Abstract
Densities (ρ) of pure liquids and their mixtures have been measured at 303.15 and 313.15 K and atmospheric pressure over the entire composition range for the binary mixtures of benzylalcohol with 1-propanol, 1-butanol, 1-pentanol, and 1-hexanol by using Rudolph Research Analytical digital densitometer (DDM-2911 model). Further, the ultrasonic sound velocities for the above said mixtures were also measured at 303.15 and 313.15 K. The measured density data were used to compute excess molar volumes (V E) and these were compared with the values obtained by Hwang equation. Isentropic compressibility (κ S) and excess isentropic compressibilities (κ ES ) were evaluated from experimental sound velocity and density data. Moreover, the experimental sound velocities were analyzed in terms of theoretic models namely, collision factor theory and free length theory. The experimental results were discussed in terms of intermolecular interactions between component molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The important role played by solvents in chemistry has long been recognized. Mixing effects for a large number of compounds and their mixtures used in the petroleum processing industry are rather difficult be known, hence knowledge of thermodynamic properties such as densities, ultrasonic sound velocity, viscosity and refractive index of many organic mixtures at various temperatures is of great importance and consideration. Mixing of the compounds with different and complex molecular structure causes various intermolecular interactions, resulting in non-ideal behavior [1–5]. We report here the excess volume and excess isentropic compressibilities data of binary mixture of benzylalcohol with 1-propanol, 1-butanol, 1-pentanol, and 1-hexanol at 303.15 and 313.15 K. The organic liquids that were used in the present investigation are having many industrial applications. Benzylalcohol is an important as a solvent for gelatin, cellulose acetate, shellac [6, 7]. 1-Alkanols are interesting simple examples of biologic and industrial important amphiphilic materials [8]. Further, it has been reported [9–11] that the strength of association in alkanols decreases as the carbon chain length in the molecule increases for 1-propanol, 1-butanol, 1-pentanol, and 1-hexanol. A survey of the literature has shown that the thermodynamic properties for binary liquid mixtures of benzylalcohol with aromatic hydrocarbons [12], 1-propanol, and 1-octanol [13] were reported earlier. To our best of knowledge, no systematic studies on excess volumes (V E) and excess isentropic compressibility (κ ES ) were reported for a series of 1-alkanols with benzylalcohol, and hence, we report here new V E and κ ES data for the binary mixtures of benzylalcohol with 1-alkanols (c3–c6). Further, the experimental sound velocity data were also compared with theoretic models proposed by Schaaff’s collision factor theory (CFT) [14] and Jacobson’s free length theory (FLT) [15, 16]. The present study was under taken to know the effect of temperature and chain length of 1-alkanols when mixed with benzylalcohol that may induce changes in sign and magnitude of excess thermodynamic functions.
Experimental
Materials and apparatus
All the chemicals used in the present study were of analytical reagent grade procured from S.D.Fine chemicals Ltd., India and Merck, and their purities were as follows: benzylalcohol 99.5 %, 1-propanol 99.5 %, 1-butanol 99.5 %, 1-pentanol 99.6 %, and 1-hexanol 99.5 %. Prior to experimental measurements, all the liquids were purified as described in the literature [17]. The purity samples were attained by fractional distillation, and the purity of chemicals were checked by comparing the measured densities and ultrasonic sound velocities, which were in good agreement with the literature values [12, 18, 19] and these are given in Table 1. The purity of the sample was further confirmed by GLC single sharp peak. Before use, the chemicals were stored over 0.4 nm molecular sieves for about 72 h to remove water and were later degassed.
All the binary liquid mixtures are prepared by weighting an appropriate amount of pure liquids and an electronic balance (Afoset, ER-120A, India) with a precision of ±0.1 mg by syringing each component into airtight stopper bottles to minimize evaporation losses. The uncertainty of the mole fraction was ±1 × 10−4. After mixing the sample, the bubble free homogenous sample was transferred into the U-tube of the densimeter through a syringe. The density measurements were reported earlier [20] with a Rudolph Research Analytical digital densimeter (DDM-2911 Model). A multi frequency ultrasonic interferometer (M-82 Model, Mittal Enterprise, New Delhi, India) operated at 2 MHz, was used to measure the ultrasonic velocities of the binary liquid mixtures at 303.15 and 313.15 K as described earlier in the literature [21, 22].
Results and discussion
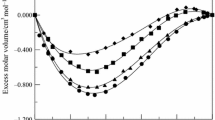
The experimental values of densities (ρ) and ultrasonic sound velocities (u) were used to calculate excess molar volume (V E) and excess isentropic compressibilities (κ ES ) for the binary liquid mixtures of benzylalcohol with 1-alcohols (c3–c6) at 303.15 and 313.15 K. The V E data are graphically represented in Fig. 1 and κ ES data in Fig. 2 for all the binary mixtures at two temperatures. The excess molar volume data of all the binary mixtures were calculated from the measured densities by using the following equation
where ρ is the density of the mixture, and x 1, M 1, ρ 1 and x 2, M 2, ρ 2 are the mole fractions, molecular masses and densities of pure components 1 and 2, respectively.
Variation of excess volume (V E) of the binary liquid mixture of benzylalcohol with 1-propanol (filled square), 1-butanol (filled circle), 1-pentanol (filled triangle), 1-hexanol (filled diamond) at 303.15 K and benzylalcohol with 1-propanol (unfilled square), 1-butanol (unfilled circle), 1-pentanol (unfilled triangle), 1-hexanol (unfilled diamond) at 313.15 K, respectively
Variation of excess isentropic compressibility (κ ES ) of the binary liquid mixture of benzylalcohol with 1-propanol (filled square), 1-butanol (filled circle), 1-pentanol (filled triangle), 1-hexanol (filled diamond) at 303.15 K and benzylalcohol with 1-propanol (unfilled square), 1-butanol (unfilled circle), 1-pentanol (unfilled triangle), 1-hexanol (unfilled diamond) at 313.15 K, respectively
From the result of ultrasonic sound velocities (u) and densities (ρ), isentropic compressibilities (κ S) were calculated as
The corresponding excess isentropic compressibilities (κ ES ) were obtained from the relation [23]:
where κ idS is the ideal value of the isentropic compressibility and was calculated from the following equation [23]:
Here, C pi and α i are the molar heat capacity and the thermal expansion coefficient of the ith component, respectively. The value of C pi and α i obtained and evaluated from the literature [18, 19, 24] and these are mentioned in Table 1.
An examination of V E data given in Fig. 1 show that it is negative over the entire composition range for the binary mixtures containing 1-propanol and 1-butanol whereas V E data exhibit an inversion in sign in the mixtures containing 1-pentanol and 1-hexanol. The sign of excess volume (V E) of system depends on the relative magnitude of expansion and contraction of two liquids. If the factors that cause expansion in volume dominate the factors creating contraction, then V E becomes positive. On the other hand, if the contractive factors dominate the expansive factors than V E become negative.
The factors those are responsible for expansion in volume, which are as follows:
(i) Loss of dipolar association i.e. rupturing of H-bonding of component by the other or breaking up of associates held by weaker forces, namely dipole–dipole or dipole-induced dipole interactions or by Vander Waals forces. (ii) The geometry of molecular structure which does not allow fitting of the one component into others. (iii) Steric hindrance which opposes proximity of the constituent molecules.
While the negative V E values arise due to dominance of the following factors:
(i) Chemical interactions between constituent molecules, such as hetero molecule association through the formation of H-bond known as strong specific interactions. (ii) Accommodation of molecules of one component into the interstitials of the molecules of the other components.
(iii) Geometry of the molecular structure that favors fitting of the component molecules with each other [25]. From the Fig. 1, it is evident that the factors which are responsible for negative V E values which are dominant in the mixtures of benzylalcohol with 1-propanol and 1-butanol. On the other hand, the factors those are responsible for both the positive and negative V E data which are competing with each other in the binary mixtures containing 1-pentanol and 1-hexanol. Further, it is observed that the magnitude of negative V E data decreases with increase in composition of benzylalcohol. According to Marcus [11], the molecules of 1-alkanols are associated through hydrogen bonding in pure state. When these alkanol molecules are mixed with polar molecule like benzylalcohol would induce mutual dissociation of the hydrogen-bonded structures present in pure alkanols with subsequent formation of strong intermolecular hydrogen bonding (O…H–O), relatively weak hydrogen bonding (π–H) between π-electrons of the benzene ring of benzylalcohol and protons of 1-alkanols [13]. Further, the curves in Fig. 1 also reveal that as the chain length of 1-alkanol molecule increases from 1-propanol to 1-hexanol, the negative excess volume tends to shift toward positive excess volume which implies that dipole–dipole interactions are becoming weak in higher 1-alkanols owing to the decrease in their polarizability [26]. Also the positive excess volumes suggest that the higher 1-alkanols pose less proton donating ability than the lower alkanols, and hence hetero-association effects decree in the binary mixtures with an increase of chain length of linear alkanols [27, 28]. The algebraic values of V E for the mixtures of benzylalcohol with 1-alkanols fall in the order:
1-hexanol > 1-pentanol > 1-butanol > 1-propanol
A perusal of κ ES data in Fig. 2 suggests that the property is negative over the entire composition range for all the binary mixtures of benzylalcohol with 1-alkanols at both the temperatures. The κ ES values ascribed to the changes in intermolecular free space defined by Jacobson. These changes occur due to structure-breaking and structure-making effects of the components and the consequent change in geometrical factors. Structure-breaking effect contributes to increases in free space between the molecules and these results in the sound waves covering shorter distance in mixtures. This leads to the positive deviation in compressibilities. On the other hand, structure-making effects would contribute to decrease in free space and a negative deviation in isentropic compressibility. The actual deviation would depend upon the balance between the two opposing effects. The experimental results indicate that the structure-making effect is dominated in all binary mixtures of benzylalcohol with 1-alkanols. The algebraic κ ES values fall in the order:
1-hexanol > 1-pentanol > 1-butanol > 1-propanol
The above order indicates that as the chain length of alkanol increases, the hetero-association decreases due to decrease in polarity of alcohols molecules. Further, it is concluded that algebraic values of excess volume (V E) and excess isentropic compressibility (κ ES ) decrease not only the increasing the chain of 1-alkanols but also decrease in polarizability values of 1-propanol (3.09 D); 1-butanol (1.75 D); 1-pentanol (1.7 D); 1-hexanol (1.55 D) [17].
An examination of κ ES data in Fig. 2 suggests that mixing of benzylalcohol with 1-alkanols leads to interstitial accommodational molecules. This leads to decreasing intermolecular free space between component molecules and formation of closer molecular aggregates [29]. The effects of increasing temperature appear the increasing κ ES values suggesting the increasing the specific molecular interactions. κ ES data become more negative which may be due to high thermal dissociation of hetero-association in liquid mixtures and more interstitial accommodation of one component into another [30]. Over the entire investigated conditions, the absolute values of excess volumes and excess isentropic compressibilities are increased with increasing the temperature in all the binary systems of benzylalcohol with 1-alkanols.
Experimental speeds of sound were analyzed in terms of the CFT [14] and FLT [15, 16]. The pure component data namely, the molar volume (V m), molar volume at absolute zero (V 0), molar available volume (V a), free length (L f), surface area (Y), collision factor (S), average molecular radius (r m), actual volume of molecules per mole (B), and molecular sound velocity (R), which were used to calculate the above said theories were collected from the literature [31]. The methods and details of calculation of theories were discussed earlier [32]. The details of various theories and relevant equations are given as follows: A comparison between experimental speed of sound and theoretic speed of sound values suggests that the model proposed by Schaaff’s CFT gives better estimation of sound velocity data. The methods of calculation of these theories were described in earlier. The merits of these theories were compared in terms of relative root mean deviation by using the following formula [19].
It is evident from the Figs. 3 and 4 that the RMSD data for all the binary systems, CFT model gives better estimation in speed of sound for the liquid mixtures under the investigation.
The experimental V E values and κ ES data have been fitted to Redlich–Kister type polynomial equation [33]
where y E are V E and κ ES , and while x 1 and x 2 refer to the mole fractions of the pure components. The equation takes value from 0 to 2; a i is the adjustable parameter of the function and is determined using the least-squares method. The corresponding standard deviations σ (yE) have been computed using the relation.
where m is the total number of experimental points, and n is the number of coefficients in Eq. (6), and the standard deviations of all the binary mixtures have been presented in Table 2.
References
Canosa JM, Rodriguez A, Iglesias M, Orge B, Tojo J. Thermodynamic properties of alkenediols+acetates at 298.15 K. J Therm Anal Calorim. 1998;52:915–32.
Nan Z, Tan ZC. Thermodynamic properties of the binary mixture of water and n-butanol. J Therm Anal Calorim. 2007;87:539–44.
Marongiu B, Piras A, Porcedda S, Tuveri E. Excess enthalpies of Chloroalkylbenzene+n-heptane(or)cyclohexane mixtures. J Therm Anal Calorim. 2007;91(1):37–46.
Dipali C, Anand A. Apparent molar volume and apparent molar adiabatic compressibility of 2-hydroxy-5 methyl acetophenone in N,N-dimethylformamide at different temperatures. J Therm Anal Cal. 2012;107:21–4.
Narendra K, Srinivasu Ch, Kalpana Ch, Narayanamurthy P. Excess thermo dynamical parameters of binary mixtures of toluene and mesitylene with anisaldehyde using ultrasonic technique at different temperatures. J Therm Anal Cal. 2012;107:25–30.
Venkatramana L, Sivakumar K, Govinda V, Dayananda Reddy K. Study on solution properties of some industrially important solvents with an aromatic alcohol. J Mol Liq. 2013;186:163–70.
Chen K-D, Lin Y-F, Tu C-H. Densities, viscosities, refractive indexes, and surface tensions for mixtures of ethanol, benzyl acetate and benzyl alcohol. J Chem Eng Data. 2012;57:1118–27.
Eads CD. Simple lattice model for salvation of non-polar molecules in hydrogen-bonded liquids. J Phys Chem. 2000;104:6653–61.
Krestov GA. Thermodynamics of solution. Chichester: Ellis Harwood; 1991.
Ali A, Anilkumar N, Vinodkumar S, Ahmad S. Study of molecular interaction in binary mixtures of 1,4-dioxane+1-alcanols. J Acoust Lett. 2000;24:9–16.
Marcus Y. Introduction to liquid state chemistry. New York: Wilwy Interscience; 1977.
Ali A, Tariq M. Thermodynamic and transport behavior of binary liquid mixtures of benzylalcohol with monocyclic aromatics at 303.15 K. J Mol Liq. 2006;128:50–5.
Ali A, Hyder AS, Kumar NA. Molecular interaction in binary mixtures of benzylalcohol with ethanol, propan-1-ol and octan-1-ol at 303 K: An ultrasonic and viscometric study. Collect Czech Chem Commun. 2002;67:1125–40.
Schaaff W. Computation of molecular radius from molar volume and velocity of sound. Z Med Phys. 1940;115:69–75.
Jacobson B. Intermolecular free lengths in the liquid state. Acta Chem Scand. 1952;8:1485–98.
Jacobson B. Intermolecular free length in liquids in relation to sound velocity. J Chem Phys. 1952;20:927–8.
Riddick JA, Bunger W, Sakano TK. Techniques of chemistry, organic solvents, physical properties and methods of purifications. 4th ed. New York: Wiley Interscience; 1986.
Sangeeta S, Martin A, Nirmala D. Densities, speeds of sound, and refractive indices for binary mixtures of 1-butyl-3-methylimidazolium methyl sulphate ionic liquid with alcohols at T = (298.15, 303.15, 308.15, and 313.15) K. J Chem Thermodyn. 2013;57:238–47.
AlTuwaim MS, Alkhaldi HAEK, Al-Jimaz SA, Mohammad AA. Comparative study of physico-chemical properties of binary mixtures of N,N-dimethylformamide with 1-alkanols at different temperatures. J Chem Thermodyn. 2012;48:39–47.
Gowrisankar M, Venkateswarlu P, Kumar KS, Sivarambabu S. Thermodynamics ofamine+ketone mixtures 3. Volumetric, speed of sound data and viscosity at (303.15 and 308.15 K)for the binary mixtures of N,N-dimethylaniline+propiophenone, +p-methylacetophenone, +p-chloroacetophenone. J Mol Liq. 2012;173:172–9.
Radhamma M, Venkatesu P, Prabhakara Rao MV, Lee MJ, Lin HM. Excess molar volumes and ultrasonic studies of dimethylsulphoxide with ketones at 303.15 K. J Chem Thermodyn. 2008;40:492–7.
Syamala V, Raja Shekar D, Venkateswarlu P. Speed of sound, isentropic compressibilities and viscosities of ternary non-electrolyte solutions at 303.15 K. J. Phys Chem Liq. 2010;48:171–82.
Benson GC, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem thermodyn. 1979;11:1061–7.
Jovanovic J, Knezevic-Stevanovic A, Grozdanic D. Prediction of high pressure liquid heat capacities of organic compounds by a group contribution method. J Serb Chem Soc. 2011;76:417–23.
Kijevcanin M, Djordjevic BD, Radovic IR, Zivkovic EM, Tasic AZ, Serbanovic SP. Experimental determination and modeling of densities and excess molar volumes of ternary systems (1-butyl+cyclohexylamine+n-heptane) and corresponding binsries from 288.15 to 323.15 K. Thermochim Acta. 2009;496:71–86.
Mecke R. Infra-red spectra of hydroxylic compounds. Discuss Faraday Soc. 1950;9:161–77.
Nikam PS, Mahale TR, Hasan M. Density and viscosity of binary mixtures of ethyl acetate with methanol, ethanol, propane-1-ol, propane-2-ol, butane-1-ol, 2-methyl propane-1-ol, and 2-methyl peopane-2-ol at9298.15,303.15 and 308.15) K. J Chem Eng Data. 1996;41:1055–8.
Ali A, Nani AK, Chand D, Lal B. Volumetric and viscometric study on N,N-diethylacetamide+1-hexanol/1-heptanol binary liquid mixtures at different temperature Indian. J Pure Appl Phys. 2003;41:928–35.
Rauf MA, Arfan M, Aziz F. Excess molar volume of (N,N-diethyl formamide+an aliphatic alcohol)at 298.15 K. J Chem Thermodyn. 1983;15:1021–3.
Ali A, Kumar NA, Abida A. Ultrasonic and volumetric studies of molecular interactions in acetonitrile+1-alkanol (C6, C8, C10) binary liquid mixtures at different temperatures. J Chin Chem Soc. 2004;51:477–85.
Syamala V, Rajasekhar D, Sivakumar K, Venkateswarlu P. Volumetric, ultrasonic and transport properties of binary liquid mixtures containing dimethylformamide at 303.15 K. Chin J Chem. 2007;25:32–43.
Syamala V, Venkateswarlu P, Kumar KS. Excess volumes, speeds of sound, isentropic compressibilities and viscosities of binary mixtures of acetophenone with chlorotoluenes and nitrotoluenes at 303.15 K. J Chem Eng Data. 2006;51:928–34.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification solutions. J Ind Eng Chem. 1948;40:345–448.
Acknowledgements
The authors express his sincere thanks to Prof. P. Venkateswarlu, Department of chemistry, S.V. University, Tirupati for providing necessary facilities to carry out the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatramana, L., Sreenivasulu, K., Sivakumar, K. et al. Thermodynamic properties of binary mixtures containing 1-alkanols. J Therm Anal Calorim 115, 1829–1834 (2014). https://doi.org/10.1007/s10973-013-3473-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3473-9