Abstract
Multicomponent cobalt phosphate–silicate glasses from P2O5–SiO2–K2O–MgO–CaO–Co2O3 system in which molar ratio of P2O5 to SiO2 is 41:6 and cobalt ions are gradually incorporated at the expense of magnesium and calcium ions were prepared via melt-quenched technique. The obtained amorphous solids were subjected to the thermal and spectroscopic studies and density measurements in order to gain information about the structure and physical properties. As DSC, XRD, FTIR and pycnometry techniques were applied, the glass transition temperature, specific heat change, crystallization temperature, density, molar volume, oxygen packing density and associated infrared spectra were collected for each glass. Then, the process of induced crystallization was performed on the selected glass samples. The related glass-crystalline materials were evaluated via XRD method. As a result, crystalline phases associated with crystallization temperature were identified. The alterations in determined parameters, stoichiometry of connected crystalline phases and features of infrared spectra allow to conclude the impact of cobalt ions on structure of analyzed glasses due to the direct dependence of structure, physical and thermal properties. The conducted research constitutes a basis for further analysis of cobalt phosphate–silicate glasses and makes a contribution to the knowledge concerning the phosphate glasses family.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since 1970, numerous studies were conducted on phosphate glasses. The results revealed the outstanding flexibility in terms of chemical composition alterations and remarkable variation of properties. Therefore, the tremendous potential for their application due to a wide range of possible adjustments was implied. The example applications include water softeners, waste storages, sealants, laser host materials, solid electrolytes for battery applications, subjects for biomedical applications. It supports the statement of the relevance of the research dedicated to this subject [1]. The favorable features stem from the material’s structure analyzable via not only spectroscopic methods—an insight can be also provided through the results of thermal analysis and density measurements.
The glasses altered by incorporation of calcium, magnesium, potassium and cobalt ions into the phospho-oxygen and silico-oxygen subnetwork are the subject of this study. The chemical composition of materials is derived from the attempt of preparing the eco-friendly fertilizer, which may be the solution for the environmental problems stemmed from the cultivation of the soil prior to obtaining the required crop. Particularly, the incorrect usage of mineral fertilizers may lead to the overdosing of nutrient elements. It has a negative impact on the plants and also leads to the distortion of a geochemical balance in natural environment. The possible solution is the substitution of conventional fertilizers by the aforementioned glassy fertilizers with lower solubility in water and controlled release of elements necessary for healthy growing of plants. Phosphorus, silicon, potassium, magnesium, calcium, and cobalt are recognized as important nutrient elements and their compounds were used in the process of acquiring the multicomponent glasses which are the subject of this study. In this glassy system, cobalt ions are introduced at the expense of magnesium and calcium ions. P2O5:SiO2:K2O and MgO:CaO molar ratios are remaining constant and the associated proportions are 41:6:6 and 1.5:1, respectively. The main network-forming compounds are SiO2 and P2O5 and based on them, the phosphorus and silica tetrahedra are formed.
The glasses can be classified as polymeric and non-polymeric [2]. Within the polymeric group, one can include: the discontinues polymeric structure where polyhedrons are joint into chains and rings (named as metaphosphates by Van Wazer [3]) as well as glasses with continues spatial network or cross-linked structure (ultraphosphate glasses). The second group (non-polymeric structure glasses) comprises polyhedrons combined into pairs (i.e., pyrophosphate units) and isolated tetrahedra (i.e., orthophosphate units). In the above-mentioned classification, inorganic glasses structure is described in terms of long range. Short-range information is provided along with the consideration about basic building element of the structure—tetrahedron, and the first shell of its neighboring groups. Within Qs notation, where s indices exhibit the number of bridging oxygens, we may distinguish singular Q0, primary Q1, secondary Q2, tertiary Q3 or quaternary tetrahedron Q4 [4]. Simultaneously, basic structural groups were assigned to monosilicates (Q0), disilicates (Q1), middle groups in chains (Q2), chain branching sites (Q3), and the three-dimensional cross-linked framework (Q4) [5, 6]. This notation was also adopted to phosphate glasses. For the purpose of this paper, Q0 is assigned to orthophosphates (PO4)3− with oxygen to phosphorus molar ratio [O]/[P] = 4, Q1 to pyrophosphates (PO3)2− with [O]/[P] = 3.5 and Q2 to metaphosphates (PO2)− with [O]/[P] = 3 [3].
The parameters designated via DSC (glass transition temperature (Tg), associated specific heat change (∆cp), crystallization temperature (Tc)) and density measurements (molar volume (Vmol) and oxygen packing density (do)) are also useful as structural indicators. Particularly, the subtle connection between Tg, ∆cp, Vmol, do parameters, the field strength of modifier ions and the ionicity of oxygen–cation bond allows to acquire an insight with regards to network connectivity, elasticity and also the level of network polymerization degree. The specific relations between Tg, ∆cp, Vmol, do parameters are also combined with the identification of phases associated with Tc through the process of induced crystallization characterization. These allow to make conclusions about the structure of analyzed glasses and are further discussed in this study. Nevertheless, glass transition temperature by itself is also one of the most important thermal properties of phosphate glasses [1] for example in industrial manufacturing when their preparation is considered.
The aim of this work is to characterize the structure of the studied phosphate–silicate glasses and to determine the role of cobalt ions in the analyzed glass system.
Experimental procedure
The glasses from P2O5–SiO2–K2O–MgO–CaO–Co2O3 system with chemical composition calculated and presented per oxides (Table 1) were prepared via melt-quenched technique. As it has been already mentioned, the molar ratios of certain components within all samples were set as constant and the values are: 41:6 for P2O5:SiO2 and 1.5:1 for MgO:CaO. Batches were prepared through mixing pure raw materials in a ceramic mortar with a pestle up to the desirable homogeneity, melting in a ceramic crucible at 1200 °C in air atmosphere in the electrical furnace, and quenching by pouring on a steel plate with preservation of the similar thermal history of materials. Amorphousness was confirmed via X-ray diffraction analysis. Homogeneity was evaluated by means of SEM microscopy. The observations were performed on the FEI Nova NanoSEM 200 scanning electron microscope with the low vacuum detector (LVD) and the accelerated voltage was 18 kV.
Thermal properties were examined by means of differential scanning calorimetry. DSC curves were designated using Netzsch STA 449 F3 Jupiter 7 operating in the heat flux DSC mode. The calibrations of the instrument were executed via the melting temperatures and melting enthalpies of high-purity materials (Al, Zn, Sn, Au, Ag). The samples (45 mg) were heated in platinum crucibles at a rate of 10 °C min−1 in air atmosphere up to 900 °C.
The glass transition temperature values were designated as the midpoint of the specific heat changes in the glass transition regions. The crystallization temperatures were ascertained at the maximum deflection point of the exothermal effect. Further data were determined by applying the Netzsch Proteus Thermal Analysis Program (version 6.0.0.)
The crystallization temperatures deduced from the DSC measurements were used and the 12 h-lasting isothermal heating process was performed on selected glass samples grinded to the size of 0.1–0.3 mm. The devitrificates were submitted to the X-ray diffraction analysis (X'Pert Pro, Empyrean, Panalytical) for the purpose of their identification.
The density measurements were conducted using Micromeritics AccuPyc II 1340 Gas Pycnometer apparatus by helium pycnometry technique with the accuracy of 0.0001 g/cm3. It was preceded by the purging with helium for removal of the impurities and temperature and volume stabilization.
FTIR spectra were acquired using a Bruker Company Vertex 70v spectrometer in room temperature within range of 1400–400 cm−1. 128 scans at the 4 cm−1 resolution were accumulated. Sample preparation was performed using standard KBr pellet method. The glass sample and KBr were precisely weighed.
Local coordination of phosphorus atoms was probed by 31P MAS NMR spectra recorded using Bruker Avance III 500.13 MHz (11.7 T) spectrometer with a resonance frequency of 202.5 MHz. Samples were packed to 4 mm rotor and spun at a speed of 10 kHz. The spectra were recorded using π/2 pulse (5 µs) and referenced to H3PO4.
Results and discussion
Preliminary evaluation of the cobalt phosphate–silicate glasses—verification of the homogeneity and amorphousness
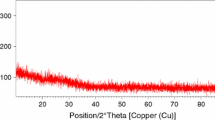
The obtained materials were verified via X-ray diffraction technique (XRD) in order to confirm the amorphousness. The results are presented in Fig. 1a. The presence of any crystalline phases was not detected.
In order to confirm the absence of the phase segregation the selected glasses were analyzed via SEM microscopy. The fracture surfaces under 170 × , 1000 × and 10,000 × magnifications are presented in Fig. 1b. The observed discontinuities are due to the mechanical damages occurred during samples preparation. The amorphous phase segregation was not observed.
Glass transition
DSC curves and designated thermal parameters are presented in Fig. 2 and Table 2, respectively. The results are typical for glasses—endothermic peaks associated with glass transition and exothermic peaks attributed to crystallization processes are recognized. In general, Tg and Tc are influenced by chemical composition [7]. With gradual cobalt ions incorporation at the expense of MgO and CaO, Tg slightly increases along with change of specific heat (Δcp) from 514 to 522 °C and from 0.394 to 0.483 J/(g K), respectively. Simultaneously, the decrease in Tc and thermal stability (∆T) calculated via Eq. (1) [8] is observed.
The transformation of glass from solid rigid body to viscoelastic state with viscosity of 1013.3 dPas is accompanied by the relaxation of internal structural strains. With increase in internal stresses, the increase in Tg and Δcp parameters is expected. Moreover, this process is strongly affected by chemical composition. Due to the mentioned dependency, further implications are established by considering the differences in ionicity of the particular cation–oxygen bond. The values determined by Görlich are applied; however, this ionicity scale is well correlated with the Pauling’s [7, 9, 10] (Table 3).
The ionicity values indicate the elasticity of the glass network—an important feature in terms of the preservation of highly polymerized network. Taking it into consideration, the exchange of ions pursued in analyzed glasses favors more covalent cobalt–oxygen bonds with lower iG and higher L parameters. This implies more of less elastic chemical bonds and therefore the increase in the rigidity of the structure is observed [9]. Thus, the internal strains increase and it also leads to the observation of the increase in Δcp and Tg. It is consistent with the obtained results.
Changes occurring in cobalt phosphate–silicate glasses after the process of induced crystallization
Upon the implementation of cobalt ions the crystallization temperature decreases along with the thermal stability. Thus it is stated that the proceeding changes in chemical composition of glasses lead to increase in susceptibility to crystallization (Table 2). The further analysis of XRD patterns of 4Co41P, 8Co41P and 15Co41P-related glass-crystalline samples obtained through the process of induced crystallization revealed that the crystalline phases associated with exothermic effects observed on DSC curves are CaMgP2O7 (JCPDS no. 42-0135), Co(PO3)2 (JCPDS no. 27-1120) and α-Co2P2O7 (JCPDS no. 49-1091). The results are shown in Fig. 3.
As it is presented on obtained XRD patterns and highlighted on its fragment within the range from 29° to 32° after subjecting it to the deconvolution, the Co(PO3)2 crystalline phase occurs only in reference to 4Co41P-related glass-crystalline sample, whereas in 8Co41P and 15Co41P-related samples only CaMgP2O7 and α-Co2P2O7 coexist.
Upon the implementation of cobalt ions at the expense of magnesium and calcium ions the increase in the peak intensity associated with α-Co2P2O7 with regards to that of CaMgP2O7 is observed. It needs to be noted that XRD pattern of α-Co2P2O7 resembles similarities to α-Mg2P2O7 (JCPDS no. 32-0626). Moreover, these two phases can easily form solid solution Co2−xMgxP2O7 [11]. Therefore, the occurrence of solid solution Co2P2O7–Mg2P2O7 is probable, especially in case of glasses with lower content of cobalt. Presumably, the decrease in number of cobalt ions in the structure of phosphate–silicate glasses contributes to the increase in “x” factor within stoichiometry of crystalized Co2−xMgxP2O7 phase. Llusar et al. [11] indicated that cell volume decrease linearly along with the increase in the number of magnesium ions and therefore the increase in x-factor in the Co2−xMgxP2O7. Taking into consideration the XRD results presented in Fig. 3, the values of d-spacing associated with the most intense peak (012) of Co2−xMgxP2O7 are 3.0172, 3.0148, and 3.0094 Å for 15Co41P, 8Co41P, and 4Co41P-derived crystalline samples. According to the reference XRD patterns the d-spacing for the aforementioned the most intense peak (012) are 3.0213 and 3.0080 Å for α-Mg2P2O7 (JCPDS no. 32-0626) and α-Co2P2O7 (JCPDS no. 49-1091), respectively. Thus, the positions of the (012) peak in the 15Co41P, 8Co41P, and 4Co41P samples are between α-Mg2P2O7 and α-Co2P2O7, and the d-spacing is increasing with the increase in the content of cobalt ions in the sample. This observation is consistent with the tendencies present in the solid solution Co2−xMgxP2O7 [11].
It is noted that Co(PO3)2 may resemble a more polymeric structure, whereas CaMgP2O7 and Co2P2O7 phases are rather connected with presence of Q1 units. Therefore, the change of the type of detected crystallize phases suggests the gradual depolymerization upon cobalt ions incorporation.
Results of density measurements and associated parameters
Selected samples were subjected to the further measurements and accurately designated density values (dr) are: 2.7123 ± 0.0032 g cm−3 (4Co41P), 2.8027 ± 0.0049 g cm−3 (8Co41P), 2.9197 ± 0.0030 g cm−3 (15Co41P), 3.0399 ± 0.0026 g cm−3 (20Co41P), and 3.1499 ± 0.0043 g cm−3 (25Co41P). Subsequently, molar volume (Vmol) and oxygen packing density (do) were calculated according to formulas 2 and 3. The summary of obtained data is presented in Fig. 4 and Table 4.
where dr is experimentally determined density, \(x_{{{\text{MO}}}}\) and \(M_{{{\text{MO}}}}\) are molar fraction and the molecular weight of the oxide, Nu is the number of the structural units per mole of glass and Vu is the volume of the structural units, respectively [12].
where \(m_{{\text{o}}}\) is the mass of oxygen atoms per mole of glass.
The subtle trend toward higher values as a consequence of the gradual cobalt ions incorporation was revealed. In reference to the dr it is due to the differences of molecular weight between magnesium, calcium and cobalt ions. Simultaneously, the changes in values of Vmol parameter usually are opposite to the alterations of dr parameter. As such tendency was not observed, the behavior of the analyzed glasses is an anomaly. However, the similar relations have been already stated for example in (100 − 2x)TeO2−xAg2O–xWO3 glass system [13].
Following [12], the number and the type of the units and related structural features are manifested via Vmol parameter. Particularly, the more depolymerized the glass network is, the higher volume of structural units is anticipated [12]. Thus, the results indicate an increase in the number of nonbridging oxygens (NBOs) [14]. In effect, phosphate–silicate glass network becomes more expanded [14]. Along with the formation of NBOs, the structure also becomes more open [13].
As the structural unit volume (Vu) is significantly influenced by the chemical composition, the second approach stemmed from the compositional variations between analyzed glasses is provided. In detail, Vu is strongly network modifier-dependent. This relation is expressed more clearly when ion field strength (Z/a2 (Z—valency, a—ionic distance for oxides [Å] [15])) is considered. Particularly, the Vu parameter is expected to decrease along with higher (Z/a2) values due to the increase in the attractive force between modifier and involved non-bridging oxygen [12].
Based on [16], the Z/a2 parameter for related cobalt ions is higher compared to the magnesium and calcium ions. The specific values vary within the type of coordination number (CN) and are presented as follows (4).
Following this consideration, the increase in Z/a2 parameter along with the gradual incorporation of cobalt ions at the expense of magnesium and calcium ions should be associated with the decrease in value of Vu parameter and presumably contribute to the decrease in value of Vmol parameter. However, a slight increase in value of Vmol parameter is observed. Thus, the effect of increase in the number of NBOs and its structural implications prevailed. It may imply that cobalt ions above all act as the structural modifiers in the studied glasses.
The increase in molar volume is also associated with increase in oxygen packing density. do is defined as mass of oxygen atoms per mole of glass. The increase in do implies that the glass network is more rigidly bonded [17], in other words, more tightly packed [13] which is consistent with the subtle increase in Tg values.
Fourier transform infrared spectroscopy results obtained at ambient temperature
Infrared spectroscopy is an invaluable technique, especially in view of the assessment of the structural features of glasses. Through the position and shape of bands present in each spectrum the information about basic groups forming the phosphate–silicate glasses network can be acquired. The characteristic bands are revealed after recording spectra collected at room temperature (Fig. 5).
In terms of chemical composition analyzed cobalt phosphate–silicate glasses are alike to iron phosphate–silicate glasses presented in [8, 18, 19]. The MIR data provided in [18 (Fig. 1)] and the spectra presented in Fig. 5 also exhibit similarities. The extensive research dedicated to iron phosphate–silicate glasses [8, 18, 19] confirmed the increase in the number of P-NBOs and progressive depolymerization along with the substitution of CaO and MgO with Fe2O3. Particularly, it was supported by the evaluation of the changes observed on the MIR spectra, which occurred due to the nonbridging and bridging oxygens oscillations and allowed to recognize the aforementioned structural alterations.
The complex interpretation of the infrared spectra of cobalt phosphate–silicate glasses and concluded structural differences between analyzed cobalt phosphate–silicate glasses has been provided below. The 1400–1200 cm−1, 1200–850 cm−1, 850–650 cm−1, 650–400 cm−1 characteristic regions have been highlighted.
1400–1200 cm−1
In the spectrum of 4Co41P glass the absorption at 1270 cm−1 is shown. Upon addition of cobalt ions a negative frequency shift to 1266 cm−1 (8Co41P) and simultaneous loss of its distinctiveness occurred. Finally, the considered band is less resolved (15Co41P) and it even cannot be precisely recognized (25Co41P). Following the [18], asymmetric stretching vibrations of nonbridging oxygen atoms bonded to phosphorus atoms (O–P–O) in PO−2 groups affiliated to metaphosphate units (νas (O–P–O) in Q2 units) is proposed as its origin. The decrease in visibility suggests that the incorporation of cobalt ions disrupts the mentioned bonds. The following interpretation indicates the gradual depolymerization within phospho-oxygen subnetwork.
1200–950 cm−1
The spectrum of 4Co41P glass also features with the bands at 1107 cm−1 and 1004 cm−1. Upon the incorporation of cobalt ions the position of these bands remains relatively stable. As absorption at 1107 cm−1 is considered, it is also indicated in 25Co41P sample, where the spectrum is more diffused and featureless compared to low-Co contained phosphate–silicate glasses. Finally, only a broad band at ca. ~ 1104 cm−1 is observed. As absorption at ~ (1270–1266) cm−1 is assigned to νas (O–P–O) in Q2 units, the origin of the bands at (1107–1100) cm−1 and at (1004–996) cm−1 is more complex.
As the SiO2–P2O5 system was studied in [20], absorption at ~ 1100 cm−1 origins from the stretching vibrations of P–O, P–O–P and P–O–Si bridging units. However, the analysis of xP2O5(1−x)SiO2 glasses (x = 0, 12.5, 25, 30, 50, 70, 81, 100%) presented in [21] indicated that the band is associated with P-O-Si vibrations. Therefore, it can be stated that in analyzed phosphate–silicate glasses three possible types of network-forming linkages are expected: P–O–P, Si–O–P, Si–O–Si. The similar assignment was carried out for ZnO- and CuO-doped phosphate–silicate glasses, particularly the combination of P–O and Si–O stretching vibration occurring in P–O–P and P–O–Si linkages in metaphosphate units was pointed out [22].
Nevertheless, based on the example of ZnO and BaO metaphosphate glasses, when Q2 units prevail the P-NBO oscillations are manifested in 1000–1150 cm−1 region [1]. Thus, as it is also concluded in [23], the assignment to the stretching vibrations of PO2 groups in Q2 units is presumably correct.
Moreover, in case of iron phosphate–silicate glasses, particularly 15Fe41P and 30Fe41P glass exhibit the broaden, featureless spectra. The band located at around 1093 cm−1 (15Fe41P) was recognized to be due to the asymmetric stretching vibrations in pyrophosphate units vas(P2O7)4− [18]. Additionally, [24] assigned the band observed at ~ 1100 cm−1 on the infrared spectra of 10Li2O-(40−x)ZnO-xCoO-50P2O5 glasses to asymmetric vibrations of the chain end groups associated with Q1 units.
It is possible, that the oscillations of P–O and Si–O in P–O–P and P–O–Si linkages and P-NBOs in phosphate units in phosphate sub-lattice may be represented by bands which are co-occurring on infrared spectra in the considered region. Thus, it was decided not to exclude any of the mentioned interpretations.
A small inconspicuous band position at ~ (1004–996) cm−1 was also indicated. It is presumably associated with symmetric stretching vibrations of PO3 end-chain groups [25,26,27].
950–650 cm−1
In the aforementioned region in the spectra of the phosphate glasses the main observed bands origins from the stretching vibrations of P–O–P linkages [1, 28]. Particularly in [1] there is a distinction that asymmetric stretching vibrations are within 950–850 cm−1, whereas symmetric modes are observed at 790–690 cm−1 frequency range. This is reflected on the obtained spectra. Particularly, the position of the bands associated with νas P–O–P in spectra of 4Co41P and 25Co41P glasses are relatively similar (922 and 927 cm−1), however, in comparison with results obtained in reference to 8Co41P and 15Co41P samples a slightly different tendency is noted (νas P–O–P at 914 cm−1). In case of the second band at 752 cm−1 (4Co41P) indicated position is even less influenced; however, the alterations of the shape allow the further assignment of the structural groups, which has been carried out below.
Thus, in the spectra of 4Co41P glass at 700–800 cm−1 frequency range, similarly to the 4Fe41P glass analyzed in [18], it can be concluded that two superimposed bands are present. The similar effects were obtained in [26, 27] works dedicated to the vanadium barium phosphate glasses and sodium titanophosphate glasses research, respectively. It was stated that this is related to the presence of the two P–O–P linkages per (P2O6)2− group associated with Q2 units. Upon the cobalt ions implementation the disruption of (P2O6)2− units is progressing and it is reflected through singular and sharper band at ~ 750 cm−1. Following [18], the band visible on 8Co41P, 15Co41P and 25Co41P spectra is presumably associated with symmetric stretching vibrations of P-O-P bridges in pyrophosphate (P2O7)4− units [26, 29].
650–400 cm−1
Within considered region the gradual tendency to bands sharpening is revealed upon the incorporation of cobalt ions. The observed bands are generally associated with various bending vibrations [22]. Particularly, a combination of bending vibrations of O–Si–O and O–P–O bonds [22], as well as bending vibrations of P–O bonds [26] and the deformation mode of P–O–(PO43−) groups [27], and harmonics of bending oscillations of O = P–O linkages [18] are possible explanations of their occurrence. It may be concluded that presumably phospho-oxygen and silico-oxygen subnetworks are reflected in this region. It may indicate that the progressive changes in chemical composition influence not only phosphor-oxygen but also silico-oxygen subnetwork.
31P MAS NMR data conducted on 4Co41P glass
The 31P MAS NMR analysis for 4Co41P glass has been conducted in order to confirm the correctness of the performed infrared spectra analysis. The characteristic parameters along with the spectrum itself are presented in Table 5 and Fig. 6.
The surrounding of phosphorus atoms and the type of the phosphorus-oxygen units are revealed. The first component band characterized by chemical shift value as − 25 ppm is associated with Q2 and the second one (− 11 ppm) is due to Q1 phosphorus-oxygen structural units [3, 22, 30]. The relative amounts of Qn sites are designated through the calculated area of associated bands. The aforementioned data validate that the 4Co41P glass was justifiably implied to be above all the type of metaphosphate structure. Nevertheless, the occurrence of component band connected to the pyrophosphate units also acknowledges the previously indicated premise.
Regarding the elaboration on infrared spectra analysis, the 31P MAS NMR data suggest the assignment of the well recognizable, distinct band at 1107 cm−1 to be due to stretching vibrations of PO2 groups in Q2 units for 4Co41P glass. As it can be observed along with the incorporation of cobalt ions, a negative frequency shift can be concluded simultaneously with broadening observed in this region. A change in position to lower wavenumbers and the already acquired knowledge regarding the similar glass family (Zn, Cu, and Fe41P phosphate–silicate glasses [18, 22]) the band at around 1100 cm−1 in case of 25Co41P is rather attributed to asymmetric vibrations of the chain end groups associated with Q1 units, similarly as it was stated for iron phosphate–silicate glasses containing 15%mol and 30%mol Fe2O3.
Conclusions
Through the establishing of the tendency of changes in values of Tg, ∆cp, Vmol, do parameters and the identification of phases associated with Tc an insight into the multicomponent phosphate–silicate glasses structure was acquired. It supports that the applied techniques are useful in view of the analysis of structural aspects of glasses.
Upon the implementation of cobalt ions at the expense of magnesium and calcium ions the increase in number of NBOs and rigidity are stated. Particularly, the depolymerization was concluded via the alterations of Vmol, observed infrared spectra and stoichiometry of associated crystalline phases obtained through the process of induced crystallization. The increase in rigidity was stated through changes of Tg, ∆cp, do parameters.
The conducted research indicated the increase in the number of P-NBOs along with the incorporation of cobalt ions at the expense of magnesium and calcium ions. Thus, the depolymerizing effect prevails. Therefore, the implemented cobalt ions act above all as modifiers.
References
Musgraves JD, Hu J, Calvez L. Springer handbook of glass. Chapter 16: Phosphate Glasses by F. Muñoz, J. Rocherullé, I. Ahmed, L. Hu. Springer Nature Switzerland AG; 2019. p. 553–94.
Stoch L, Aboud T. Structural classification of phosphate glasses. Ceramics. 1993;43:267–75.
Brow RK. Review: the structure of simple phosphate glasses. J Non-Cryst Solids. 2000;263&264:1–28.
Liebau F. Structure and Bonding in crystals. In: O'Keeffe M, Navrotsky A, editors. Chapter 13: The influence of cation properties on the conformation of silicate and phosphate anions. Industrial Chemistry Library, Elsevier; 1981. p. 198.
Lippmaa E, Maegi M, Samoson A, Engelhardt G, Grimmer AR. Structural studies of silicates by solid-state high-resolution silicon-29 NMR. J Am Chem Soc. 1980;102(15):4889–93.
Nocuń M. Structural studies of phosphate glasses with high ionic conductivity. J Non-Cryst Solids. 2004;333:90–4.
Stoch L. Thermal analysis and thermochemistry of vitreous into crystalline state transition. J Therm Anal Calorim. 2004;77:7–16.
Kuczek J, Sułowska J, Lach R, Szumera M. The glass formation and crystallization studies on iron phosphate–silicate glasses. J Therm Anal Calorim. 2019;138:1953–64.
Stoch L, Wacławska I, Środa M. Thermal study of the influence of chemical bond ionicity on the glass transformation in (Na2O, CaO, MgO)–Al2O3–SiO2 glasses. J Therm Anal Calorim. 2004;77:57–63.
Görlich E. The effective nuclear charges and the electronegativity. Kraków: Polish Academy of Art and Science; 1997.
Llusar M, Zielinska A, Tena MA, Badenes JA, Monrós G. Blue-violet ceramic pigments based on Co and Mg Co2−xMgxP2O7 diphosphates. J Eur Ceram Soc. 2010;30:1887–96.
Doweidar H. Density–structure correlations in silicate glasses. J Non-Cryst Solids. 1999;249:194–200.
Upender G, Ramesh S, Prasad M, Sathe VG, Mouli VC. Optical band gap, glass transition temperature and structural studies of (100–2x)TeO2–xAg2O–xWO3 glass system. J Alloy Compd. 2010;504:468–74.
Li HJ, Liang XF, Yu HJ, Yang DQ, Yang SY. Studies of structure of calcium–iron phosphate glasses by infrared, Raman and UV–Vis spectroscopies. Indian J Phys. 2016;90(6):693–8.
Varshneya AK. Fundamentals of inorganic glasses. In: Chapter 3: Glass Formation Principles. Elsevier, 1994. p. 37–69.
Scholze H. Glass nature, structure, and properties. In: Chapter 2: Nature and structure of glass. New York: Springer-Verlag; 1991. p. 108–9
Fredholm YC, Karpukhina N, Law RV, Hill RG. Strontium containing bioactive glasses: glass structure and physical properties. J Non-Cryst Solids. 2010;356:2546–51.
Kuczek J, Jelen P, Sułowska J, Szumera M. Correlation between glass transition effect and structural changes in multicomponent iron phosphate silicate glasses. J Therm Anal Calorim. 2019;138:4145–53.
Kuczek J, Jelen P, Stoch P, Błachowski A, Wacławska I, Szumera M. Raman and Mossbauer studies of iron phosphate-silicate glasses. J Mol Struct. 2018;1170:82–9.
Chakraborty IN, Condrate RA. The vibrational spectra of glasses in the Na2O-SiO2-P2O5 system with a 1:1 SiO2:P2O5 molar ratio. Phys Chem Glasses. 1985;26(3):68–73.
Wong J. Vibrational spectra of vapor-deposited binary phosphosilicate glasses. J Non-Cryst Solids. 1976;20:83–100.
Szumera M, Wacławska I, Sułowska J. Influence of CuO and ZnO addition on the multicomponent phosphate glasses: spectroscopic studies. J Mol Struct. 2016;1114:78–83.
Hudgens JJ, Martin SW. Glass transition and infrared spectra of low-alkali, anhydrous lithium phosphate glasses. J Am Ceram Soc. 1993;76(7):1691–6.
Jerroudi M, Bih L, Yousfi S, Manoun B, Lazor P. Structure-property correlations in lithium zinc cobalt metaphosphate glasses and glass-ceramics. Phys B. 2021;610:412949.
Moustafa YM. Characterization of iron oxychloride potassium phosphate glasses. J Phys D Appl Phys. 1999;32:2278–86.
Shaim A, Et-tabirou M. Role of titanium in sodium titanophosphate glasses and a model of structural units. Mater Chem Phys. 2003;80:63–7.
Majjane A, Chahine A, Et-tabirou M, Echchahed B, Do T-O, Mc BP. X-ray photoelectron spectroscopy (XPS) and FTIR studies of vanadium barium phosphate glasses. Mater Chem Phys. 2014;143:779–87.
Doweidar H, Moustafa YM, El-Egili K, Abbas I. Infrared spectra of Fe2O3–PbO–P2O5 glasses. Vib Spectrosc. 2005;37:91–6.
Gabelica-Robert M, Tarte P. Infrared spectrum of crystalline and glassy pyrophosphates: preservation of the pyrophosphate group in the glassy structure. J Mol Struct. 1982;79:251–4.
Brow RK, Tallant DR, Myers ST, Phifer CC. The short-range structure of zinc polyphosphate glass. J Non-Cryst Solids. 1995;191:45–55.
Acknowledgements
The work was partly supported by program "Excellence initiative—research university" for the AGH University of Science and Technology and the EU Project POWR.03.02.00-00-I004/16. Authors would also like to acknowledge PhD Piotr Jeleń from AGH University of Science and Technology, Faculty of Material Science and Ceramics for the conducting of FTIR measurements.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by JK, MS, JS, DR-Z and MG. MG conducted the 31P MAS NMR measurements and prepared the data for the analysis. JS provided the density data and performed additional analysis. The first draft of the manuscript was written by JK. MS and DR-Z commented on previous versions and the final version of the manuscript. JK and MS mainly contributed to the study conception and design. All authors participated in the process of introducing amendments into the manuscript due to revision process. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuczek, J., Szumera, M., Rutkowska-Zbik, D. et al. Thermal and spectroscopic behavior of glasses from P2O5–SiO2–K2O–MgO–CaO–Co2O3 system. J Therm Anal Calorim 148, 1435–1444 (2023). https://doi.org/10.1007/s10973-022-11362-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11362-z