species richness and the proportion cover of native vegetation (E i = 1.43), whereas the relationship with road density was negative and the BCIs overlapped one widely (Fig. 2). There was no clear relationship between species richness and distance to the nearest wetland with all effect sizes close to one.
Multiplicative effect sizes of a five landscape and b three local habitat variables (means and 95 % credible intervals) on amphibian species richness at wetlands in the South Nowra area, Australia. Model numbers refer to models presented in Table 1. ACC_HAB accessible habitat, NATIVE_VEG total area of native vegetation within 1000 m radius, ROAD road density within 1000 m radius (numbers refer to models 4 and 6); HWY_DIST distance to the highway. Only effect sizes predicted from best-ranked models (ΔDIC <2) are presented. Multiplicative effect sizes >1 indicate a positive effect of the explanatory variable on species richness; effect sizes <1 indicate negative effects
Local habitat relationships
There was a relatively strong negative relationship between species richness and wetland type, with a mean multiplicative effect size of 0.55–0.59 for models 1 to 5 (Fig. 2). This translates to the prediction that a concrete pond would only have around half the number of species than a non-concreted pond. There was also a strong negative relationship between species richness and electrical conductivity, with a mean multiplicative effect size of 0.42–0.56 across five models (Fig. 2). The relationship with wetland type was clear with no BCIs overlapping one, although BCIs in four of the five models of conductivity slightly overlapped one (Fig. 2). Hydroperiod had no clear relationship with species richness.
Species composition
The species dissimilarity values (D) ranged from 0.76 at five wetlands with two species, to 0.00 at three sites with seven identical species. The average \(\overline{D}\) was 0.28 indicating that although the wetlands were not identical in their species composition, there was a high degree of similarity among them.
The first RDA axis (axis 1; eigenvalue = 0.166) explained 16.6 % of the variability in the species data and 60.9 % of the variability in the species-environment relationship. Axis 1 described a gradient from wetlands with a high proportion of both accessible habitat and diverse aquatic vegetation to wetlands constructed of concrete (Fig. 3). Four species were positively associated with accessible habitat and aquatic vegetation (Crinia signifera, Limnodynastes peronii, Litoria aurea and L. fallax), whereas all species were negatively associated with concrete ponds. Three species were not associated with axis 1 (Litoria peronii, L. tyleri and L. verreauxii). The second RDA axis (axis 2) explained 4.7 and 17.5 % of the variability in the species data and species-environment relationships, respectively. Axis 2 represented a gradient from wetlands with high electrical conductivity to concreted wetlands (Fig. 3); no species were associated with axis 2.
Redundancy analysis (RDA) biplot of the composition of amphibian communities relative to accessible habitat, distance to the nearest wetland and five local habitat variables at 52 wetlands in the South Nowra area, Australia. Axis 1, indicated by the heavy dashed line and heavy dot (accessible habitat–wetland type), explained 60.9 % of variability in the species-environment relationship. Species codes C. = Crinia; Lim. = Limnodynastes; Lit. = Litoria
Discussion
The results of this study demonstrated a positive relationship between accessible habitat and species richness and composition of an amphibian community at wetlands near a major highway. There was also a positive relationship between species richness and distance to the highway, and a smaller uncertain relationship with proportion cover of native vegetation. Models that included both cover of native vegetation and distance to the highway had no relative importance on species richness based on multi-model inference. These results mirror those by Eigenbrod et al. (2008b) in that accessible habitat was a better predictor of amphibian species richness than total remnant habitat in the landscape. Had accessible habitat not been considered, based on effect sizes and credible intervals there would be the mistaken conclusion that there was no relationship between species richness and the amount of remnant native vegetation. This result leads me to reiterate the finding of Eigenbrod et al. (2008b) that failing to consider accessible habitat will result in studies of habitat loss and road effects that underestimate the effect of habitat availability on species richness. This result also highlights the importance of the availability of remnant natural wetland and terrestrial habitats at the landscape scale for maintaining amphibian diversity in rapidly-developing landscapes.
The positive relationships between accessible habitat and species richness and community composition infers that the highway is having a barrier-effect on species distributions in the study area, and that frog populations are likely being negatively affected by both habitat loss and roads. Mortality along the highway caused by passing traffic is likely contributing to this barrier effect; for example, a road-killed Litoria aurea was observed on the highway prior to the upgrade in 2012 (J. Stokes pers. comm.). There are two possibilities on how the highway is acting as a barrier. Either, mortality rates while crossing are so high that there is near-negligible exchange of individuals across the highway, or that individuals are deterred from attempting to cross the highway because of road traffic or unsuitable terrain for movement. Mortality rates of amphibians attempting to cross motorways can be >90 % (Hels and Buchwald 2001), and the probability of an amphibian being killed on a road increases with higher traffic volumes (Fahrig et al. 1995; Mazerolle 2004; Sutherland et al. 2010). The positive relationship between species richness and distance to the highway implies that there may be other road-related impacts on species distributions. Aside from barrier effects, there may be impacts from traffic noise and indirect road effects within a ‘road-effect zone’ (Trombulak and Frissell 2000; Eigenbrod et al. 2009). However, distance to the highway was strongly correlated with wetland type (Table S3), and so the relationship with species richness may be confounded by the stronger negative effect of concrete ponds.
Four species were positively associated with accessible habitat: two relatively large-bodied and vagile species (Limnodynastes peronii and Litoria aurea) and two small-bodied species (Crinia signifera and Litoria fallax). Litoria aurea has been recorded moving up to 5 km within the study area (A.J. Hamer pers. obs.) and is therefore likely to encounter roads while dispersing. Carr and Fahrig (2001) suggested that more vagile species may be more vulnerable to road mortality than less vagile species. Although underpasses for L. aurea have been installed at potential movement points along the highway (e.g., along drainages), it is unknown as to their effectiveness in facilitating movement under the highway. The wide usage of terrestrial habitats by Crinia signifera places it at particular risk of fragmentation effects (Baker and Lauck 2006); hence, its association with larger areas of intact remnant habitat. Negative impacts on Crinia signifera from the highway would likely occur due to a barrier effect. Large-bodied tree frogs that were not associated with accessible habitat (e.g., Litoria peronii) may be able to climb over fencing and access habitats on the far side of the highway. However, road-killed L. peronii have been observed on smaller roads in the study area and so some mortality would be expected if individuals attempt to cross the highway. It is therefore likely that the highway is acting as a filter for species movement rather than being a complete barrier.
There were clear relationships between landscape variables and species richness only when local habitat factors were also considered in models. This result underscores the importance of local habitat quality together with landscape habitat availability for amphibian communities. I found strong negative relationships between wetland type and water conductivity with both species richness and community composition. Concrete ponds constructed recently as replacement habitat for Litoria aurea were designed to exclude ground-dwelling frogs such as Limnodynastes peronii which have the potential to introduce chytrid fungus into wetlands (Stockwell et al. 2010). Pond age would likely exclude species according to their dispersal abilities and distance to source ponds, and there can be temporal variation in amphibian colonisation of replacement habitat near highways, indicating species-specific colonisation ability and habitat requirements (Lesbarrères et al. 2010). A negative association between amphibian communities and conductivity has been demonstrated in other modified landscapes (Hamer and Parris 2011; Ficken and Byrne 2013). In the study area, higher conductivity at wetlands in low-lying areas is likely the result of groundwater infiltration, whereas high conductivity in creeks likely results from road run-off. The results of the RDA showed contrasting relationships among the species pool in their response to local habitat variables, thereby highlighting the importance of using complementary measures of community diversity to account for species-specific habitat preferences within amphibian communities (Hamer and Parris 2011). For example, Litoria fallax was associated with highly-vegetated wetlands whereas L. verreauxii was not. The effects of landscape predictors in disturbed landscapes are generally much stronger and clearer than are predictors of local habitat, which are more likely to vary between species (Price et al. 2004; Pillsbury and Miller 2008; Smallbone et al. 2011). However in this study, wetland type was a much stronger predictor of species richness than landscape variables.
Although I aimed to include as many wetlands as possible within 1000 m of the highway, it was not possible to include all wetlands due to constraints on site access; consequently there was a strong positive correlation between the proportion of accessible habitat and native vegetation (r s = 0.81). However, despite the correlation, there was a slightly larger and clearer effect of accessible habitat on species richness. Many wetlands had overlapping buffers, so landscape predictor variables were not entirely independent. While this has the potential to reduce the statistical power to detect an effect (Eigenbrod et al. 2011), I still found positive effects of accessible habitat and distance to the highway on species richness in separate models. Furthermore, variation in predictor variables was high; site values of accessible habitat ranged from 0.00 to 0.93. There was no relationship between species richness and distance to the nearest wetland, which may be because not every wetland in the study area was sampled, so that a non-sampled wetland may have been located closer to a sampled wetland than its nearest neighbour.
Twenty seven (52 %) of the landscape buffers included the highway (c.f. 59 % in Eigenbrod et al. 2008b), so that accessible habitat within 1000 m of the road was measured from around half the total sample size. Within buffers that included the highway, and in the majority of buffers elsewhere, the dominant vegetation community was dry forest (Tozer et al. 2010). Therefore, the relationship observed between accessible habitat in proximity to the highway and species richness was derived on the amount of terrestrial habitat surrounding wetlands, furthermore underscoring the importance of non-breeding habitat for amphibian communities, which can be used for movement, shelter and foraging (Pope et al. 2000).
Finally, the present study was conducted in a peri-urban area containing a diverse selection of wetland types and other roads, whereas the Eigenbrod et al. (2008b) study was conducted in a rural area containing one motorway and where wetlands considered unsuitable for amphibian breeding were excluded (e.g., fish hatcheries). Had Eigenbrod et al. (2008b) included more wetland types the effect of accessible habitat they observed may have become less significant if, for instance, quarry sites containing fish had strong effects on species richness. This study also demonstrated a much clearer relationship between species richness and accessible habitat than with the density of roads in both urban and rural areas. Eigenbrod et al. (2008b) were unable to assess this relationship because there were no roads other than the highway within 1000 m of their sampling ponds with traffic volumes that would present a barrier to amphibian movement.
Management implications
While the results of this study suggest that the highway is a near-complete barrier for movement by some frog species, it is unclear whether the underpasses that were installed as road mitigation are functioning as intended. For instance, although they were installed during road construction within 6 months of fieldwork commencing, there may be a time lag until they are used effectively by some species. Hence, accessible habitat was calculated as if underpasses were not facilitating frog movement, and so the highway was still considered to be a barrier. It is probable that the highway was historically a barrier to frog movement prior to the highway upgrade, as pre-upgrade traffic volumes would be comparable to those experienced post-construction despite the increased road width and speed limit. Time lags in the response of some species to road construction may further complicate our ability to detect road effects on species (Findlay and Bourdages 2000).
By assessing the effects of a major road together with local habitat variables, this study demonstrated that maintaining viable frog populations close to a highway should focus on conserving wetland and terrestrial habitats that can be accessed through movement by a range of species. The ability of species to access these breeding and non-breeding habitats will be dependent on the creation or maintenance of functional connectivity to ensure regional persistence of species populations in areas bisected by roads.
References
Adams MJ, Richter KO, Leonard WP (1997) Surveying and monitoring amphibians using aquatic funnel traps. In: Olson DH, Leonard WP, Bury RB (eds) Sampling amphibians in lentic habitats. Northwest Fauna Number 4, Society for Northwestern Vertebrate Biology, Olympia, pp 47–54
Allen GR, Midgley SH, Allen M (2003) Field guide to the freshwater fishes of Australia. Western Australian Museum, Perth
Anstis M (2002) Tadpoles of south-eastern Australia: a guide with keys. Reed New Holland, Sydney
Australian Bureau of Statistics (2015) ABS.Stat beta: Nowra (SA2). http://stat.abs.gov.au. Accessed 12 Oct 2015
Baker S, Lauck B (2006) Association of common brown froglets, Crinia signifera, with clearcut forest edges in Tasmania, Australia. Wildl Res 33:29–34
Becker CG, Fonseca CR, Haddad CFB, Batista RF, Prado PI (2007) Habitat split and the global decline of amphibians. Science 318:1775–1777
Beebee TJC (2013) Effects of road mortality and mitigation measures on amphibian populations. Conserv Biol 27:657–668
Brand AB, Snodgrass JW (2010) Value of artificial habitats for amphibian reproduction in altered landscapes. Conserv Biol 24:295–301
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Bureau of Meteorology (2015) Climate statistics for Australian locations: Nowra RAN Air Station. http://www.bom.gov.au/climate/averages/tables/cw_068076.shtml. Accessed 12 Oct 2015
Carr LW, Fahrig L (2001) Effect of road traffic on two amphibian species of differing vagility. Conserv Biol 15:1071–1078
Clarke KR, Gorley RN (2001) PRIMER v5: user manual/tutorial. PRIMER-E, Plymouth
Cosentino BJ, Marsh DM, Jones KS, Apodaca JJ, Bates C, Beach J, Beard KH, Becklin K, Bell JM, Crockett C, Fawson G, Fjelsted J, Forys EA, Genet KS, Grover M, Holmes J, Indeck K, Karraker NE, Kilpatrick ES, Langen TA, Mugel SG, Molina A, Vonesh JR, Weaver RJ, Willey A (2014) Citizen science reveals widespread negative effects of roads on amphibian distributions. Biol Conserv 180:31–38
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Daly G (2014) From rags to riches and back again: fluctuations in the green and golden bell frog Litoria aurea population at Nowra on the south coast of New South Wales. Aust Zool 37:157–172
Eigenbrod F, Hecnar SJ, Fahrig L (2008a) The relative effects of road traffic and forest cover on anuran populations. Biol Conserv 141:35–46
Eigenbrod F, Hecnar SJ, Fahrig L (2008b) Accessible habitat: an improved measure of the effects of habitat loss and roads on wildlife populations. Landscape Ecol 23:159–168
Eigenbrod F, Hecnar SJ, Fahrig L (2009) Quantifying the road-effect zone: threshold effects of a motorway on anuran populations in Ontario, Canada. Ecol Soc 14:24. http://www.ecologyandsociety.org/vol14/iss1/art24/
Eigenbrod F, Hecnar SJ, Fahrig L (2011) Sub-optimal study design has major impacts on landscape-scale inference. Biol Conserv 144:298–305
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L, Pedlar JH, Pope SE, Taylor PD, Wegner JF (1995) Effect of road traffic on amphibian density. Biol Conserv 73:177–182
Fahrig L, Rytwinski T (2009) Effects of roads on animal abundance: an empirical review and synthesis. Ecol Soc 14:21.http://www.ecologyandsociety.org/vol14/iss1/art21/
Ficken KLG, Byrne PG (2013) Heavy metal pollution negatively correlates with anuran species richness and distribution in south-eastern Australia. Aust Ecol 38:523–533
Findlay CS, Bourdages J (2000) Response time of wetland biodiversity to road construction on adjacent lands. Conserv Biol 14:86–94
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–231
Gagné SA, Fahrig L (2007) Effect of landscape context on anuran communities in breeding ponds in the National Capital Region, Canada. Landscape Ecol 22:205–215
Gibbs JP (1998) Distribution of woodland amphibians along a forest fragmentation gradient. Landscape Ecol 13:263–268
Glista DJ, DeVault TL, DeWoody JA (2008) Vertebrate road mortality predominantly impacts amphibians. Herpetol Conserv Biol 3:77–87
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815
Guerry AD, Hunter ML (2002) Amphibian distributions in a landscape of forests and agriculture: an examination of landscape composition and configuration. Conserv Biol 16:745–754
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Conserv 141:2432–2449
Hamer AJ, Parris KM (2011) Local and landscape determinants of amphibian communities in urban ponds. Ecol Appl 21:378–390
Hamer AJ, Langton TES, Lesbarrères D (2015) Making a safe leap forward: Mitigating road impacts on amphibians. In: van der Ree R, Smith DJ, Grilo C (eds) Handbook of road ecology. Wiley, Chichester, pp 261–270
Hartel T, Schweiger O, Öllerer K, Cogalniceanu D, Arntzen JW (2010) Amphibian distribution in a traditionally managed rural landscape of Eastern Europe: probing the effect of landscape composition. Biol Conserv 143:1118–1124
Heard GW, Scroggie MP, Malone BS (2012) Classical metapopulation theory as a useful paradigm for the conservation of an endangered amphibian. Biol Conserv 148:156–166
Hels T, Buchwald E (2001) The effect of road kills on amphibian populations. Biol Conserv 99:331–340
Jackson SD, Langen TA, Marsh DM, Andrews KM (2015) Natural history and physiological characteristics of small animals in relation to roads. In: Andrews KM, Nanjappa P, Riley SPD (eds) Roads and ecological infrastructure. Concepts and applications for small animals. Johns Hopkins University Press, Baltimore, pp 21–41
Knutson MG, Sauer JR, Olsen DA, Mossman MJ, Hemesath LM, Lannoo MJ (1999) Effects of landscape composition and wetland fragmentation on frog and toad abundance and species richness in Iowa and Wisconsin, U.S.A. Conserv Biol 13:1437–1446
Kruger DJD, Hamer AJ, Du Preez LH (2015) Urbanization affects frog communities at multiple scales in a rapidly developing African city. Urban Ecosyst 18:1333–1352
Lesbarrères D, Fowler MS, Pagano A, Lodé T (2010) Recovery of anuran community diversity following habitat replacement. J Appl Ecol 47:148–156
Marsh DM, Jaeger JAG (2015) Direct effects of roads on small animal populations. In: Andrews KM, Nanjappa P, Riley SPD (eds) Roads and ecological infrastructure. Concepts and applications for small animals. Johns Hopkins University Press, Baltimore, pp 42–56
Mazerolle MJ (2004) Amphibian road mortality in response to nightly variations in traffic intensity. Herpetologica 60:45–53
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, Cambridge
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Parris KM (2006) Urban amphibian assemblages as metacommunities. J Anim Ecol 75:757–764
Pellet J, Guisan A, Perrin N (2004) A concentric analysis of the impact of urbanization on the threatened European tree frog in an agricultural landscape. Conserv Biol 18:1599–1606
Pellet J, Schmidt BR (2005) Monitoring distributions using call surveys: estimating site occupancy, detection probabilities and inferring absence. Biol Conserv 123:27–35
Pillsbury FC, Miller JR (2008) Habitat and landscape characteristics underlying anuran community structure along an urban–rural gradient. Ecol Appl 18:1107–1118
Pope SE, Fahrig L, Merriam HG (2000) Landscape complementation and metapopulation effects on leopard frog populations. Ecology 81:2498–2508
Price SJ, Marks DR, Howe RW, Hanowski JM, Niemi GJ (2004) The importance of spatial scale for conservation and assessment of anuran populations in coastal wetlands of the western Great Lakes, USA. Landscape Ecol 20:441–454
Pyke GH, White AW (2000) Factors influencing predation on eggs and tadpoles of the endangered green and golden bell frog Litoria aurea by the introduced Plague Minnow Gambusia holbrooki. Aust Zool 31:496–505
Rytwinski T, Fahrig L (2012) Do species life history traits explain population responses to roads? A meta-analysis. Biol Conserv 147:87–98
Rytwinski T, Fahrig L (2015) The impacts of roads and traffic on terrestrial animal populations. In: van der Ree R, Smith DJ, Grilo C (eds) Handbook of road ecology. Wiley, Chichester, pp 237–246
Smallbone LT, Luck GW, Wassens S (2011) Anuran species in urban landscapes: relationships with biophysical, built environment and socio-economic factors. Landsc Urban Plan 101:43–51
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc B 64:583–639
Spiegelhalter D, Thomas A, Best N, Lunn D (2007) Open BUGS user manual. Version 3.0.2. MRC Biostatistics Unit, Cambridge
Stockwell MP, Clulow J, Mahony MJ (2010) Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Anim Conserv 13(Suppl. 1):62–71
Sutherland RW, Dunning PR, Baker WM (2010) Amphibian encounter rates on roads with different amounts of traffic and urbanization. Conserv Biol 24:1626–1635
ter Braak CJF (2000) Ordination. In: Jongman RHG, ter Braak CJF, van Tongeren OFR (eds) Data analysis in community and landscape ecology. Cambridge University Press, Cambridge, pp 91–173
ter Braak CJF, Šmilauer P (1998) CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination (version 4). Microcomputer Power, Ithaca
Tozer MG, Turner K, Keith DA, Tindall D, Pennay C, Simpson C, MacKenzie B, Beukers P, Cox S (2010) Native vegetation of southeast NSW: a revised classification and map for the coast and eastern tablelands. Cunninghamia 11:359–406
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Wintle BA, Elith J, Potts JM (2005) Fauna habitat modelling and mapping: a review and case study in the Lower Hunter Central Coast region of NSW. Aust Ecol 30:719–738
Acknowledgments
I thank Josie Stokes and Shaun Walsh (New South Wales Roads and Maritime Services) for financial and logistical assistance; JS also provided valuable comments on the manuscript. Lee Harrison, Rodney Wattus, Briony Mitchell, Joanne Ainley and Caroline Wilson assisted with fieldwork. Rodney van der Ree provided support with initial planning of the study. Amy Hahs provided GIS support and helpful comments on the manuscript. I thank the landowners who kindly assisted with access to their properties. Phil Craven (NSW National Parks and Wildlife Service) provided information on local site conditions prior to fieldwork. Steve Swearer and James Shelley provided assistance with fish identification. The Baker Foundation provided additional support. This study was conducted under NSW National Parks and Wildlife Service scientific licence number SL101064, and NSW Department of Primary Industries animal ethics approval number 12/4900.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamer, A.J. Accessible habitat delineated by a highway predicts landscape-scale effects of habitat loss in an amphibian community. Landscape Ecol 31, 2259–2274 (2016). https://doi.org/10.1007/s10980-016-0398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-016-0398-2