Abstract
This paper presents a systematic method for the estimation of theoretical value of temperature produced in the gas turbine combustor through MATLAB simulation. The fuel used is natural gas. The assumption of adiabatic combustion has been used for the simplicity of the analysis. For getting accurate results, the combustion process has been analyzed considering the variation of specific heat of gases with temperature. The theoretically calculated temperature has been compared with the actual temperature produced in an actual gas turbine combustor to validate the thermodynamic model. One of the most hazardous pollutants from a gas turbine is nitrogen oxides (NOx) whose formation takes place due to dissociation of nitrogen at high temperature and its subsequent reaction with oxygen. To avoid NOx formation, the flame temperature has been lowered to 1355 K by increasing the amount of excess combustion air. Therefore, the effect of excess air on the combustion temperature and power output has been investigated in this study. The theoretical power output has also been compared with the actual output. The method described in this paper may prove to be useful not only to the designers of gas turbine but also to the researchers analyzing other types of combustion systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy in the form of electricity is the most essential requirement of the people in the present-day civilization. It is considered as a basic input for any country to keep the wheels of its economy moving. Due to rapid industrialization of the world, the demand of electricity is increasing exponentially. The gas turbine represents perhaps the most satisfactory means to develop very large quantity of power in a self-contained and compact unit. The working of a gas turbine is based on Brayton cycle. The major fields in which these turbines are most applicable are—aviation, power generation and marine propulsion. A gas turbine plant has poor efficiency as compared to other thermal power generation systems. In aviation and marine applications, the efficiency of the gas turbine is not the criteria for its choice. In these fields, light weight, compactness, low weight to power ratio, easy starting, easy control and ability to fit into the compact shape of the structure are the main requirements. A gas turbine satisfies all these requirements and hence used in these fields. Heppenstall [1] provided a critical review of some of the important concepts of gas turbines.
In thermal power plants, the basic gas turbine cycle is not used due to its poor efficiency. Heat recovery schemes are the most important ways of increasing the efficiency of the power generation process. In this regard, exhaust recuperation, steam injection into the combustion chamber, use of evaporation cycle, chemical recuperation and use of inlet air chillers are some of the early concepts that were introduced in gas turbine plants for improving efficiency. McDonald and Wilson [2] discussed the role that recuperators and regenerators could play in improving the efficiency of gas turbines and stressed the necessity of utilizing ceramic-composite heat-exchanger configurations. Nguyen and Otter [3] described the conceptual designs of a prototype “closed-loop” steam injection water recovery (SIWR) system that was developed for gas turbine applications. Frutschi and Plancherel [4] studied an evaporative gas turbine (EGT) in which water was injected into the compressor outlet and evaporated there and a steam injection gas turbine (STIG) in which the steam was raised in a heat recovery steam generator downstream of the turbine and then injected into the combustion chamber or into the turbine nozzle guide vanes. Kesser et al. [5] investigated a basic chemically recuperated gas turbine (CRGT) and showed that the basic CRGT has a thermal efficiency higher than the steam-injected gas turbine (STIG) and simple cycles but not as high as the combined cycle. Ondryas et al. [6] investigated the augmentation of gas turbine power in a cogeneration plant with inlet air chilling.
Even after adopting the above means, the exhaust gases still contain substantial amount of heat that goes waste. Therefore, there is a scope for further improving the efficiency of power generation, if this waste exhaust heat could be utilized for power generation. The technology adopted nowadays for the utilization of the waste exhaust heat is the “combined cycle”. In combined cycle plants, the Brayton cycle is combined with Rankine cycle. Khaliq and Kaushik [7] used the second law approach for the thermodynamic analysis of a reheat combined Brayton/Rankine power cycle and investigated the effects of pressure ratio, cycle temperature ratio, number of reheats and cycle pressure drop on performance of the cycle. Reddy and Mohamed [8] performed exergy analysis of a combined cycle power plant to investigate the effect of gas turbine inlet temperature and pressure ratio on exergetic efficiency of the plant and evaluated the exergy destruction in different components of the plant. The combined cycle technology is now well established and offers superior efficiency to the basic gas turbine cycle for large-scale power generation applications. Singh and Kaushik [9] theoretically integrated Kalina cycle with the existing Brayton–Rankine combined cycle of a 330 MW natural gas-fired thermal power plant and examined the energy- and exergy-based performances of the resulting triple cycle system. Both energy and exergy efficiencies were found to increase further. Singh and Kaushik [10] also carried out the thermoeconomic analysis and optimization of this triple cycle system.
The gas turbines used for power generation generally use natural gas as fuel. The fuel is burnt in the combustor of the gas turbine, and the hot gases so produced act on the blades of the turbine to develop power. The combustor is one of the most important components of the gas turbine plant. The power output of the turbine greatly depends on the temperature of the gases produced in the combustor. The highest combustion temperature in the combustor is possible with stoichiometric air–fuel mixture and under adiabatic condition. This highest possible temperature is called adiabatic flame temperature [11]. In actual practice, the flame temperature is maintained much below this temperature as the possibility of NOx formation is more at high temperature due to dissociation of nitrogen at high temperature. Many Indian gas turbine power plants are quite aged and operating with lower efficiency due to lower turbine inlet temperature. Increasing the turbine inlet temperature increases the possibility of NOx formation. To resolve this problem, it is necessary to improve the combustor design so that high turbine inlet temperature may be used without NOx formation. In the combustor design, accurate determination of the temperature of gases produced in the combustor is absolutely necessary as it will help in accurate prediction of power output and other performance parameters of the plant and, therefore, help in designing the whole power plant. The work presented in this paper aims to provide a comprehensive methodology for the estimation of temperatures produced in the combustor of the gas turbine under different operating conditions. This methodology and the mathematical relations and data used therein will prove to be useful in the analysis of other combustion systems as well. Improved combustor design has also been suggested.
System description
The schematic diagram of the gas turbine unit under consideration is shown in Fig. 1 which is actually a part of the Brayton–Rankine combined cycle of natural gas-fired power plant situated in New Delhi, India. The gas turbine plant consists of a compressor, combustor and turbine. The air compressed by the compressor is supplied to the combustor for the purpose of combustion of fuel which is natural gas. The high temperature gases produced due to combustion of fuel are passed through the turbine. The gases expand in the turbine to low pressure, and shaft work is produced due to enthalpy drop of the gases. A part of the work developed by the turbine is used to run the compressor, and the remaining is used to generate electricity. The gases leaving the turbine still have high heat content and are, therefore, used to generate steam in the Rankine bottoming cycle of the combined cycle plant for further power production. The conditions and operating data of this gas turbine unit are presented in Table 1.
Thermodynamic modeling and analysis
Analysis of the combustion process
Assumptions
-
1.
The operation of combustor is a steady-state operation.
-
2.
Mass flow rate through the control volume is constant.
-
3.
There is no heat transfer through the boundary of the control volume, i.e., the process of combustion is adiabatic.
-
4.
Changes in potential and kinetic energies between inlet and exit of the control volume are neglected.
-
5.
The combustion takes place at constant pressure.
-
6.
The dissociation of the combustion products at high temperature is neglected.
-
7.
Pressure drops in pipe lines are neglected.
Combustion equation and stoichiometric air–fuel ratio
Air contains 21.0 % oxygen and 79 % nitrogen by volume. Therefore, for each kmol of oxygen, \( \frac{79}{21} = 3.7619 \) kmol of nitrogen are involved.
Therefore, the combustion equation for complete combustion of 1 kmol of natural gas having a % CH4, b % C2H6, c % C3H8, d % C4H10 and e % N2 can be written as [12]
With f % excess air, the above combustion equation becomes
Therefore, air–fuel ratio on a mole basis
and air–fuel ratio on a mass basis,
Mass balance
The mass balance for the control volume is given by
or
For the given fuel consumption rate, the molar flow rates of the reactants with chemically correct air, 25 % excess air, 50 % excess air, 75 % excess air, 100 % excess air, 125 % excess air, 150 % excess air, 159 % excess air, 175 % excess air and 200 % excess air are determined and presented in Table 2. Molar flow rates of the products are determined by applying the mass balance and presented in Table 3.
Energy balance and adiabatic flame temperature
First law of thermodynamics or energy balance equation for a steady-state, steady-flow reacting system neglecting the changes in potential and kinetic energies is given by
When the mixture of fuel and air enters the adiabatic steady-flow combustor and if the fuel is completely burned into products, the adiabatic flame temperature can be found by equating the enthalpy of the reactants at the reference state condition to the enthalpy of the products at adiabatic flame temperature. Therefore,
or
where, R and P refer to reactants and products, respectively.
The enthalpy of a component at given temperature is the sum of enthalpy of formation and sensible enthalpy. Therefore, Eq. (9) can be further written as
Since, the reactants enter the combustion zone at the reference state (T 0 = 298.15 K and p 0 = 101,325 Pa), Eq. (10) can be rewritten as
or
Specific heat \( \overline{C}_{\text{p}}^{{}} \) of gases is a function of temperature.
Following equations for \( \overline{C}_{\text{p}}^{{}} \) for various gases [13] have been used:
where, \( \theta = \frac{T}{100}{\text{ K}} \).
Using Eqs. (12)–(16), the adiabatic flame temperature of the gases in the combustor with chemically correct combustion and with excess air can be found.
Isentropic efficiencies of compressor and gas turbine
The isentropic efficiency of compressor is given by
The compressor outlet air temperature with isentropic compression, \( T_{{2^{{\prime }} }} \), is given by
Using Eqs. (17) and (18), the actual temperature of air at outlet from the compressor (T 2) can be found.
The turbine inlet gas temperature is same as the adiabatic flame temperature, i.e.,
The isentropic efficiency of gas turbine is given by
The turbine outlet gas temperature with isentropic expansion, \( T_{{4^{{\prime }} }} \), is given by
Using Eqs. (20) and (21), the actual temperature of gases at outlet from the gas turbine (T 4) can be found.
Power output and thermal efficiency
Net power output = power developed by the turbine − power consumed by the compressor
Heat supplied in the combustor = increase in enthalpy during combustion
or
Emissions and their control
The major pollutants emitted from a gas turbine engine are carbon monoxide (CO), oxides of sulfur (SOx) and nitrogen oxides (NOx). CO formation results from incomplete combustion of fuel which can be avoided by using excess combustion air to ensure that the combustion process is chemically complete. Oxides of sulfur (SOx), mainly SO2, will appear only when the fuel contains sulfur. As the gas turbine plant under consideration in this work uses sulfur-free natural gas, there is no emission of SOx. As regards NOx formation, there are two ways in which it is created in the gas turbine combustor:
-
Thermal NOx which forms due to the oxidation of atmospheric nitrogen present in the combustion air.
-
Conversion of nitrogen present in the fuel into NOx.
Studies have been conducted by various researchers to understand the chemical mechanism behind NOx formation. The four well-recognized mechanisms for NOx formation are Zeldovich mechanism [14], prompt or Fenimore mechanism [15], fuel-bound nitrogen mechanism [16] and nitrous oxide mechanism [17]. Zeldovich mechanism very well explains the formation of thermal NOx in which the dissociation of nitrogen present in the combustion air takes place which subsequently reacts with oxygen to form NOx. Following chemical reactions are involved in this mechanism:
The reaction rate increases exponentially with the flame temperature, and the NOx so generated is called “thermal NOx”. The variation of NOx with flame temperature is shown in Fig. 2 [18]. From the graph plotted in Fig. 2, it is clear that if the flame temperature is maintained below 1500 K, the “thermal NOx” formation would not occur. Fuel-bound NOx is usually of less importance for normal fuels. The gas turbine fuel considered in this study is natural gas. The molecular nitrogen presents in natural gas does not contribute significantly to NOx [19].
MATLAB simulation
For the calculation of the adiabatic flame temperature and subsequently the power output and thermal efficiency for different percentages of excess air, a computer program in MATLAB was developed. Figure 3 shows the flow chart of the simulation procedure. This program uses iteration technique to compute adiabatic flame temperature. Since, the reactants enter the combustion zone at the reference state (T 0 = 298.15 K and p 0 = 101,325 Pa), their total enthalpy \( (\sum\nolimits_{\text{R}} {n_{\text{i}} \bar{h}_{\text{i}} } ) \) is calculated at these conditions of temperature and pressure. The program then assumes a random value of adiabatic flame temperature (T 3), and using this value of the adiabatic flame temperature, the enthalpy of the products \( (\sum\nolimits_{\text{P}} {n_{\text{e}} \bar{h}_{\text{e}} } ) \) is calculated and compared with that of reactants. If there is a difference between these values, a new value of T 3 is assumed and the whole procedure is repeated. The simulation is regarded as convergent when the criterion of \( \left| {\sum\nolimits_{\text{P}} {n_{\text{e}} \bar{h}_{\text{e}} } - \sum\nolimits_{\text{R}} {n_{\text{i}} \bar{h}_{\text{i}} } } \right| \le 10^{ - 4} \) is satisfied. The value of adiabatic flame temperature so obtained is the true value, and corresponding to this value of T 3, the net power output and thermal efficiency are calculated. This procedure is repeated for different values of percent excess air.
Results and discussion
In gas turbines, it is necessary to know the maximum temperature that can be produced by the combustion of fuel as the maximum permissible temperature in the combustor is limited by the metallurgical considerations. With the given assumption of adiabatic combustion, no heat exchange is taking place with the surroundings so that the products leave the combustor at highest possible temperature called adiabatic flame temperature. The maximum value of adiabatic flame temperature can be achieved only when the fuel is burned with the stoichiometric or chemically correct amount of oxygen. With natural gas of given composition, the maximum value of adiabatic flame temperature that can be achieved is 2584 K. Although a high power output and hence a high thermal efficiency would be obtained at this temperature, due to the possibility of incomplete combustion with stoichiometric mixture and the dissociation of the combustion products at such a high temperature leading to NOx formation, a lower value of flame temperature is used in practice. The maximum permissible temperature is also determined by the metallurgical considerations. It is, therefore, very important to have a close control of the temperature of the products. In practice, the method adopted for controlling the adiabatic flame temperature is by controlling the amount of excess air that is used. Table 4 presents the effect of increasing excess air percentage on adiabatic flame temperature, exhaust gas temperature, turbine power, compressor power, net power output and thermal efficiency of the plant. The variation of adiabatic flame temperature and thermal efficiency with excess air are plotted in Fig. 4. It is found that both adiabatic flame temperature and the thermal efficiency decrease with the increase in the excess combustion air. The maximum permissible temperature in the gas turbine plant considered in this work is 1355 K which is theoretically obtained with 159 % excess air. The actual value of excess air used to attain this temperature in the gas turbine plant under consideration is 150 %. This slight deviation is due to the dissociation that takes place in the combustion products which has significant effect on the adiabatic flame temperature. The theoretical value of excess air obtained in this work (159 %) is fairly close to that used in actual practice (150 %) which closely validates this thermodynamic model developed in this work. The theoretical value of thermal efficiency obtained with these operating conditions is around 34 %, and the theoretical value of net power output is 130 MW.
The variation of power developed by the turbine, power required by the compressor and net power output of the plant with excess air percent is plotted in Fig. 5. With the increase in excess percent air, both the power developed by the turbine and the power required by the compressor increase due to increase in mass flow rates through these machines. But the net power output of the plant decreases because the rate of increase in compressor work is more than the rate of increase in the turbine power. This is also indicated by the decreasing gap between the turbine power curve and compressor power curve in Fig. 5.
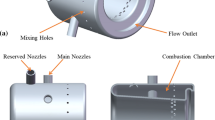
The plant under consideration uses the conventional combustor as shown in Fig. 6. In traditional combustor, about 70 % of the combustion air bypasses the combustion zone. Only 30 % of it is used for combustion, resulting in high flame temperature (about 2500 K). As the flame travels further, the remaining 70 % combustion air dilutes the gases lowering their temperature below the NOx forming temperature. But due to the presence of localized areas of high temperature, i.e., hot spots in the combustion zone, the NOx formation takes place. Therefore, in conventional combustor, it is not possible to avoid NOx formation. The combustor most suitable for lowering the flame temperature by running fuel lean is the dry low NOx combustor (DLN) [19] which is also shown in Fig. 6. In this combustor, premixing the fuel and air is done in such a way that the mixture becomes homogeneous to avoid the presence of hot spots in the flame (i.e., localized areas of high fuel-to-air ratios that produce NOx). An overview of dry low-emission combustors may be found in [20–22].
Conclusions
In this work, a procedure for calculating the flame temperature of the gases in the combustor of a gas turbine with adiabatic conditions is developed. With natural gas of given composition, the maximum value of adiabatic flame temperature is found to be 2584 K. This value is achieved only when the fuel is burned with the stoichiometric or chemically correct amount of air. In the gas turbine plant under consideration, the maximum permissible temperature is limited to 1355 K due to the metallurgical considerations and also due to the possibility of NOx formation above 1500 K. Therefore, to achieve this temperature, the amount of excess combustion air is increased. The theoretical value of excess air obtained to attain this temperature is found to be 159 % which is fairly close to that used in actual practice, i.e., 150 % which closely validates the thermodynamic model developed in this work. The highest value of thermal efficiency and the net power output are obtained with the stoichiometric amount of air. The values of these parameters decrease with the increase in excess air. The theoretical value of thermal efficiency and the net power output obtained with the given operating conditions are found to be 34 % and 130 MW, respectively. The actual power output of the plant is 100 MW. This deviation is may be because of so many assumptions made in the analysis. For the purpose of lowering the flame temperature by increasing excess combustion air to avoid NOx formation, the use of a dry low NOx combustor (DLN) is proposed. The method described in this paper may prove to be useful not only to the analysts and designers in conducting the analysis and design of a gas turbine power plant and in devising ways and means to control emissions but also to the researchers for analyzing other types of combustion systems.
Abbreviations
- \( \overline{C}_{\text{p}} \) :
-
Isobaric molar specific heat (kJ kmol−1 K−1)
- H :
-
Enthalpy (kJ)
- \( \bar{h} \) :
-
Molar specific enthalpy (kJ kmol−1)
- \( \Delta \bar{h} \) :
-
Change in molar specific enthalpy (kJ kmol−1)
- \( \bar{h}_{\text{f}}^{0} \) :
-
Standard molar specific enthalpy of formation (kJ kmol−1)
- m :
-
Mass of a component (kg)
- \( \dot{m} \) :
-
Mass flow rate (kg s−1)
- M :
-
Molecular mass of a component
- n :
-
kmol of a component
- \( \dot{n} \) :
-
Molar flow rate (kmol s−1)
- \( p_{0} \) :
-
Actual environmental pressure (Pa)
- \( \dot{Q} \) :
-
Heat transfer rate (kW)
- \( r_{\text{p,C}} \) :
-
Pressure ratio of the compressor
- \( r_{\text{p,T}} \) :
-
Pressure ratio of the gas turbine
- \( T \) :
-
Absolute temperature (K)
- T 0 :
-
Actual environmental temperature (K)
- \( \dot{W} \) :
-
Work done rate (kW)
- T 0 :
-
Standard temperature (K)
- η isen,C :
-
Isentropic efficiency of compressor (%)
- η isen,T :
-
Isentropic efficiency of gas turbine (%)
- η Th :
-
Thermal efficiency (%)
- γ :
-
Specific heat ratio
- Δ:
-
Used for a change in any parameter
- CV:
-
Control volume
- e:
-
Exit
- i:
-
Inlet
- P:
-
Product
- R:
-
Reactant
- 1, 2, 3, 4:
-
Nodal points
References
Heppenstall T. Advanced gas turbine cycles for power generation: a critical review. Appl Therm Eng. 1998;18:837–46.
Mcdonald CF, Wilson DG. The utilization of recuperated and regenerated engine cycles for high- efficiency gas turbines in the 21st century. Appl Therm Eng. 1996;16(8/9):635–54.
Nguymen HB, den Otter A. Development of a gas turbine steam injection water recovery (SIWR) dystem. ASME J Eng Gas Turb Power. 1994;16(1):68–74.
Frutschi H, Plancherei A. A comparison of combined cycles with steam injection and evaporation cycles. In: Proceedings of ASME Turbo-Cogen Conference, Montreux, Switzerland. 1988.
Kesser K, Hoffman M, Baughn J. Analysis of a basic chemically recuperated gas turbine power plant. ASME J Eng Gas Turb Power. 1994;16(2):277–84.
Ondryas IS, Wilson DA, Kawamoto M, Haub GL. Options in gas turbine power augmentation using inlet air chilling. ASME J Eng Gas Turb Power. 1991;113:203–11.
Khaliq A, Kaushik SC. Second-law based thermodynamic analysis of Brayton/Rankine combined power cycle with reheat. Appl Energy. 2004;78:179–97.
Reddy BV, Mohamed K. Exergy analysis of natural gas fired combined cycle power generation unit. Int J Exergy. 2007;4(2):180–96.
Singh OK, Kaushik SC. Reducing CO2 emission and improving exergy based performance of natural gas fired combined cycle power plants by coupling Kalina cycle. Energy. 2013;55:1002–13.
Singh OK, Kaushik SC. Thermoeconomic evaluation and optimization of a Brayton–Rankine–Kalina combined triple power cycle. Energy Convers Manag. 2013;71:32–42.
Nag PK. Power plant engineering. New Delhi: McGraw-Hill; 2007.
Kaushik SC, Singh OK. Estimation of chemical exergy of solid, liquid and gaseous fuels used in thermal power plants. J Therm Anal Calorim. 2014;115:903–8.
Sonntag RE, Borgnakke C, Van Wylen GJ. Fundamentals of classical thermodynamics. New York: Wiley; 2000.
Zeldovich YB, Sadovnikov PY, Frank-Kamentskii DA. Oxidation of nitrogen in combustion. Moscow: Academy of Sciences of the USSR; 1947.
Fenimore CP, Hilt MB, Johnson RH. Formation and measurements of nitrogen oxides in gas turbines. 1971;12(4):38–41.
Toof JL. Model for the prediction of thermal, prompt, and fuel NOx emissions from combustion turbines. In: 30th international gas turbine conference and exhibit. New York, NY: ASME; 1985. p. 8.
Corr RA, Malte PC, Marinov NM. Evaluation of NOx mechanisms for lean premixed combustion. In: International gas turbine and aeroengine congress and exposition. New York, NY: ASME; 1991. p. 12.
Lefebvre AH. Fuel effects on gas turbine combustion—liner temperature, pattern factor and pollutant emissions. In: AIAA/SAE/ASME 20th joint propulsion conference. New York, NY: AIAA; 1984. p. 18.
Meher-Homji CB, Zachary J, Bromley AF. Gas turbine fuels—system design, combustion and operability. In: Proceedings of the thirty-ninth turbomachinery symposium; 2010.
Popovic P, Myers G, Citeno J, Symonds R, Campbell A. Proceedings of the ASME Turbo Expo, Glasgow, UK. ASME paper no. GT2010-22267; 2010.
Campbell A, Goldmeer J, Healy T, Washman R, Moliere M, Citeno J. Heavy duty gas turbine fuel flexibility. In: Proceedings of the ASME turbo expo, Berlin, Germany. ASME paper no. GT2008-51368; 2008.
Wisniewski KJ, Handelman S. Expanding fuel flexibility capability in GE’s aeroderivative engines. In: Proceedings of the ASME turbo expo, Glasgow, UK. ASME paper no. GT2010-23546; 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, O.K. Combustion simulation and emission control in natural gas fuelled combustor of gas turbine. J Therm Anal Calorim 125, 949–957 (2016). https://doi.org/10.1007/s10973-016-5472-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5472-0