Abstract
Different aqueous deicers in the range of 20–50 mass% were investigated by calorimetric methods. A dynamic differential scanning calorimetry (DSC) procedure was developed to determine the thermal properties (freezing point, melting enthalpy) of aqueous brines. Phase change temperatures were characterized for the broad melting peak determining two specific temperatures: extremum peak temperature (T peak) and the temperature corresponding to the inflection point of heat flow curve, which represents the end of the phase change (T end). Heating rates of 0.2, 0.5 and 1.0 °C min−1 were used to extrapolate the characteristic temperatures at zero heating rate. For each sample, at least three runs were performed to check the reproducibility. Thermal analyses were conducted with Setaram DSC and with Netzsch DSC. Additionally, the influence of different kinds of sample cells was compared. The results demonstrated that the developed calorimetric method is suitable for the characterization of aqueous deicers. Our studies confirmed that the enthalpy of fusion is appropriate to assess the performance of aqueous deicers. The extrapolated end temperatures at zero heating rate matched the literature values very well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing use of chemicals containing chloride salts used in the snow and ice control operations, which are also called deicers, raised concerns about their impact on transportation infrastructure, vehicles and the environment. Deicer products are used to remove the ice from streets and roads once it has formed, while anti-icing products are used to prevent the formation of ice.
According to field studies in Finland, some agencies have begun to voice preference for potassium-based deicers [1–3]. Lindroos and Nysten [3] recommend in their report that groundwater quality problems associated with deicing can also be mitigated by using alternative deicing chemicals. Long-term monitoring studies showed that if potassium formate is applied as winter maintenance, this results in low load of organic carbon into surface water bodies [3, 4]. One of the key conclusions by Nysten et al. [4] is that potassium formate is more efficient against black ice compared to sodium salt. On the other hand, it was shown that for the removal of packed snow from roads, sodium salt is more effective.
The performance of a deicer is mainly determined from its eutectic and effective (cf. description further below) temperature. At ambient temperatures below the eutectic or effective temperature, a deicer will not perform at its best. The eutectic temperature is defined in the phase diagram of a eutectic system by the eutectic point and the eutectic concentration. Liquid and solid curves merge at the eutectic point, i.e., the eutectic of a deicer defines the lowest temperature at which it can melt ice. Effective deicers have eutectic temperatures well below the expected ambient temperature range. In Table 1, the eutectic temperatures and concentrations of three deicers are summarized [5].
Know-how on the practices in deicing and cost-efficient use of potassium formate has to be developed. Potassium-based deicers may increase costs, but last longer and can be more powerful at melting ice, especially at cold temperatures. There are many parameters playing a role in the effectiveness of deicer products used in winter maintenance. Understanding the performance characteristics and negative impacts of deicers is crucial for effective and sustainable winter maintenance operations. A more precise selection of deicers should then be possible.
We present a study to evaluate various deicers in terms of their performance, like thermal properties or ice-melting capacity. Differential scanning calorimetry (DSC) has been identified as a promising laboratory test for evaluating deicers, and it was demonstrated how such results can be utilized in decision-making framework [6–12].
Experimental
Materials
Analytical grade sodium chloride (NaCl >99.8 mass%) was purchased from Carl Roth, Germany, and used without further purification as a reference. Different aqueous deicers in the range of 20–50 mass% were provided by Evonik Industries AG, Germany, used without further purification, and are summarized in Table 2.
DSC experimental setup and protocol
A Setaram DSC (microDSC7evo) and a Netzsch DSC (204 Phoenix) were used in our study. The Setaram DSC operates between −45 and 120 °C and the Netzsch DSC between −150 and 600 °C. The DSC devices were selected according to temperature requirements. All experiments with deicing solutions were conducted at atmospheric pressure.
In the Setaram DSC device, two Hastelloy cells were enclosed in the same temperature-controlled furnace. Both cells are sealed during the measurement with the sample in the sample cell, while the reference cell is left empty. The Setaram DSC was equipped with a thermostat for cooling, and dry nitrogen was used as purge gas.
The furnace of the Netzsch DSC is a disk-shaped sensor on which the crucibles are placed. The reference crucible is left empty during the experiments. The Netzsch DSC uses liquid and gaseous nitrogen for cooling purposes. The influence of different kinds of sample cells was studied, too. We used cold-weldable aluminum crucibles being sealed with a handy sealing press, and stainless steel crucibles (high-pressure cells) that could be joint together gastight with a mounting tool. The calibrations of both DSC were made using high-purity gallium and pure distilled water at different heating rates.
For the dynamic experiments in the Setaram DSC, the furnace temperature was ramped down from room temperature down to −45 °C at rate of 1 °C min−1. The temperature was kept for 20 min to freeze the liquid completely and subsequently raised from −45 to 20 °C with heating rates of 0.2, 0.5 and 1 °C min−1. As an example, a thermal analysis curve of the Setaram DSC for sodium chloride (20 mass%) with a heating rate of 1.0 °C min−1 is shown in Fig. 1. Two endothermic peaks are observed during heating in Fig. 1. The first peak, at around T = −21 °C, is due to the melting of the eutectic mixture of ice and NaCl. The subsequent peak is due to the non-isothermal melting of ice, as a result of progressive dilution of NaCl in the aqueous phase [13, 14]. The Netzsch DSC was used in a lower temperature range down to −80 °C, and three different heating rates for the dissociation were used as well. A representative dissociation curve of the experiments conducted with Netzsch DSC including furnace temperature and heat flux over time of potassium formate (50 mass%) is given in Fig. 2. All samples were run in triplicate, at least. If there were deviations between repeated measurements in the used DSC device, further experiments were conducted in the other DSC device for control purposes.
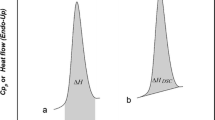
For pure, eutectic or stoichiometric composition, DSC traces show a sharp melting peak, and the onset temperature T onset corresponds to the phase transition temperature, i.e., the start of melting. For non-stoichiometric and non-eutectic compositions, the DSC curve shows a broad melting peak, and the equilibrium temperature is defined by the end of melting: It is the temperature when the last crystals vanish. A representative DSC dissociation curve is shown in Fig. 3, in which the phase change temperatures were characterized following the definitions given in [13, 14]: The onset temperature was determined in case of eutectic melting. T onset can be determined at the intersection of the auxiliary line through the descending peak slope with the baseline. For the progressive melting peak, we determined two different temperatures (T peak, T end). Tpeak denotes the extremum peak temperature, whereas T end is the temperature corresponding to the inflection point of heat flow curve. T end (β = 0) is the inflection point temperature at zero heating rate with β as heating rate. This temperature was extrapolated by the inflection point temperature T end at 0.2, 0.5 and 1.0 °C min−1. The inflection point is the peak point of the first derivative of the heat flow curve, which was calculated based on the heat flow and temperature data [13, 14].
Representative DSC dissociation curve with characteristic temperatures, reproduced from [13]
Results and discussion
The effective temperature of a deicer is the lowest temperature limit at which the deicer remains effective and achieves ice melting. It corresponds to the temperature threshold below which ice crystals start to form in diluted deicer solution. Above the effective temperature, there is no ice crystal present, so that there is no risk of slippery pavement.
If the apparent transition temperature is plotted as a function of heating rate (Fig. 4), the extrapolation to zero heating rates (β = 0) should give the best estimation for the phase transition temperature. In the literature, it has been pointed out that T peak does not represent the end of the phase transition, but the recorded temperature indicates the maximum of energy exchange [13–16]. The last solid particle dissolves at the time corresponding to the inflection point T end. With higher heating rates, peaks are shifted to higher temperature. Then, the peak area gets bigger (broader and higher) and the converted mass per time unit is larger. The influence of heating rate on temperature shifts can be eliminated by the extrapolation to zero heating rate [17]. The extrapolation to zero heating rate followed the GEFTA procedure described in [18], where the thermal inertia of the measuring system (“thermal lag”) is considered. The extrapolation to zero heating rates (β = 0) should give the best estimation for the phase transition temperature and was compared to the literature data.
Extrapolation to zero heating rate for melting of potassium formate (20 mass%) compared to the literature value [5]
In general, we observed in our investigations that the DSC curves were very reproducible for each heating rate. The experiments with different kinds of sample cells with the Netzsch DSC resulted in better reproducible curves when using the high-pressure cells assuming better contact face between bottom of cell and disk-shaped oven. Seven aqueous deicers gave similar curve shapes, so that we present here only curves with potassium formate (20 mass%). The melting temperatures of aqueous deicers were underestimated using T peak (β = 0). Extrapolating T end (β = 0) to the zero heating rate gives better results than using T peak (β = 0). In Fig. 4, it can be seen that the extrapolated T end (β = 0) for potassium formate (20 mass%) is very close to the literature value given in [5]. The freezing points and heat of fusion of aqueous deicers studied are summarized in Table 3, where freezing points given in the literature are listed as well [5].
The freezing point of sodium chloride used as a reference material in the concentration of 20 mass% was determined to be approximately −18 °C. Compared to two other aqueous deicers with the same concentration of 20 mass% potassium formate and potassium carbonate, the DSC measurements showed up with clearly higher freezing points (KF approx. −11 °C, KC approx. −8 °C). In general, the freezing points decrease with increasing salt concentration, which was stated in our studies. The potassium formate-based deicer with the highest concentration (50 mass%) results in the lowest freezing point (KF approx. −55 °C) as the lowest effective temperature of all studied aqueous deicers.
Figure 5 shows a deviation plot with [(T Lit − T (β = 0))/T Lit] × 100 % on the ordinate and the solution concentration on the abscissa. It was found that besides one value (T peak for K2CO3, 30 mass%), all other values of [(T Lit − T (β = 0))/T Lit] are smaller than 1 %, whatever the solute concentrations were. This temperature difference is highly acceptable.
During measurements of K2CO3 (30 mass%), we observed with both DSC devices that white crystals were left as residue in the sample cells. The heat flow curves of K2CO3 (30 mass%) showed some disturbances, thereby preventing determination of the inflection point and T end, respectively. Only T peak was determined in this case.
By using calorimetric methods, time-consuming performance tests following SHRP can be overcome. An empirical correlation of DSC measurements and ice-melting capacity tests according to SHRP at 0 °C is described in the literature [6, 11], where the extremum peak temperature T peak is used as the characteristic temperature and a heating rate of 3.6 °C min−1 in the DSC measurements. In our DSC experiments, we used three different heating rates (0.2, 0.5 and 1.0 °C min−1). We characterized two temperatures, T peak and T end, and found that those temperatures underestimate and overestimate the melting temperatures of aqueous deicers. Compared to the literature values, the extrapolated T end (β = 0) at zero heating rate generated clearly better results than T peak and T end. The effective temperature of a deicer should therefore use extrapolated T end (β = 0) to assess the deicer performance under certain road weather conditions.
The change in heat flow was calculated in [6, 11] by subtracting the total heat of fusion for pure water from the measured heat flow of the characteristic peak. It is noted in [6, 11] that the more powerful a deicer is, the less external heat is needed to melt the ice. This was confirmed by our experiments. The potassium formate-based deicer with the highest concentration (KF 50 mass%) results in the lowest melting enthalpy of all studied aqueous deicers.
Conclusions
Calorimetric methods were used to study different aqueous deicers in the range of 20–50 mass% to determine the thermal properties (freezing point, melting enthalpy) of aqueous brines. We developed procedures for two different DSC apparatuses and extrapolated the characteristic temperatures T end and/or T peak to zero heating rate. T end (β = 0) at zero heating rate, which was extrapolated with three different heating rates, generated better results than T peak and T end. The extrapolated temperature T end (β = 0) should be used as effective temperature for the selection of a deicer.
Freezing points of three different aqueous solutions with varying concentrations were determined. The results matched the literature data very well. The DSC curves were highly reproducible for each heating rate.
A more powerful deicer needs less external heat for melting, i.e., the deicer with the highest concentration (KF 50 mass%) results in the lowest melting enthalpy.
The deicer performance was characterized by calorimetric methods, with the advantages of DSC measurements: being quick and easy to perform.
Abbreviations
- c :
-
Concentration (mass%)
- GEFTA:
-
Gesellschaft für Thermische Analyse (German Society for Thermal Analysis)
- m :
-
Mass (mg)
- M R :
-
Molecular mass (g mol−1)
- t :
-
Time (min, h)
- T :
-
Temperature (°C, K)
- T onset :
-
Onset temperature (°C)
- T peak :
-
Peak extremum temperature (°C)
- T end :
-
Inflection point temperature (°C)
- β :
-
Heating rate (°C min−1)
References
Hellsten P, Nysten T, Salminen J, Grandlund K, Huotari T, Vallinkoski VM. Biodegradation of potassium formate in soil and groundwater. In: Final report of studies on alternative deicing chemicals. Finnish Environment Institute (SYKE); 2004. http://hdl.handle.net/10138/40448. (Accessed 13 Oct 2015).
Salminen J, Nysten T, Tuominen S. Alternative deicing agents and groundwater protection. In: Final report of MIDAS2-project. Finnish Environment Institute (SYKE); 2010. http://hdl.handle.net/10138/37972. (Accessed 13 Oct 2015).
Lindroos N, Nysten T. The trends of chloride concentrations of groundwater and factors affecting them in the first Salpausselkä ice-marginal formation. In: Research reports of the Finnish Transport Agency, Infrastructure and Environment; 2015. http://www2.liikennevirasto.fi/julkaisut/pdf8/lts_2015-11_salpausselan_pohjaveden_web.pdf. (Accessed 8 Feb 2016).
Nysten T, Salminen J, Tuominen, S. Migration of alternative deicing chemicals in aquifers (MIDAS). In: Project migration of alternative deicing chemicals in aquifers (MIDAS) of Finnish Environment Institute (SYKE); 2013. http://syke.fi/projects/midas. (Accessed 8 Feb 2016).
Melinder A. Thermophysical properties of aqueous solutions used as secondary working fluids. Stockholm: KTH; 2007.
Fay L, Shi X. Laboratory investigation of performance and impacts of snow and ice control chemicals or winter road service. J Cold Reg Eng. 2011. doi:10.1061/(ASCE)CR.1943-5495.0000025.
Akin M, Shi X. Development of standardized test procedures for evaluating deicing chemicals. In: Report of Western Transportation Institute. Montana State University; 2010. http://clearroads.org/project/development-of-standardized-test-procedures-for-evaluating-deicing-chemicals/. (Accessed 8 Feb 2016).
Akin M, Shi X. Development of standard laboratory testing procedures to evaluate the performance of deicers. J Test Eval. 2012. doi:10.1520/JTE103615.
Muthumani A, Shi X. Effectiveness of liquid agricultural by-products and solid complex chlorides for snow and ice control. In: TRB 94th annual meeting compendium of papers. Washington, DC: Transportation Research Board; 2015. http://docs.trb.org/prp/15-5815.pdf. (Accessed 14 Oct 2015).
Fay L, Akin M, Wang S, Shi X, Williams D. Correlating lab testing and field performance for deicing and anti-icing chemicals (phase 1). In: Final report of Montana State University; 2010. http://wisdotresearch.wi.gov/wp-content/uploads/10-17deicingcorrelation-f.pdf. (Accessed 8 Feb 2016).
Shi X, Fay L, Gallaway C, Volkening K, Peterson MM, Pan T, Creighton A, Lawlor C, Mumma S, Liu Y, Nguyen TA. Evaluation of alternative anti-icing and deicing compounds using sodium chloride and magnesium chloride as baseline deicers (phase 1). In: Final report of Colorado Department of Transportation, Denver; 2009. http://www.westerntransportationinstitute.org/documents/reports/4W1095_Final_Report.pdf. (Accessed 14 Oct 2015).
Shi X, Jungwirth S, Akin M, Wright R, Fay L, Veneziano D, Zhang Y, Gong J, Ye Z. Evaluating snow and ice control chemicals for environmentally sustainable highway maintenance operations. J Transp Eng. 2014. doi:10.1061/(ASCE)TE.1943-5436.0000709.
Lin W, Dalmazzone D, Fürst W, Delahaye A, Fournaison L, Clain P. Accurate DSC measurement of the phase transition temperature in the TBPB–water system. J Chem Thermodyn. 2013. doi:10.1016/j.jct.2013.02.005.
Lin W, Dalmazzone D, Fürst W, Delahaye A, Fournaison L, Clain P. Thermodynamic properties of semiclathrate hydrates formed from the TBAB + TBPB + water and CO2 + TBAB + TBPB + water systems. Fluid Phase Equilib. 2015. doi:10.1016/j.fluid.2014.03.026.
Kousksou T, Jamil A, Zeraouli Y, Dumas JP. Equilibrium liquidus temperatures of binary mixtures from differential scanning calorimetry. Chem Eng Sci. 2007. doi:10.1016/j.ces.2007.07.008.
Kousksou T, Jamil A, Zeraouli Y, Dumas JP. DSC study and computer modelling of the melting process in ice slurry. Chem Eng Sci. 2006. doi:10.1016/j.tca.2006.07.004.
Wielgosz E, Kargul T. Differential scanning calorimetry study of peritectic steel grades. J Therm Anal Calorim. 2015. doi:10.1007/s10973-014-4302-5.
Höhne GWA, Flammersheim HJ, Hemminger W. Differential scanning calorimetry: an introduction for practitioners. 2nd ed. Berlin: Springer; 2003.
Han B, Choi JH, Dantzig JA, Bischof JC. A quantitative analysis on latent heat of an aqueous binary mixture. Cryobiology. 2006. doi:10.1016/j.cryobiol.2005.09.007.
Acknowledgements
We thank Evonik Industries AG for the sample materials of different aqueous deicers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egenolf-Jonkmanns, B., Graen-Heedfeld, J. & Bruzzano, S. Development of calorimetric methods for the characterization of different aqueous deicers. J Therm Anal Calorim 127, 857–862 (2017). https://doi.org/10.1007/s10973-016-5426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5426-6