Abstract
In the present work, burning of LPG mixture (50% each of propane C3H8 and butane C4H10) in a gas turbine type environment is simulated and validated against the benchmark continuous combustion unit (P. A. Hilton) experiment. Ansys Fluent® has been used to carry out the numerical study with major validation parameters limited to axial profiles of exhaust gas temperature and adiabatic flame temperature with equivalence ratio. It is observed that both exhaust gas and flame temperatures follow the theoretical trend of increasing with equivalence ratio, achieving their peak values at equivalence ratio near to unity and then subsequently decreasing. A pressure-based steady-state solver based on finite volume approach has been used to model the fluid flow with eddy dissipation limiters and realizable k-ɛ equations defining the combustion and turbulence phenomenon respectively. Axial profiles of important combustion parameters like temperature, pressure, and component mass fractions are obtained to draw out conclusions. The developed numerical methodology has been further extended to study the behavior of CNG in place of LPG and effect of air preheating on combustion. The study focuses on robustness of the solver and can be further expanded to include burning of liquid fuel sprays and their blends, variation of NOx, and study of flame characteristics in a continuous combustion-type environment.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In modern gas turbine engines, basic performance parameters are thermal efficiency and emissions [1, 2]. For improvement, various experimental and numerical solutions have been tried and tested for given operating conditions, for example designing of modern gas turbine combustors may employ advanced technologies such as catalytic reduction and RQL, etc. [1]. Another option for controlling NOx emissions is the employment of steam injection [3]. Retrofitting technologies (inlet evaporative cooling system and steam-injected gas turbine) have been applied on simple gas turbine cycle for performance improvement followed by parametric analysis. The performance improvement has been thermodynamically analyzed and discussed for retrofitted techniques. The parametric study predicts that retrofitting technique (STIG) improves net power output, thermal efficiency, power generation efficiency, first law efficiency, and exergy efficiency (Second law efficiency) while heat rate falls with a considerable increment in fuel consumption. Exergy analysis further showed that combustion chamber and turbine are most sensitive components of retrofitted system. The power output, thermal efficiency, exergy efficiency, and fuel-air ratio improved using STIG technology [4].
Numerical simulation of gas turbine combustor is a challenging task due to inherent complex processes taking place simultaneously [1,2,3]. Hence, the prime objective of the present study aims at numerically simulating a realistic ‘continuous combustion unit’ (experimental set up provided by P. A. Hilton, UK) and to validate the experimental results with those obtained through simulation. The study involves validation of a basic experimental setup moving onto inclusion of advanced variables. The information generated can be further used to analyze modern gas turbine combustion units. For present simulation, grid independency test and use of proper turbulence and chemical kinetics model becomes important. Furthermore, optimization of the solver to account maximum possible flow physics, heat transfer, and collision phenomena with an efficient CPU time [5] has been ensured.
2 Problem Formulation
2.1 Experimental Setup
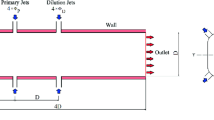
Figure 1 shows the continuous combustion unit setup at Combustion Lab of the Department of Mechanical Engineering, Aligarh Muslim University. It comprises of an air and fuel compression system, a preheater, a gas turbine combustion chamber, and an exhaust outlet. Compressed air is injected into the combustion chamber followed by fuel injection. A stable burning flame is observed which can be witnessed through watch glass on the walls of chamber. There is heat loss inform of convection and radiation. A water jacket running around the walls of the chamber cools the combustion products before they are vented out into the atmosphere through an exhaust system. The experimental setup is meant to study the energy balance, effect of equivalence ratio on combustion characteristics, and the effect of preheating, compression ratio, and fuel type on flame characteristics.
2.2 Numerical Setup
A numerical methodology based on commercial code of Fluent® has been developed to validate the experimental setup. The intent is to diversify the effect and choice of variables on combustion phenomenon beyond the capacity of experimental setup with the help of a robust numerical solver. A pressure-based semi-implicit formulation has been used to solve the dynamics of the flow. Due to axisymmetric properties of the flow and gas turbine chamber, only half section is considered as the computational domain. Figure 2 shows the symmetry in the complete domain and Fig. 3 shows a structured grid that has been generated using ICEM CFD comprising of 65 K elements and least non-dimensional height of 1e-04. A grid independence study was used to arrive at the number of cells (65 K) appropriate for this simulation. The equations for conservation of momentum, energy, and species have been solved with k-ɛ turbulence model. SIMPLE coupling scheme has been used with second and third-order spatial discretization of evolution equations. Eddy dissipation model is used to inhibit turbulence chemistry interaction. Details of geometrical parameters of combustion chamber are given in Table 1.
The species transport model predicts the transport of all species along with their consumption and production in the combustion zone. It solves conservation equation for every chemical species in the reaction. The eddy dissipation model is suitable for reactions which occur in short time; the flow is turbulent and non-premixed. The progress of elementary reaction is to determine reactant and products limiter, based on conservation equation for mass fraction. Major advantage of eddy dissipation model is that it calculates Arrhenius rate once and thus provides early results than other models. Boundary conditions for species are given in Table 2. The top wall is modeled as a mixed heat transfer (convection and radiation) boundary condition, while the outlet is given a pressure outlet BC averaging the gauge pressure to zero.
3 Simulation Results of the Present Study for LPG-Air Mixture Combustion Along Combustion Chamber Length (Axial Profile)
A continuous combustion unit (gas turbine type) is simulated with respect to the initial conditions of fuel (LPG) and air mass fractions, temperature, and pressure. Figure 4 shows the contours of total temperature in the computational domain, and Fig. 5 indicates the variation of temperature along the axis of combustion chamber. The profile obtained is a standard for constant pressure-type gas turbine combustion chambers. The decrease in the magnitude can be attributed to convection and radiation heat losses from the chamber to water jacket and surroundings. A flat pressure profile in Fig. 6 validates the constant pressure assumption taken for the model, with a minor bump near 0.2 m position arising due to turbulence created from fuel injection and combustion. Figures 7 and 8 show reactant mass fractions, and Fig. 9 shows product mass fractions along the axis of combustion chamber. The values in these figures are in accordance with rate kinetics of the chemical reactions.
3.1 Validation of Adiabatic Flame Temperature (AFT) Versus Equivalence Ratio for Air-Propane Mixture
Tables 3 and 4 show results of present study and study [1] for validation purpose (Fig. 10).
3.2 Simulation Result for Combustion of CNG-Air Mixture (Axial Profile)
Density of CNG = 0.8 kg/m3, mass flow rate of fuel = 0.00194 kg/s, mass flow rate for air at equivalence ratio 0.91 = 0.0368 kg/s, hydraulic diameter for fuel inlet = 0.01, hydraulic diameter for air inlet = 0.128.
The adiabatic flame temperature of air-propane combustion achieves its maximum value near to equivalence ratio 1 (Fig. 11). The temperature first increases till equivalence ratio 1 and then decreases gradually which is similar to AFT versus equivalence ratio plot of [1], thus results obtained show a good validation.
3.3 Comparison of Experimental Data (Hilton Continuous Combustion Unit) with Respect to Exhaust Gas Temperature and Present Simulation Study for Different Equivalence Ratios (E. R)
Table 5 show results of experimental and simulation studies in terms of exhaust gas temperature as a function of equivalence ratio. Figure 12 illustrates the comparison of exhaust gas temperature in the experiment with the numerical study. The polynomial curves fit obtained for both of the data sets helps in realization of validation studies within predefined numerical certainty.
3.4 Effect of Preheating of Air on Exhaust Gas Temperature of LPG-Air Mixture
Table 6 shows values of exhaust gas temperature in cases of air preheating and with no preheating.
As expected, due to preheating of air, the exhaust gas temperature increases as compared to the case of without preheating (Fig. 13). Also, the flame temperature increases with air preheating in the same proportion. The temperature is maximum at equivalence ratio near to unity. Preheating air before it reaches the combustion chamber can improve mixture fuel economy and efficiency. Preheating the intake air can be used for different purposes such as anti-icing, low temperature operations, and problems caused by the low load operations can be avoided or at least significantly reduced among other positive effects [6].
4 Conclusions
A numerical study is performed on a state-of-the-art experimental setup comprising of ‘continuous combustion unit’ (more detailed versions of which are employed in gas turbine engines). The simulation results are obtained with respect to adiabatic flame temperature and exhaust gas temperature as a function of equivalence ratio. Both these performance parameters play a vital role in the design of gas turbine combustion chamber. Literature review indicates that past studies [1,2,3] have used complex CFD models to evaluate the performance parameters requiring large CPU time. The same results are obtained in the present study requiring relatively less CPU time. A 3D model of the CCU used in the present study can be evolved which could help study the flame characteristics and compare schlieren images from that of experiment. Some important conclusions drawn from the study are listed below:
-
Numerical study of LPG-Air combustion in a continuous combustion unit (P. A. Hilton) is carried out using Ansys Fluent®. Results indicate that both flame temperature and exhaust gas temperature become maximum near the stoichiometric equivalence ratio. The results are compared with experimental and theoretical data and are found to be in a good agreement.
-
Profiles of temperature, pressure, reactants, and products mass fractions have been used to draw out conclusions for both LPG-Air and CNG-Air mixtures.
-
Effect of inlet air preheating shows that both the flame temperature and exhaust gas temperature increase with an increase in equivalence ratio peaking at equivalence ratio of about unity.
-
Future scope of this study involves studying the behavior of liquid fuel (kerosene) in similar conditions by employing discrete phase models. The rate of heat release and NOx variation with air-fuel ratio, preheating, and change of fuel (including blends) can also be quantified.
References
Turns SR (2012) An introduction to combustion concepts and applications. McGraw Hill International Edition
Lefebvre AH (2010) Gas turbine combustion, Second edn. Taylor and Francis group
Moore RPGD. Gas turbine emission and control, GE Energy Services Atlanta, GA
Agarwal S, Mishra RS (2012) Thermodynamic analysis for improvement in thermal performance of a simple gas turbine cycle through retrofitting techniques. In: Proceedings of the national conference on trends and advances in mechanical engineering, YMCA University of Science & Technology, Faridabad, Haryana, Oct 19–20
Khodabandeh A (2011) CFD modelling of generic gas turbine combustor, Master thesis, ISSN 1652-8557
Droubot P, Denhez M (2015) Gas turbine with intake air preheating. European patent application, EP2881562A1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Alam, S.S., Rehman, S., Varshney, A. (2021). Numerical Study of Gas Turbine Type Continuous Combustion Unit Burning LPG Mixture. In: Sikarwar, B.S., Sundén, B., Wang, Q. (eds) Advances in Fluid and Thermal Engineering. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-0159-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-0159-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0158-3
Online ISBN: 978-981-16-0159-0
eBook Packages: EngineeringEngineering (R0)